Published online Feb 21, 2015. doi: 10.3748/wjg.v21.i7.2108
Peer-review started: July 15, 2014
First decision: August 15, 2014
Revised: August 26, 2014
Accepted: October 14, 2014
Article in press: October 15, 2014
Published online: February 21, 2015
Processing time: 211 Days and 9.9 Hours
AIM: To assess the efficacy of endocytoscopic narrow-band imaging (EC-NBI) for evaluating the severity of inflammation in ulcerative colitis (UC).
METHODS: This retrospective study was conducted at a single tertiary care referral center. We included UC patients who underwent colonoscopy with endocytoscopy from July 2010 to December 2013. EC-NBI was performed, and the images were evaluated by assessing visibility, increased vascularization, and the increased calibers of capillaries and were classified as Obscure, Visible or Dilated. Obscure was indicative of inactive disease, while Visible and Dilated were indicative of acute inflammation. This study received Institutional Review Board approval. The primary outcome measures included the diagnostic ability of EC-NBI to distinguish between active and inactive UC on the basis of histological activity. The conventional endoscopic images were classified according to the Mayo endoscopic score. A score of 0 or 1 indicated inactive disease, whereas a score of 2 indicated active disease.
RESULTS: Fifty-two patients were enrolled. There was a strong correlation between the EC-NBI findings and the histological assessment (r = 0.871, P < 0.01). The sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of EC-NBI for diagnosing acute inflammation were 84.0%, 100%, 87.1%, 100%, and 92.3%, respectively, while those for the Mayo endoscopic score were 100%, 40.7%, 100%, 61.0%, and 69.2%, respectively. Compared with conventional endoscopy, EC-NBI was superior in diagnostic specificity, negative predictive value, and accuracy (P < 0.001, P = 0.001 and P = 0.047, respectively).
CONCLUSION: The EC-NBI finding of capillaries in the rectal mucosa was strongly correlated with histological inflammation and aided in the differential diagnosis between active and inactive UC.
Core tip: Endocytoscopic narrow-band imaging (EC-NBI) is a new technique, in which ultramagnified EC images are used in combination with NBI. The EC-NBI finding associated with the capillaries showed strong correlations with the presence of histological inflammation and the ability to distinguish between inactive and active ulcerative colitis. The sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of the EC-NBI findings for diagnosing acute inflammation were 84.0%, 100%, 87.1%, 100% and 92.3%, respectively. Compared with conventional endoscopy, EC-NBI was superior in diagnostic specificity, negative predictive value, and accuracy.
- Citation: Maeda Y, Ohtsuka K, Kudo SE, Wakamura K, Mori Y, Ogata N, Wada Y, Misawa M, Yamauchi A, Hayashi S, Kudo T, Hayashi T, Miyachi H, Yamamura F, Ishida F, Inoue H, Hamatani S. Endocytoscopic narrow-band imaging efficiency for evaluation of inflammatory activity in ulcerative colitis. World J Gastroenterol 2015; 21(7): 2108-2115
- URL: https://www.wjgnet.com/1007-9327/full/v21/i7/2108.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i7.2108
Surveillance colonoscopy is important for patients with ulcerative colitis (UC) because endoscopic appearance is a predictor of future clinical relapse. Moreover, the goal of therapy in these patients has shifted from symptom control alone to clinical remission in conjunction with mucosal healing. Although most studies on mucosal healing have focused on endoscopic scores, it has been suggested that histological inflammation is a valuable therapeutic goal[1,2]. Studies have indicated a higher risk of relapse in patients with persistent active microscopic inflammation compared with that in patients with normal histology[3,4]. However, it has also been reported that endoscopic and histological assessments differ in patients with active and inactive disease. Therefore, the routine diagnosis of histological activity in UC requires multiple biopsy specimens[5]; however, the collection of biopsy specimens is invasive. Therefore, it is important to identify a surrogate marker of histological activity.
Endocytoscopy (EC; Olympus Medical Systems, Tokyo, Japan)[6] enables the real-time observation of cells and nuclei in vivo using × 450 ultramagnification. This device can facilitate the differentiation of neoplastic and non-neoplastic lesions, and it can also guide the observations of celiac disease, amebic colitis, serrated polyps, and rectal carcinoids[7-14].
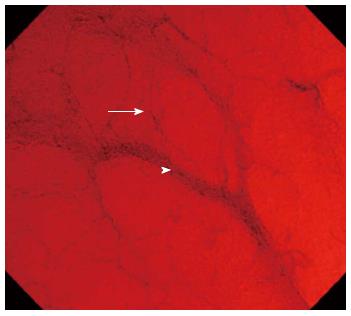
Furthermore, a correlation between EC classification and paired histological sample findings in UC patients has been reported[15]. Narrow-band imaging (NBI; Olympus Medical Systems) is a technique that uses spectral narrow-band optical filters instead of the full spectrum of white light. The applied light wavelengths are restricted to those specific to hemoglobin absorption, thereby clarifying mucosal vascular patterns. The NBI system was developed as an in vivo approach for visualizing the morphological changes in microvessels located in the superficial neoplasia or inflammation[16-19]. Endocytoscopic NBI (EC-NBI) is a new technique in which ultramagnified EC images are used in combination with NBI. It can help in visualizing microvessel structures and assist in the differentiation of capillaries from ascending arterioles and descending veins (Figure 1)[20]. This pilot study aimed to assess the efficacy of EC-NBI for evaluating the severity of inflammation in UC.
This study was conducted at the Digestive Disease Center of the Showa University Northern Yokohama Hospital from July 2010 to December 2013. Using EC-NBI images to distinguish active from inactive UC according to histological activity, a total of 52 patients with a confirmed diagnosis of UC and a Mayo endoscopic score[21] of 0-2 were included; patients with a Mayo endoscopic score of 3 were not recruited. Informed consent was obtained from all patients before the procedure was performed, and ethical approval was granted by the local ethics review committee. This study was conducted in accordance with the Declaration of Helsinki.
In this study, total colonoscopy and routine observation were performed with conventional endoscopy (CF-H260AZI; Olympus Medical Systems) to define the Mayo endoscopic score and target area, which was the most severely inflamed area in the rectum. The selected active section of the rectal area was observed using EC-NBI. The rectal mucosa was washed with excess water, and EC-NBI was performed without spraying or staining. Subsequently, a biopsy specimen was obtained from the rectal mucosa for histological analysis of the same area.
As mentioned in a previous publication from our facility[10], the integrated-type EC (CF-Y-0020; Olympus Medical Systems) has an adjustable scope with an outside diameter of 13.6 mm at the distal end and a working length of 1330 mm. This scope enables consecutive conventional and ultramagnified endoscopic observation using one-touch switch operation. In the endocytoscopic mode, the scope has a magnification capability of × 450 displayed on a 14-inch monitor; the light focusing depth of field is 50 μm, and the surface field of view is 400 μm × 400 μm. Moreover, EC-NBI facilitates the increased visualization of microvessel structures, allowing for the differentiation of capillaries from ascending arterioles and descending veins. The principle of the endocytoscopic mode is similar to that of contact endoscopy and is set up with a fixed focus. The scope tip is applied to the target area after switching to the NBI mode. The EC-NBI images are immediately obtained using the mode-changing switch on the handle.
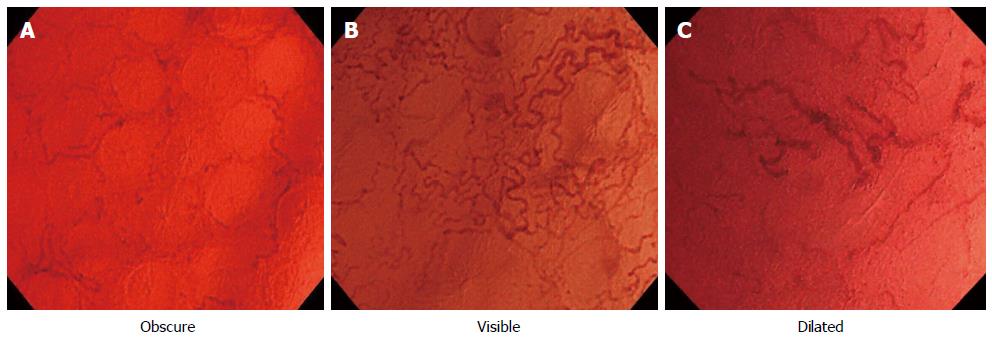
For each case, multiple EC-NBI images were obtained because the diameters of capillaries may change gradually. These images were obtained using the integrated image capture system and saved on the server that hosted the database in JPEG format with a pixel array of 640 × 480 and 16-bit color (Olympus Medical Systems). The EC-NBI and conventional endoscopic images for each case were separately downloaded from the server for analysis by a reader (Ya M), who was a trained endoscopist blinded to the findings of the histological analysis. From the multiple images obtained for each case, the reader selected the image that showed the most microvessel distension. All EC-NBI and conventional endoscopic images from the target area were randomly allocated. The EC-NBI images were classified by grade of visibility, increased vascularization, and the increased calibers of capillaries as follows: Obscure (Figure 2A), the capillaries were not clearly visible; Visible (Figure 2B), the capillaries were clearly visible and/or increased vascularization was observed; Dilated (Figure 2C), the caliber of the capillaries was unusually large (three times as large as that of the surrounding capillaries). Obscure was indicative of inactive disease, while Visible and Dilated were indicative of acute inflammation in our EC-NBI findings. The conventional endoscopic images were classified according to the Mayo endoscopic score (Table 1). A score of 0 or 1 indicated inactive disease, whereas a score of 2 indicated active disease[22].
| 0 | Normal or inactive disease |
| 1 | Mild disease: erythema, decreased vascular pattern, mild friability |
| 2 | Moderate disease: marked erythema, absent vascular pattern, friability, erosions |
| 3 | Severe disease:spontaneous bleeding, ulceration |
All biopsy specimens were fixed in 10% formalin, embedded in paraffin, serially sectioned, and stained using hematoxylin and eosin (HE). Histological examination was performed by an experienced pathologist (S.H.) who was blinded to both the clinical and endoscopic data.
Histological assessment was based on the Geboes index (Table 2)[23]. The scale included six grades, and each grade was divided into 4-5 subgroups[23,24]. The patients were divided into two groups according to their final indices; one group was comprised of those with an index of ≤ 3.0, and the other contained those with an index of > 3.0. A grade of 3.1 indicated the presence of neutrophils in the epithelium, which was representative of acute inflammation and a predictor of relapse[25]. A Geboes index of ≤ 3.0 indicated inactive disease, while an index of > 3.0 indicated active disease.
| Grade 0 | Structural (architectural change) |
| Subgrades | |
| 0.0 | No abnormality |
| 0.1 | Mild abnormality |
| 0.2 | Mild or moderate diffuse or multifocal abnormalities |
| 0.3 | Severe diffuse or multifocal abnormalities |
| Grade 1 | Chronic inflammatory infiltrate |
| Subgrades | |
| 1.0 | No increase |
| 1.1 | Mild but unequivocal increase |
| 1.2 | Moderate increase |
| 1.3 | Marked increase |
| Grade 2 | Lamina propria neutrophils and eosinophils |
| 2A Eosinophils | |
| 2A. 0 | No increase |
| 2A.1 | Mild but unequivocal increase |
| 2A.2 | Moderate increase |
| 2A.3 | Marked increase |
| 2B Neutrophils | |
| 2B. 0 | None |
| 2B.1 | Mild but unequivocal increase |
| 2B.2 | Moderate increase |
| 2B.3 | Marked increase |
| Grade 3 | Neutrophils in epithelium |
| 3.0 | None |
| 3.1 | < 5% crypts involved |
| 3.2 | < 50% crypts involved |
| 3.3 | > 50% crypts involved |
| Grade 4 | Crypt destruction |
| 4.0 | None |
| 4.1 | Probable-local excess of neutrophils in part of crypt |
| 4.2 | Probable-marked attenuation |
| 4.3 | Unequivocal crypt destruction |
| Grade 5 | Erosion or ulceration |
| 5.0 | No erosion, ulceration, or granulation tissue |
| 5.1 | Recovering epithelium + adjacent inflammation |
| 5.2 | Probable erosion-focally stripped |
| 5.3 | Unequivocal erosion |
| 5.4 | Ulcer or granulation tissue |
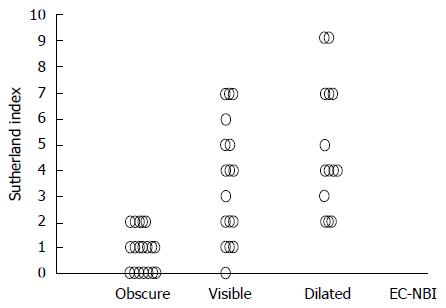
Diagnoses obtained by conventional endoscopy and EC-NBI were compared with those based on the Geboes index, and the diagnostic abilities of conventional endoscopy and EC-NBI to differentiate between inactive and active disease were assessed. Next, the Spearman rank correlation coefficient between the Mayo endoscopic score and the Geboes index and that between EC-NBI findings and the Geboes index were calculated. We compared the EC-NBI findings with the Sutherland index for the clinical severity of inflammation in UC. The Spearman rank correlation coefficient between the EC-NBI findings and Sutherland index was calculated[26]. All clinical data were collected from medical records.
In a subanalysis, the inter- and intraobserver agreements for conventional endoscopy and EC-NBI were calculated for the three endoscopists (Ya M., K. W., and Yu. M.). Thirty images each from conventional endoscopy and EC-NBI were randomly selected from all images and randomly arranged for assessments among the three endoscopists. To assess intraobserver agreement, the same images were randomly allocated to the endoscopists for reassessment more than a month after the initial assessment. Interobserver agreement was calculated from the results of the first reading, and intraobserver agreement was calculated by comparing the first and second assessments and presented as the kappa value.
For the statistical analysis, a computerized database was designed using R (The R Foundation for Statistical Computing, Vienna, Austria, v. 2.13.0). The quantitative data were expressed as the mean ± SD values. Statistical significance was evaluated using Fisher’s exact test or Student’s t-test as appropriate. P values (two-tailed) of < 0.05 were considered statistically significant. The agreements were interpreted as follows: no agreement (0), slight agreement (< 0.20), fair agreement (0.21-0.40), moderate agreement (0.41-0.60), substantial agreement (0.61-0.80), and almost perfect agreement (≥ 0.81), as proposed by Landis and Koch[27].
Examination with the new prototype EC was uneventful in all cases. The clinical features of the 52 patients are shown in Table 3. The additional time required to perform a further examination with EC-NBI was approximately 3 min. According to the EC-NBI findings, the images were classified into 21 obscure cases, 17 visible cases, and 14 dilated cases. With regard to histological activity, 25 and 27 cases had Geboes indices of ≤ 3.0 and > 3.0, respectively. The diagnoses obtained using EC-NBI were compared with the histological diagnoses. All obscure cases had Geboes indices of ≤ 3.0. However, 76.5% (13/17) of the visible and all of the dilated cases had a Geboes indices of > 3.0. There was a strong correlation between EC-NBI finding and the Geboes index (r = 0.871, P < 0.01; Table 4). We also assessed the correlation between conventional endoscopic findings and histological activity (r = 0.665, P < 0.01; Table 4). The sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of the EC-NBI findings for diagnosing acute inflammation were 84.0%, 100%, 87.1%, 100%, and 92.3%, respectively (Table 5), while those for the Mayo endoscopic score were 100%, 40.7%, 100%, 61.0%, and 69.2%, respectively (Table 5). Compared with conventional endoscopy, EC-NBI was superior in terms of diagnostic specificity, negative predictive value, and accuracy (Table 6). The correlations between EC-NBI finding and the Sutherland index as an indicator of clinical disease activity are shown in Figure 3. The EC-NBI findings showed a positive correlation with the Sutherland index (r = 0.721, P < 0.01).
| Total number of patients | 40 |
| Sex, M/F | 19/21 |
| Age, yr | 46.7 |
| Disease duration, yr | 20 |
| Type of UC | |
| Total colitis | 20 |
| Left-sided | 6 |
| Proctitis | 14 |
| Clinical course | |
| Relapsing–remitting type | 21 |
| Chronic continuous type | 11 |
| One attack only | 8 |
| Treatment | |
| 5-ASA | 39 |
| SASP | 10 |
| Mesalazine | 33 |
| Prednisolone | 2 |
| AZA | 4 |
| CAP | 1 |
| No medication | 1 |
| Geboes index | EC-NBI | Mayo endoscopic score | Total | ||||
| Obscure | Visible | Dilated | 0 | 1 | 2 | ||
| 1 | 17 | 1 | 0 | 12 | 6 | 0 | 18 |
| 2 | 4 | 3 | 0 | 5 | 2 | 0 | 7 |
| 3 | 0 | 6 | 1 | 2 | 5 | 0 | 7 |
| 4 | 0 | 7 | 11 | 1 | 7 | 10 | 18 |
| 5 | 0 | 0 | 2 | 0 | 1 | 1 | 2 |
| the Spearman rank correlation coefficient | |||||||
| r = 0.871, P < 0.01 | r = 0.665, P < 0.01 | ||||||
| Acute inflammation | Acute inflammation | ||||
| EC-NBI finding | - | + | Mayo score | - | + |
| Obscure | 21 | 0 | 0/1 | 25 | 16 |
| Visible/dilated | 4 | 27 | 2 | 0 | 11 |
| EC-NBI | Mayo score | P value | |
| Sensitivity | 84.0% | 100% | P = 0.110 |
| Specificity | 100% | 40.7% | P < 0.001 |
| PPV | 87.1% | 100% | P = 0.568 |
| NPV | 100% | 61.0% | P = 0.001 |
| Accuracy | 92.3% | 69.2% | P = 0.047 |
To assess the reproducibility of EC-NBI, the kappa values for the interobserver agreements were assessed for the three readers. A kappa value of 0.823 (95%CI: 0.682-0.965) and very high agreements were observed in association with the EC-NBI findings. There was also excellent intraobserver agreement, with kappa values of 0.850 (95%CI: 0.689-1.011) for Ya M, 0.750 (95%CI: 0.549-0.950) for K.W., and 0.801 (95%CI: 0.620-0.983) for Yu M.
To our knowledge, this is the first study to show the potential applicability of the newly developed EC-NBI system for assessing the histological disease activity of UC. Recent studies have shown that an increase in the severity of inflammation, as detected both endoscopically and histologically, correlated with an increased frequency of relapse or of dysplasia[28,29]. However, the consistency between the endoscopic and histological findings in UC patients has not been elucidated. It has been suggested that conventional colonoscopy is not reliable for assessing acute inflammation and the potential for relapse[1]. We confirmed a strong correlation between the EC-NBI findings and histological activity and also found that this system has a high diagnostic ability to differentiate between active and inactive UC. EC-NBI was superior to conventional endoscopy in terms of sensitivity, negative predictive value, and accuracy. The results obtained in this study may be serve as a simple and objective foundation for the optimization of therapeutic strategies for the treatment of UC.
Employing an approach similar to that used in the present study, Bessho et al[15] have shown the benefits of classifying the histological activity of UC using EC. In their report, the shape and distance of the crypt were also assessed, and an excellent correlation was revealed between EC and histological diagnosis. However, their study had some limitations because the EC observations required pretreatment with methylene blue or toluidine blue staining. The additional time required for EC observation was reported to be approximately 20 min in one area[15]. Therefore, a more practical procedure for assessing the histological activity of UC is necessary. EC-NBI images can be immediately obtained using the mode-changing switch on the handle without the need for spraying or for staining. EC-NBI can be performed in approximately 3 min; therefore, this procedure may be intuitive because it involves the evaluation of visibility, increased vascularization, and the increased calibers of capillaries in the target area. Both the inter- and intraobserver agreements for the EC-NBI finding were excellent in this study. However, there were only three types of EC-NBI finding, which may have contributed to its high reproducibility.
In this study, capillaries in the rectal mucosa were assessed. Previous studies have evaluated. The colorectal microvasculature with NBI [30], demonstrating a good correlation with Histological diagnosis. However, the difference between the capillaries and other types of microvasculature was not considered. This differentiation is very important for assessing the visibility and the increased calibers of microvessels. The mucosal capillary plexus of the large intestine is typically arranged in a regular, hexagonal, honeycomb pattern surrounding the mucosal glands. The plexus is supplied by arteries that are divided within the submucosa to form the subepithelial capillaries. Venous drainage occurs through the venules immediately originating under the mucosal surface and leading to the submucosal veins[20]. To our knowledge, this is the first report to assess the differences between the capillaries and other vessels using the ultramagnification afforded by EC in combination with the detailed microvessel visualization permitted by NBI.
Recent data have provided evidence that the differential regulation of angiogenic mediators involved in inflammatory bowel disease-associated chronic inflammation causes this pathological angiogenesis. Many factors are involved in this phenomenon, including growth factors/cytokines, chemokines, adhesion molecules, integrins, matrix-associated molecules, and signaling targets[31]. Our EC-NBI findings were based on the evidence of increased vascularization and the increased calibers of the capillaries in the target area.
This study has some limitations. First, the number of subjects was small. This was because there was only one integrated-type endocytoscope in the facility. Second, in the present study, the EC-NBI images were obtained in vivo but were not actually diagnosed in vivo. We aimed to assess the accuracy of diagnosis solely on the basis of EC-NBI, with the assessors blinded to the conventional endoscopic findings. Moreover, the lack of enrollment of patients with severe active UC may have biased the results. The presence of ulcers or the prominent spontaneous hemorrhaging of the colorectal mucosa is important for the diagnosis of the severe active stage. These lesions can be recognized easily with conventional endoscopy. Furthermore, the blue light emitted around the wavelength used for NBI is mainly absorbed by hemoglobin, and the prominent hemorrhaging of the surface mucosa can be a major disadvantage to NBI assessments. Therefore, patients with a Mayo endoscopic score of 3 were not recruited in this study. The EC-NBI findings were compared with histological activity as a predictive factor of long-term clinical prognosis; however, the findings were not compared directly with the long-term clinical prognosis.
In conclusion, the EC-NBI finding of capillaries in the rectal mucosa was strongly correlated with the presence of histological inflammation and the ability to distinguish between inactive and active UC. Furthermore, EC-NBI is easy to perform and has high reproducibility. EC-NBI findings can be effective in the on-site evaluation of inflammatory activity in UC. To assess the clinical efficacies of the EC-NBI findings, further multicenter prospective clinical trials involving larger patient samples and direct comparisons with long-term clinical prognosis are required.
Surveillance colonoscopy is important for patients with ulcerative colitis (UC) because endoscopic appearance is a predictor of future clinical relapse. Although most studies on mucosal healing have focused on endoscopic scores, it has been suggested that histological inflammation is a valuable therapeutic goal. However, it has also been reported that endoscopic and histological assessments differ in patients with active and inactive disease. Therefore, it is important to identify a surrogate marker of histological activity. The efficacy of endocytoscopic narrow-band imaging (EC-NBI) for evaluating the severity of inflammation in UC has not been evaluated.
EC-NBI is a new technique in which ultramagnified endocytoscopy images are used in combination with NBI. It can help in visualizing microvessel structures and assist in the differentiation of capillaries from ascending arterioles and descending veins.
EC-NBI can help in visualizing microvessel structures and assist in the differentiation of capillaries from ascending arterioles and descending veins. The additional time required toperform a further examination with EC-NBI was approximately 3 min. There was a strong correlation between EC-NBI findings and histlogical diagnosis. Compared with conventional endoscopy, EC-NBI was superior in terms of diagnostic specificity, negative predictive value, and accuracy. The correlations between EC-NBI findings and the Sutherland index as an indicator ofclinical disease activity. The EC-NBI findings showed a positive correlation with the Sutherland index.
The study results suggest that the EC-NBI finding of capillaries in the rectal mucosa was strongly correlated with the presence of histological inflammation and the ability to distinguish between inactiveand active UC. EC-NBI findings can be effective in the on-site evaluation of inflammatory activity in UC.
EC (Olympus Medical Systems, Tokyo, Japan) is a novel endoscopy enables the real-timeobservation of cells and nuclei in vivo using 450 × ultramagnification. NBI (Olympus Medical Systems) is a technique that uses spectral narrow-band optical filters instead of the full spectrum of white light. The applied light wavelengths are restricted to those specific to hemoglobin absorption, thereby clarifying mucosal vascular patterns.
Maeda et al have assessed the efficacy of endocytoscopic narrow-band imaging findings to evaluate the severity of inflammation in ulcerative colitis. The study has been well designed and written.
P- Reviewer: Kayadibi H, Koulaouzidis A S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
| 1. | D’Haens G, Sandborn WJ, Feagan BG, Geboes K, Hanauer SB, Irvine EJ, Lémann M, Marteau P, Rutgeerts P, Schölmerich J. A review of activity indices and efficacy end points for clinical trials of medical therapy in adults with ulcerative colitis. Gastroenterology. 2007;132:763-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 722] [Cited by in RCA: 795] [Article Influence: 44.2] [Reference Citation Analysis (1)] |
| 2. | Hanauer SB, Kirsner JB. Treat the patient or treat the disease? Dig Dis. 2012;30:400-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Riley SA, Mani V, Goodman MJ, Dutt S, Herd ME. Microscopic activity in ulcerative colitis: what does it mean? Gut. 1991;32:174-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 396] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 4. | Bitton A, Peppercorn MA, Antonioli DA, Niles JL, Shah S, Bousvaros A, Ransil B, Wild G, Cohen A, Edwardes MD. Clinical, biological, and histologic parameters as predictors of relapse in ulcerative colitis. Gastroenterology. 2001;120:13-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 353] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 5. | Itzkowitz SH, Present DH. Consensus conference: Colorectal cancer screening and surveillance in inflammatory bowel disease. Inflamm Bowel Dis. 2005;11:314-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 395] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 6. | Inoue H, Kudo SE, Shiokawa A. Technology insight: Laser-scanning confocal microscopy and endocytoscopy for cellular observation of the gastrointestinal tract. Nat Clin Pract Gastroenterol Hepatol. 2005;2:31-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 84] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 7. | Inoue H, Sasajima K, Kaga M, Sugaya S, Sato Y, Wada Y, Inui M, Satodate H, Kudo SE, Kimura S. Endoscopic in vivo evaluation of tissue atypia in the esophagus using a newly designed integrated endocytoscope: a pilot trial. Endoscopy. 2006;38:891-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 86] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 8. | Neumann H, Fuchs FS, Vieth M, Atreya R, Siebler J, Kiesslich R, Neurath MF. Review article: in vivo imaging by endocytoscopy. Aliment Pharmacol Ther. 2011;33:1183-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 9. | Sasajima K, Kudo SE, Inoue H, Takeuchi T, Kashida H, Hidaka E, Kawachi H, Sakashita M, Tanaka J, Shiokawa A. Real-time in vivo virtual histology of colorectal lesions when using the endocytoscopy system. Gastrointest Endosc. 2006;63:1010-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 103] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 10. | Kudo SE, Wakamura K, Ikehara N, Mori Y, Inoue H, Hamatani S. Diagnosis of colorectal lesions with a novel endocytoscopic classification - a pilot study. Endoscopy. 2011;43:869-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 103] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 11. | Hosoe N, Kobayashi T, Kanai T, Bessho R, Takayama T, Inoue N, Imaeda H, Iwao Y, Kobayashi S, Mukai M. In vivo visualization of trophozoites in patients with amoebic colitis by using a newly developed endocytoscope. Gastrointest Endosc. 2010;72:643-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Kutsukawa M, Kudo SE, Ikehara N, Ogawa Y, Wakamura K, Mori Y, Ichimasa K, Misawa M, Kudo T, Wada Y. Efficiency of endocytoscopy in differentiating types of serrated polyps. Gastrointest Endosc. 2014;79:648-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Matysiak-Budnik T, Coron E, Mosnier JF, Le Rhun M, Inoue H, Galmiche JP. In vivo real-time imaging of human duodenal mucosal structures in celiac disease using endocytoscopy. Endoscopy. 2010;42:191-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Maeda Y, Kudo SE, Mori Y. In vivo assessment of a carcinoid tumor using endocytoscopy. Dig Endosc. 2013;25:465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 15. | Bessho R, Kanai T, Hosoe N, Kobayashi T, Takayama T, Inoue N, Mukai M, Ogata H, Hibi T. Correlation between endocytoscopy and conventional histopathology in microstructural features of ulcerative colitis. J Gastroenterol. 2011;46:1197-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Gono K, Obi T, Yamaguchi M, Ohyama N, Machida H, Sano Y, Yoshida S, Hamamoto Y, Endo T. Appearance of enhanced tissue features in narrow-band endoscopic imaging. J Biomed Opt. 2004;9:568-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 634] [Cited by in RCA: 611] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 17. | Sano Y, Muto M, Tajiri H, Ohtsu A, Yoshida S. Optical/digital chromoendoscopy during colonoscopy using narrow-band imaging system. Dig Endosc. 2005;17 Suppl:S43-S48. [RCA] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 18. | Kudo T, Matsumoto T, Esaki M, Yao T, Iida M. Mucosal vascular pattern in ulcerative colitis: observations using narrow band imaging colonoscopy with special reference to histologic inflammation. Int J Colorectal Dis. 2009;24:495-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 19. | Wada Y, Kudo SE, Kashida H, Ikehara N, Inoue H, Yamamura F, Ohtsuka K, Hamatani S. Diagnosis of colorectal lesions with the magnifying narrow-band imaging system. Gastrointest Endosc. 2009;70:522-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 148] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 20. | Konerding MA, Fait E, Gaumann A. 3D microvascular architecture of pre-cancerous lesions and invasive carcinomas of the colon. Br J Cancer. 2001;84:1354-1362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 207] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 21. | Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317:1625-1629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1958] [Cited by in RCA: 2250] [Article Influence: 59.2] [Reference Citation Analysis (0)] |
| 22. | Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, Travers S, Rachmilewitz D, Hanauer SB, Lichtenstein GR. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462-2476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2744] [Cited by in RCA: 2885] [Article Influence: 144.3] [Reference Citation Analysis (2)] |
| 23. | Geboes K, Riddell R, Ost A, Jensfelt B, Persson T, Löfberg R. A reproducible grading scale for histological assessment of inflammation in ulcerative colitis. Gut. 2000;47:404-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 575] [Cited by in RCA: 691] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 24. | Bessissow T, Lemmens B, Ferrante M, Bisschops R, Van Steen K, Geboes K, Van Assche G, Vermeire S, Rutgeerts P, De Hertogh G. Prognostic value of serologic and histologic markers on clinical relapse in ulcerative colitis patients with mucosal healing. Am J Gastroenterol. 2012;107:1684-1692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 222] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 25. | Li CQ, Xie XJ, Yu T, Gu XM, Zuo XL, Zhou CJ, Huang WQ, Chen H, Li YQ. Classification of inflammation activity in ulcerative colitis by confocal laser endomicroscopy. Am J Gastroenterol. 2010;105:1391-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 119] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 26. | Sutherland LR, Martin F, Greer S, Robinson M, Greenberger N, Saibil F, Martin T, Sparr J, Prokipchuk E, Borgen L. 5-Aminosalicylic acid enema in the treatment of distal ulcerative colitis, proctosigmoiditis, and proctitis. Gastroenterology. 1987;92:1894-1898. [PubMed] |
| 27. | Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43944] [Cited by in RCA: 41922] [Article Influence: 873.4] [Reference Citation Analysis (0)] |
| 28. | Rutter M, Saunders B, Wilkinson K, Rumbles S, Schofield G, Kamm M, Williams C, Price A, Talbot I, Forbes A. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology. 2004;126:451-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 895] [Cited by in RCA: 882] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 29. | Gupta RB, Harpaz N, Itzkowitz S, Hossain S, Matula S, Kornbluth A, Bodian C, Ullman T. Histologic inflammation is a risk factor for progression to colorectal neoplasia in ulcerative colitis: a cohort study. Gastroenterology. 2007;133:1099-1105; quiz 1340-1341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 617] [Cited by in RCA: 570] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 30. | Esaki M, Kubokura N, Kudo T, Matsumoto T. Endoscopic findings under narrow band imaging colonoscopy in ulcerative colitis. Dig Endosc. 2011;23 Suppl 1:140-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 31. | Chidlow JH, Shukla D, Grisham MB, Kevil CG. Pathogenic angiogenesis in IBD and experimental colitis: new ideas and therapeutic avenues. Am J Physiol Gastrointest Liver Physiol. 2007;293:G5-G18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 124] [Article Influence: 6.9] [Reference Citation Analysis (0)] |