Published online Feb 14, 2015. doi: 10.3748/wjg.v21.i6.1972
Peer-review started: June 19, 2014
First decision: July 9, 2014
Revised: July 21, 2014
Accepted: September 18, 2014
Article in press: September 19, 2014
Published online: February 14, 2015
Processing time: 238 Days and 4.3 Hours
AIM: To use existing hepatitis C virus (HCV) antiviral therapies as access to new treatments is limited.
METHODS: A PubMed search for randomised control trials or meta-analysis related to response-guided therapy of HCV genotype 1 patients was undertaken using pegylated interferon and ribavirin (PR), boceprevir (B) and telaprevir (T) and lead-in where response-guided therapy at TW4(TW4), 8(TW8), 10(TW10), or 12(TW12) based on HCVRNA(+) or HCVRNA(-). Studies presented at major conferences were also used. Where necessary, a post-hoc analysis was performed. A response-guided management roadmap was created based on sustained virological response (SVR).
RESULTS: Starting with PR, those with HCVRNA(-) at TW4 have > 86% SVR, while those are HCVRNA(+) have 34%-41.7% SVR. HCVRNA(-) TW4 patients can have 24 wk PR if HCVRNA < 400000 IU/mL. Alternatively, 28 wk BPR has similar SVR. If HCVRNA(+) at TW4, 72 wk PR leads to 53% SVR, hence BPR is a better option, and if HCVRNA(-) by TW8, 28 wk therapy is sufficient. If HCVRNA(+) at TW8, then HCVRNA should be checked at TW10 and TW12. By TW12, HCVRNA ≥ 100 IU/mL activates the stopping rule. This roadmap is applicable for treatment-naïve, treatment failures and cirrhotic patients. Validation from an Asia Pacific early access boceprevir program confirmed the findings that HCVRNA(-) at TW4, or TW8 conferred > 80% SVR, leading to the “80-80” rule.
CONCLUSION: Using a roadmap based on HCVRNA(-) at TW4 or TW8 (the “80-80” rule), high SVR can be achieved, and guide the best choices for treatment, and also reduces drug exposure in poor responders.
Core tip: Lex management of hepatitis C virus (HCV) genotype 1 using a simplified road map and "80-80" rule can help physicians manage their patients better. This roadmap distills the essential findings from using pegylated interferon and ribavirin as well as boceprevir using treatment week 4 and 8 virological responses based on whether HCVRNA is detectable or not.
- Citation: Lim SG. Chronic hepatitis C genotype 1 treatment roadmap for resource constrained settings. World J Gastroenterol 2015; 21(6): 1972-1981
- URL: https://www.wjgnet.com/1007-9327/full/v21/i6/1972.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i6.1972
Chronic Hepatitis C is the one of most common causes of chronic viral liver disease worldwide afflicting 130-170 million persons[1], leading to significant morbidity and mortality due to liver disease and hepatocellular carcinoma of infected persons. The standard of care for hepatitis C therapy has long been pegylated interferon and ribavirin for 48 wk until 2011, but this therapy has been suboptimal in efficacy, safety and tolerance[2]. In 2011, the first generation protease inhibitors, boceprevir and telaprevir[3] were approved, then in 2013, simeprevir[4] and sofosbuvir[5] were approved by the United States Food and Drug Adminstration and then by the European Medicines Agency. They represent a substantial advance in efficacy with better tolerability, but come at a high cost. While resource rich countries can afford to use these treatments as first line therapy, access to such expensive, may be limited in resource constrained countries. Resource constrained countries are defined as those countries where the latest therapies sofosbuvir and simeprevir and not currently available nor likely in the near future, and where pegylated interferon and ribavirin (PR) ± boceprevir, are are still the standard of care. The roles of these new agents, in the context of PR with or without boceprevir and telaprevir is unclear in treatment naïve patients and in treatment failures, particularly where there is little experience on its use in resource constrained countries. The first generation protease inhibitors may still have a role in current management of chronic hepatitis C genotype 1 patients, although telaprevir is less widely available, and does not use a lead-in strategy. Hence, it is a somewhat less optimal therapeutic choice in a resource constrained setting. Alternatively, the complex treatment strategy for boceprevir based on presence of cirrhosis and previous treatment failure is compounded by the differences between the United States[6] and the European label[7] with regards to the duration of therapy. Different algorithms have been proposed for different types of hepatitis C virus (HCV) patients: treatment naïve, treatment experienced and for cirrhotics. Consequently, there is an important unmet need to simplify treatment strategy using a roadmap based management that can provide a practical guide for decision making at fixed timepoints (weeks 4, 8, 10 and 12) during HCV treatment. Undetectable or detectable HCVRNA was used as a decision tool as this only relied on the test result at that timepoint, in contrast to previous decision tools such as reduction of HCVRNA from baseline of or by stratifying the reduction in HCVRNA which requires at least two results.
A PubMed search for meta-analyses or randomised control trials of hepatitis C genotype 1 treatment with PR for 24, 48 and 72 wk, where treatment week 4 (TW4) HCVRNA results and SVR were reported. Where no published articles were available, studies published in abstract format from key international liver meetings were utilised.
For boceprevir treatment, randomised control trials of phase 2 and 3 studies were used. In studies where the information on detectable or undetectable HCVRNA at TW4, 8, 10 or 12 were not presented, a post-hoc analysis was performed and data was extracted from the following studies: the phase 2 study (SPRINT-1) and the phase 3 (SPRINT-2 and RESPOND-2) boceprevir clinical trials[8-10], and PROVIDE studies[11]. Data was also included from analyses from a meta-analysis of cirrhotic patients[12] derived from five phase 3 boceprevir trials (SPRINT-2, RESPOND-2, peg2a, PROVIDE and anemia management study). The detailed methodology and primary outcomes from these studies have been are available in clinical trials.gov: NCT00423670, NCT00708500; NCT00705432, and NCT00910624. For telaprevir therapy, there is little information on lead-in and response-guided therapy.
The primary end point in all studies was SVR, defined as undetectable HCVRNA 24 wk after completing treatment. Plasma HCVRNA levels were measured using COBAS TaqMan or COBAS TaqMan 2.0 (Roche Diagnostics) with respective lower limits of detection of 15 IU/mL and 9.3 IU/mL. Virologic response rates were assessed at various time points during therapy, at TW4, TW8, TW10 and TW12 after starting PR, and during boceprevir or telaprevir therapy.
TW4 has been validated as the most important predictor of SVR[13]. With regards to predictors of SVR[14]. multivariate analysis showed that the strongest predictor of SVR in Caucasians and Black patients given boceprevir triple therapy was ≥ 1 log reduction in HCVRNA, even stronger that the IL28B cc genotype. In the largest Asian study of peginterferon and ribavirin[15], multivariate analysis again showed that week 4 undetectable HCVRNA was a stronger predictor of SVR than IL28B cc genotype. Consequently while IL28B genotype is still an important predictor, it may not be crucial in the decision to start therapy since week 4 HCVRNA is the stronger predictor.
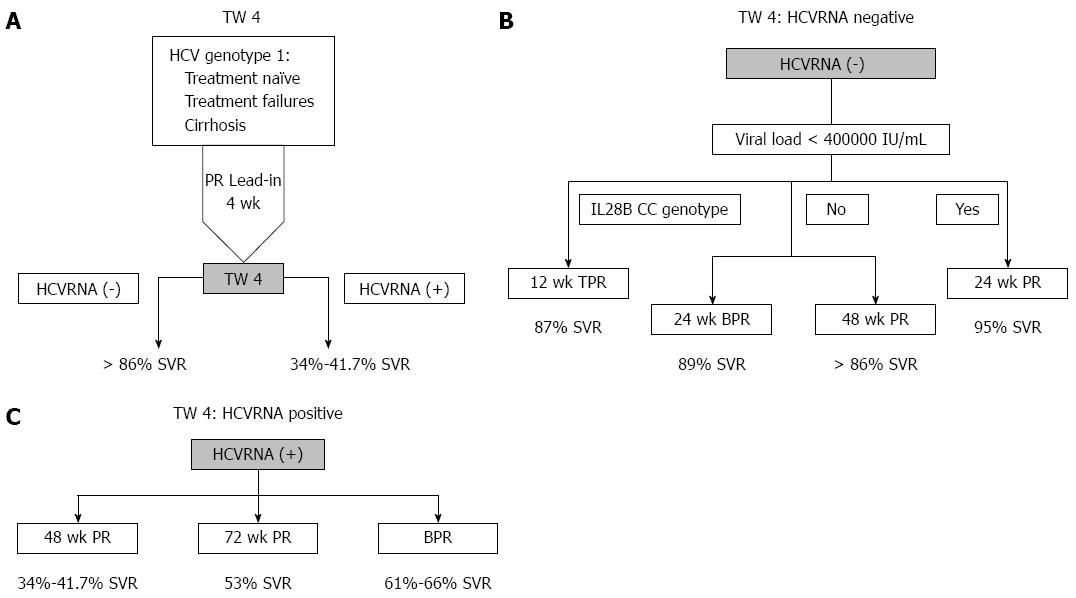
The largest randomized control trial (RCT) of 48 wk PR[13] had few Asians, but the SVR rate in those with undetectable HCVRNA at TW4 was 86% while those with detectable HCVRNA had SVR of 33.9%. In the largest Asian RCT of 48 wk PR[15], those with undetectable HCVRNA at TW4 had 98% SVR, but if HCVRNA was detectable, SVR was 34.5%, remarkably similar to non-Asians. Consequently the first decision point of the roadmap is TW4 (Figure 1A). The difference in the two studies was that undetectable HCVRNA at TW4 was achieved in only 8.9% of patients in the IDEAL study, but 54.8% in the Asian study due to the high prevalence of the IL28B good response genotype[16].
With a high SVR of 86%-98% with 48 wk PR, can therapy be shortened? A meta-analysis[17] showed that SVR was significantly higher with 48 wk PR, 94.1% vs 79.7% for 24 wk PR (RR = 1.15; 95%CI: 1.07-1.24; P < 0.0001). But those with low baseline viral load (< 40000 IU/mL), had no difference in SVR, 95.5% for 48 wk PR vs 90.6% in the 24 wk PR (RR = 1.05; 95%CI: 0.99-1.11; P = not significant). Consequently, only those low baseline viral load can have 24 wk PR.
Can BPR for 24 wk be an alternative? In the SPRINT-2 study[9], those who achieved undetectable HCVRNA at TW4 and TW8 had 24 wk BPR had 88% SVR, compared to 97% SVR of 97% in those who had 48 wk BPR (P = NS), making this an alternative to 48 wk PR (Figure 1B).
An alternative to boceprevir is telaprevir. In the CONCISE study[18], treatment naïve or relapser patients with the IL28B CC genotype who had undetectable HCVRNA at TW4 who were randomised to 12 wk of telaprevir and PR (TPR) achieved 87% SVR compared to 97% in the 12 wk TPR+12 wk PR group (P = NS). This is the only study telaprevir was used with lead-in based on undetectable HCVRNA at TW4.
A Cochrane systematic review[19] addressed whether 72 wk PR was superior to 48 wk PR in these patients. The meta-analysis showed 41.7% SVR for 48 wk PR, with a risk ratio of 1.27 (95%CI: 1.07-1.50), or 53% (95%CI: 44.6%-62.6%) SVR for 72 wk PR (Figure 1C). Importantly, this meta-analysis included both Caucasian and Asian studies with no significant difference between them in tests of heterogeneity.
The SVR rate of patients who have detectable HCVRNA at TW4 in the SPRINT-2 study[9], was 65% in the RGT group and 66% in 48 wk fixed duration therapy group, while in RESPOND-2, no analysis was performed of SVR rates in those with detectable HCVRNA at TW4, as the analysis was based on interferon responsiveness, defined as HCVRNA decline at TW4 by < 1log10≥. The PROVIDE study[11] was analysed in a similar manner. Fortunately, a post hoc analysis can be performed using the abstract presented by Vierling et al[20] which showed the different levels of interferon responsiveness at TW4 for RESPOND-2. SVR in those with detectable HCVRNA at TW4 was calculated to be 61% (95/156) (range: 31%-90%) in the RGT group, and 66% (103/156) (range: 13%-100%) for the 48 wk fixed duration group. Consequently, the SVR rate in those who have detectable HCVRNA at TW4 is remarkably similar, 61%-66% regardless whether patients are treatment naïve or failures, and regardless of RGT or 48 wk fixed duration therapy (Figure 1C). However, SVR could be as low as 33% if the patient was a “null responder” or as high as 80% in “partial responders” at TW4[21].
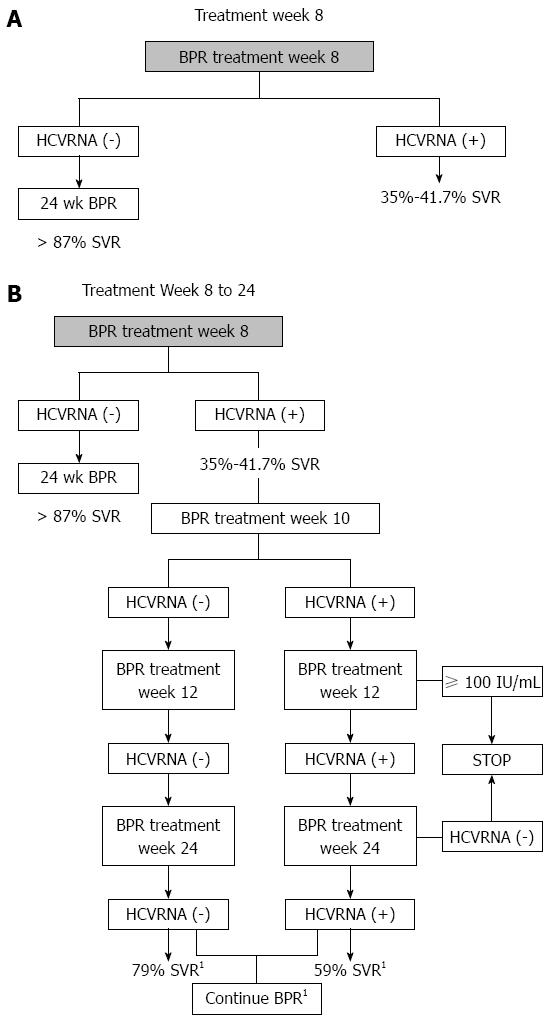
HCVRNA undetectable with BPR: In SPRINT-2[9], treatment naïve patients with undetectable HCVRNA at TW8, had 88% SVR with RGT compared to 90% SVR with BPR 48 wk. In the RESPOND-2 study[10], prior treatment failure patients with undetectable HCVRNA at TW8, had 86% SVR with RGT compared to 88% SVR with 48 wk BPR. These findings confirm that those with undetectable HCVRNA will benefit from 24 wk BPR (Figure 2A). Even treatment naïve poor interferon responders at TW4, or “null responders” (< 1log HCVRNA reduction at TW4), in a post-hoc analysis of the SPRINT-2 and RESPOND-2 studies, who have undetectable HCVRNA at TW8, achieve 83% SVR[22].
HCVRNA detectable with BPR: In the SPRINT-2[9], treatment naïve late responders (HCVRNA detectable at TW8 but undetectable at treatment week 24) achieved 73.1% SVR with RGT compared to 75% SVR with 48 wk fixed duration therapy. However, when all patients with detectable HCVRNA at TW8 are examined in Th SPRINT-2 study, the SVR rate is only 36% for RGT and 40% for 48 wk fixed dose therapy, as these include patients who have detectable HCVRNA at TW24 (which invoke the futility rule for stopping therapy). However, we do not need to wait to TW24, as by TW12 the futility rule can also be applied to patients with HCVRNA ≥ 100 IU/mL[23] (Figure 2B). In the RESPOND-2 study[10], SVR data on those with detectable HCVRNA at TW8 were not presented but can be extracted as a post-hoc analysis. Since the total SVR rates were known for group 2 (95/162, 58.6%) and group 3 (107/161, 66.4%) and the rates for patients with undetectable HCVRNA at TW8 are known, for group 2 (74/161, 46%), and group 3 (84/161, 52%), as well as SVR rates are known for group 2 (64/74, 86%), and group 3 (74/84, 88%), we can calculate SVR rates in those with detectable HCVRNA at TW8 in group 2 (31/88, 35%), and group 3 (33/80, 41.2%). Group 2 patients were in the RGT arm, and group 3 were in the fixed duration 48 wk therapy arm. Pooling group 2 and 3 together we can calculate that 168/323 (52%) of prior treatment failure patients had detectable HCVRNA at TW8.
Consequently, both SPRINT-2 and RESPOND-2 studies demonstrate remarkably similar results for those with detectable HCVRNA at TW8, with SVR rates of 35%-36% for RCT and 40%-41.2% for fixed duration 48 wk therapy, regardless of their prior response to therapy.
For patients who have detectable HCVRNA at TW8, rather than wait for TW12, undetectable HCVRNA at TW10 can also be used to determine if SVR is likely (Figure 2B). In an post-hoc analysis combining SPRINT-2 and RESPOND-2 studies[24], those with detectable HCVRNA at TW8 were evaluated at TW10 and undetectable HCVRNA at TW10 and at TW12 conferred SVR of 79% while a detectable HCVRNA at TW10 followed by undetectable HCVRNA at TW12 conferred a SVR of 59% (Figure 2B), provided they maintained undetectable HCVRNA through to treatment week 24.
Based on the data above, it would seem that response-guided management using undetectable HCVRNA successively at TW4, 8, 10 and 12 predict a high chance of SVR regardless of whether a patient is treatment naïve or a prior treatment failure, but does this apply to cirrhotics?
It is well known that SVR is impacted by the presence of progressive fibrosis and cirrhosis. Subanalyses from randomised control trials show that RVR is lower in cirrhotic patients compared to non-cirrhotics[25,26]. In the CHARIOT study[25], RVR in F0-2 patients was 24% compared to 18% in those with F3-4. Overall SVR was much lower in those with cirrhosis (10%) compared to those without fibrosis, F0 (70%). Those with RVR and F0-2 achieved 80% SVR but those with F3-4 had only 63% SVR. Bruno et al[26], collated three RCTs showing that RVR in those without advanced fibrosis was 23.6% compared to 11% with advanced fibrosis. SVR was lower in those with cirrhosis (33%) compared to those without bridging fibrosis (60%). Notably, those who achieved RVR without advanced fibrosis had SVR of 95% compared to 89% in those with advanced fibrosis.
Consequently, responses to boceprevir in cirrhotics needs evaluation. A meta-analysis of all boceprevir treated patients with cirrhosis[12] found that all those with undetectable HCVRNA at TW8 has similar SVR, 86% for F0-2, 85% for F3 and 89% for F4. Undetectable HCVRNA at TW8 was the strongest predictor of SVR by multivariate analysis with OR = 10.57 (95%CI: 5.23-21.36). Consequently, we can be confident that the findings from non-cirrhotics are similar in cirrhotics (Figure 2A).
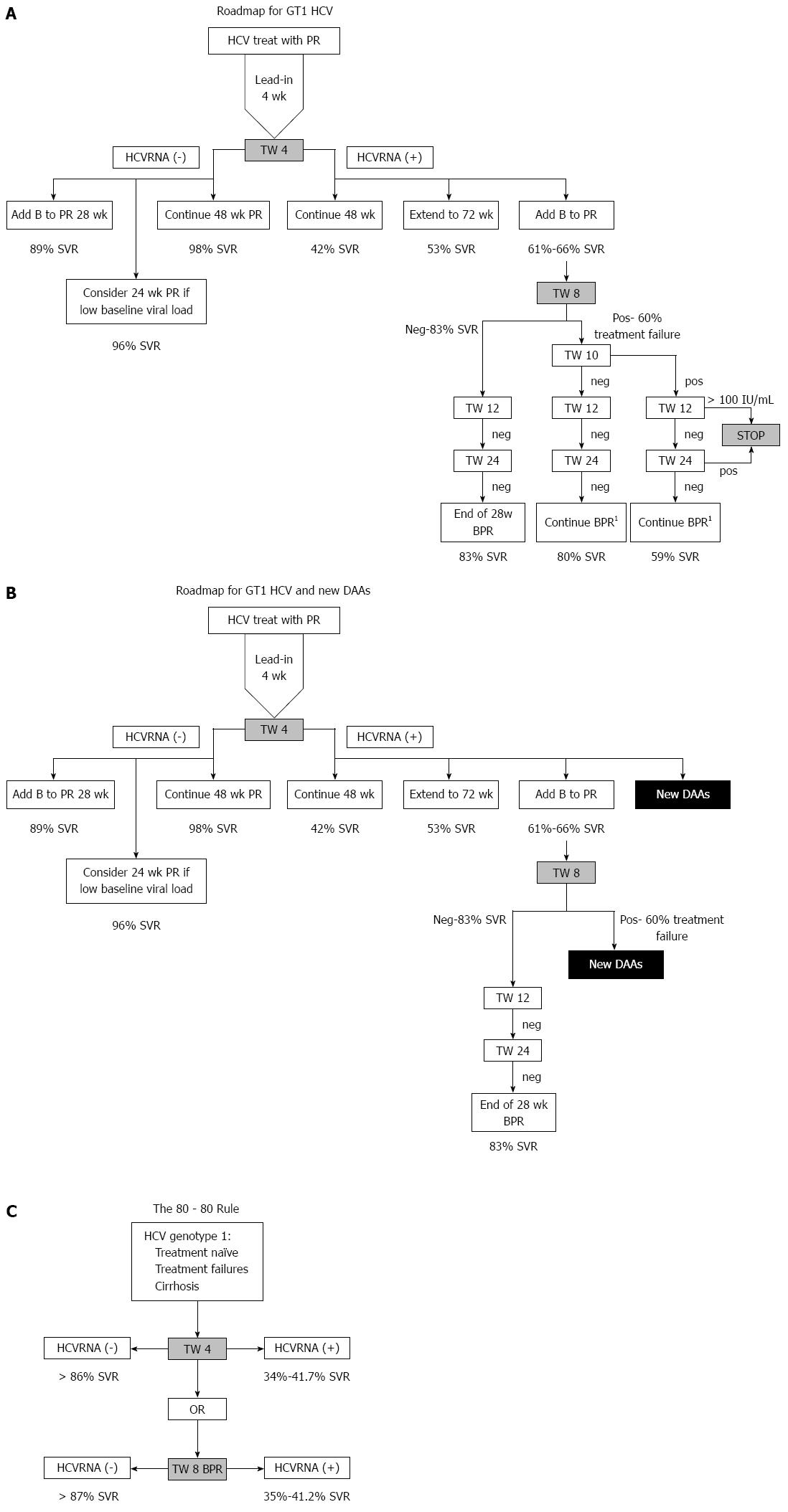
Based on the response-guided management proposed for boceprevir (Figures 2B and 3), patients who have undetectable HCVRNA at TW8 can shorten therapy to 24 wk of BPR (after lead-in). This is true even for patients with cirrhosis and even for those who had previous PR treatment failure. However, it is important to note that the boceprevir label[23] advises duration of boceprevir triple therapy based on whether the patient is treatment naïve, is an early or late responder, has prior treatment failure or has cirrhosis. Only in treatment naïve early responders is it recommended to for 24 wk BPR. In cirrhosis and previous null responders, it is recommended to have 44 wk of BPR (after 4 wk lead-in). For the remainder, late responders, prior treatment failures the recommendation is for 32 wk of BPR (after 4 wk lead-in) with or without an extra 12 wk PR tail. We should be mindful that the 32 wk BPR proposal is not supported by evidence, only by modeling. This confusing recommendation leaves much to be desired since it does not simplify management, and that the prior documentation of null response can be rather poor, not to mention that diagnosis of cirrhosis with non-invasive markers is not always optimal, making it difficult at times to select the correct duration of therapy. Although our proposed roadmap simplifies therapy considerably in those who are good responders regardless of prior treatment failure or presence of cirrhosis, when in doubt, the package insert should be followed[23].
How long should those with detectable HCVRNA at TW8 be treated with BPR? Based on the package insert, at least 32 wk of BPR ± 12 wk of PR tail. Given these patients are the most likely to fail therapy, they should be given the maximum benefit with 44 wk BPR, as supported by the SPRINT-2 and RESPOND-2 studies.
In Figure 3A, a consolidated management approach to HCV genotype 1 is proposed regardless of the baseline characteristics of treatment naïve, treatment failure or cirrhosis.
Using the roadmap based management, we can invoke an “80-80” rule, that is, if undetectable HCVRNA is achieved at TW4, > 80% SVR will be obtained, or using boceprevir, undetectable HCVRNA is achieved at TW8, > 80% SVR will also be obtained (Figure 3C). The possibility of failure if HCVRNA is detectable at TW8 is higher. In a post-hoc analysis from SPRINT-2, there were 64% (83/129) failures in the RGT arm, and 60% (79/131) in the fixed duration arm, while in RESPOND-2, failures in the RGT arm were 64.7% (57/88), and the fixed duration arm was 58.8% (47/80). Overall, with detectable HCVRNA at TW8, the chance of failure is approximately 60%.
The proposed roadmap was derived from randomised control trials but how would it perform in real life? In an early access program for boceprevir, the Boceprevir Named Patient Program (BNPP), investigators from Singapore, Thailand, Malaysia and Australia decided to pool their patients into a study, Boceprevir Early-Access For Advanced Fibrosis/Cirrhosis To Evaluate Outcomes In Asia-Pacific HCV Genotype 1 Non-Responders And Relapser Patients (BEACON study). A total of 150 patients (Asians = 86, Caucasians = 63) were enrolled and these patients had to have previous treatment failure with PR, and had to have advanced fibrosis or cirrhosis, but no decompensated liver disease. The final data analysis is currently being prepared for publication (manuscript in press, World J Gastroenterology). Applying the roadmap strategy to the BEACON study, those who have undetectable HCVRNA at TW4, have 100% SVR (14/14), while those who have undetectable HCVRNA at TW8 have 87% SVR (74/85). Those who have detectable HCVRNA at TW4 have 58% SVR (72/124) and those who have detectable HCVRNA at TW8 have 22% SVR. These patients represent the most difficult to treat subtypes, as they have had previous treatment failure, and also have advanced fibrosis or cirrhosis. The findings of BEACON confirm that in real-life settings, the roadmap strategy is indeed valid.
When the “80-80” rule is not met, treatment failure becomes more likely. This rule can be met as early as TW4, when an alternative is to introduce new DAAs (Figure 3B) such as sofosbuvir and PR, however, there is no data on the SVR rates in treatment-experienced patients. An alternative is simeprevir and PR, but in null responders, SVR was 58.8%[27]. As patients who are HCVRNA positive at TW4 are enriched for null responders, simeprevir and PR would not be an optimal alternative. At TW8, detectable HCVRNA also increases the likelihood of treatment failure to 60% making alternative new DAAs an option (Figure 3B). Again such patients would be enriched for null responders hence simeprevir and PR is a suboptimal, nor is there data on such patients treated with sofosbuvir and PR. However, such patients can be rescued in the near future with the new generation of interferon-free oral treatments, and null responders achieve > 90% SVR with the Abbvie quad regimen[28], the sofosbuvir-ledispavir ± ribavirin combination[29], and the sofosbuvir-simeprevir combination[30]. Sofosbuvir and ledispavir ± ribavirin was also effective in patients with baseline protease inhibitor resistance associated variants[31].
These are rather exciting times for patients who suffer from chronic hepatitis C. Sofosbuvir[5] and simeprevir[4] have both been very recently been approved for treatment of chronic hepatitis C which appear to be a substantial improvement over the current DAAs, telaprevir and boceprevir. However, access to the newly approved DAAs will be gradual as approval in different countries and regions are likely to take years. In the interim, hepatitis C treatment in each country or region needs to adapt to the evolving situation and to determine the best strategy for SVR, bearing in mind cost of therapy is a critical factor in resource constrained settings. In the immediate and near term, PR is still likely to be the mainstay of therapy in resource constrained settings since not only is it the only available therapy, but even boceprevir and telaprevir constitute a considerable cost, not taking into consideration adverse events, tolerability and toxicity.
The current analysis is a distillation of existing literature showing that by using TW4 and TW8 HCVRNA, dichotomised to detectable or undetectable, provides a high level of predictability for SVR, simpliflying the complexity and confusion of using log reduction in HCVRNA, and stratifying by type of prior treatment failure or presence of absence of cirrhosis. This response-guided approach is particularly appealing in countries where IL28B CC genotype is prevalent, since a greater proportion of patients have undetectable HCVRNA at TW4. In countries where the IL28B T genotype is more prevalent, the TW4 response is likely to be poor, and more reliance will be placed on the TW8 response. At TW4 or TW8, if HCVRNA is undetectable, SVR > 80%, leading to the “80-80” rule, making this reassuring for both physicians and patients that SVR is likely, encouraging compliance, and making tolerability of adverse events more bearable. Moreover, the roadmap allows patients to terminate treatment as early as TW12 if HCVRNA ≥ 100 IU/mL, if response to therapy is unfavorable, based on the stopping rule. Consequently the roadmap also can provide at each timepoint the likelihood of treatment failure and such patients can have the option of stopping therapy and consider the new generation of DAAs, where data is available.
There are some caveats to the roadmap strategy since it provides likelihood of SVR in those who are able to tolerate therapy. A significant proportion of patients have adverse events to pegylated interferon, ribavirin and boceprevir or the combination, and discontinuation of therapy occurs in a substantial proportion of patients due to adverse events. In the CUPIC study[32] patients who were at risk of developing severe complications including sepsis and death, had a low serum albumin < 35 g/dL and a low platelet count < 100000/L. Consequently boceprevir or telaprevir therapy is not recommended in such patients due to the high risk of these complications. In such patients, they should look to interferon-free therapy and the new generation of DAAs.
With the new generation of DAAs around the corner, the era of interferon-free regimens is around the corner. Moreover, approval of the new DAAs may take as long as 3-4 years in some Asian countries. Consequently the new DAAs, which currently come with a high cost of therapy, having cheaper but almost as effective alternatives a choice to be considered. Consequently, PR is still likely to be a first line therapy for cost reasons, both in countries with and without re-imbursement. One can speculate that the current first generation DAAs could be used as second-line therapy when RVR fails, and the new DAAs could become a third line therapy when both first and second line are ineffective. Clearly this can change if cost structures alter. In the ideal world, interferon based regimes are inconvenient and carry adverse events which makes tolerance and compliance an important issue. While the future points to an interferon-free HCV treatment, we should discount the utility of interferon based regimens as an interim measure for patient management.
Although new all oral antiviral agents for hepatitis C virus (HCV) are available in some countries, many countries who have constrained resources still are using pegylated interferon and ribavirin and first generation direct-acting antivirals.
There is still confusion on how to use pegylated interferon, ribavirin with or without first generation direct-acting antivirals, and a distillation of the available best evidence can provide a practical guide for on-treatment management.
On-treatment response based on detectable or undetectable HCVRNA at week 4 or 8 can guide continuing or changing treatment. This can be summarised as the “80-80” rule which means SVR ≥ 80% can be achieved if HCVRNA is negative at week 4 or 8 of therapy.
This roadmap is applicable to all HCV genotype 1 patients regardless of whether.
Dr. Lim SG described the road map for antiviral therapy to HCV genotype 1 patients. This study is review article. As the author pointed out, direct acting antivirals present substantial advance in efficacy with better tolerability, though they are very expensive.
P- Reviewer: Abe H, O'Connor KS, Torres C S- Editor: Qi Y L- Editor: A E- Editor: Ma S
| 1. | World Health Organization. WHO headquarters fact sheet: Hepatitis C. WHO Health Topics 2010. Available from: http://www.euro.who.int/en/health-topics/communicable-diseases/hepatitis/data-and-statistics/hepatitis-c. |
| 2. | Ghany MG, Strader DB, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335-1374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2320] [Cited by in RCA: 2242] [Article Influence: 140.1] [Reference Citation Analysis (1)] |
| 3. | Casey LC, Lee WM. Hepatitis C virus therapy update 2013. Curr Opin Gastroenterol. 2013;29:243-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Food And Drug Administration. FDA approves new treatment for hepatitis C virus. 2013; Available from: http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm376449.htm. |
| 5. | Food And Drug Administration. Approval of Sovaldi (sofosbuvir) tablets for the treatment of chronic hepatitis C. 2013; Available from: http://www.fda.gov/forconsumers/byaudience/forpatientadvocates/ucm377920.htm. |
| 6. | Food and Drug Administration, USA. Product label for Vitcrelis. 2011. . |
| 7. | European Medicines Authority, European Union. Product label for Victrelis. 2011. . |
| 8. | Kwo PY, Lawitz EJ, McCone J, Schiff ER, Vierling JM, Pound D, Davis MN, Galati JS, Gordon SC, Ravendhran N. Efficacy of boceprevir, an NS3 protease inhibitor, in combination with peginterferon alfa-2b and ribavirin in treatment-naive patients with genotype 1 hepatitis C infection (SPRINT-1): an open-label, randomised, multicentre phase 2 trial. Lancet. 2010;376:705-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 517] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 9. | Poordad F, McCone J, Bacon BR, Bruno S, Manns MP, Sulkowski MS, Jacobson IM, Reddy KR, Goodman ZD, Boparai N. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1195-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1996] [Cited by in RCA: 1981] [Article Influence: 141.5] [Reference Citation Analysis (0)] |
| 10. | Bacon BR, Gordon SC, Lawitz E, Marcellin P, Vierling JM, Zeuzem S, Poordad F, Goodman ZD, Sings HL, Boparai N. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1207-1217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1306] [Cited by in RCA: 1308] [Article Influence: 93.4] [Reference Citation Analysis (0)] |
| 11. | Vierling J, Davis M, Flamm S, Gordon S, Lawitz E, Yoshida E, Galati J, Luketic V, McCone J, Jacobson I. Sustained Virologic Response (SVR) in Prior PegInterferon/Ribavirin (PR) Treatment Failures After Retreatment with Boceprevir (BOC) PR: PROVIDE Study Interim Results. Boston: American Association for Study of Liver Dsiease 2011; 931. |
| 12. | Vierling JM, Zeuzem S, Poordad F, Bronowicki JP, Manns M, Bacon BR, Esteban R, Flamm SL, Kwo PY, Pedicone LD. Safety and Efficacy Of Boceprevir/Peginterferon/Ribavirin (Boc/P/R) Combination Therapy for Chronic HCV G1 Patients With Compensated Cirrhosis: A Meta-Analysis of five Phase 3 Clinical Trials. Amsterdam: European Association for Study of Liver 2013; abstract 1430. |
| 13. | McHutchison JG, Lawitz EJ, Shiffman ML, Muir AJ, Galler GW, McCone J, Nyberg LM, Lee WM, Ghalib RH, Schiff ER. Peginterferon alfa-2b or alfa-2a with ribavirin for treatment of hepatitis C infection. N Engl J Med. 2009;361:580-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 894] [Cited by in RCA: 886] [Article Influence: 55.4] [Reference Citation Analysis (0)] |
| 14. | Poordad F, Bronowicki JP, Gordon SC, Zeuzem S, Jacobson IM, Sulkowski MS, Poynard T, Morgan TR, Molony C, Pedicone LD. Factors that predict response of patients with hepatitis C virus infection to boceprevir. Gastroenterology. 2012;143:608-18.e1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 150] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 15. | Liu CH, Liang CC, Liu CJ, Tseng TC, Lin CL, Yang SS, Su TH, Hsu SJ, Lin JW, Chen JH. Interleukin 28B genetic polymorphisms and viral factors help identify HCV genotype-1 patients who benefit from 24-week pegylated interferon plus ribavirin therapy. Antivir Ther. 2012;17:477-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Thomas DL, Thio CL, Martin MP, Qi Y, Ge D, O’Huigin C, Kidd J, Kidd K, Khakoo SI, Alexander G. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature. 2009;461:798-801. [PubMed] |
| 17. | Di Martino V, Richou C, Cervoni JP, Sanchez-Tapias JM, Jensen DM, Mangia A, Buti M, Sheppard F, Ferenci P, Thévenot T. Response-guided peg-interferon plus ribavirin treatment duration in chronic hepatitis C: meta-analyses of randomized, controlled trials and implications for the future. Hepatology. 2011;54:789-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | Nelson DR, Poordad F, Feld JJ, Fried MW, Jacobson IM, Pockros P, Sulkowski MS, Zeuzem S, Bengtsson L, George S. High SVR Rates (SVR4) for 12-week Total Telaprevir Combination Therapy in IL28B CC Treatment-naive and Prior Relapsers with G1 Chronic Hepatitis C: CONCISE Interim Analysis. Amsterdam: EASL 2013; . |
| 19. | Katz LH, Goldvaser H, Gafter-Gvili A, Tur-Kaspa R. Extended peginterferon plus ribavirin treatment for 72 weeks versus standard peginterferon plus ribavirin treatment for 48 weeks in chronic hepatitis C genotype 1 infected slow-responder adult patients. Cochrane Database Syst Rev. 2012;9:CD008516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Vierling JM, Lawitz EJ, Poordad F, Sulkowski MS, Bourliere M, Buti M, Cooper C, Galati JS, Albrecht JK, Boparai N. Four-week Therapy With Peginterferon Alfa-2b/Ribavirin Effectively Predicts Sustained Virologic Response in Previously Untreated and Previous-Treatment-Failure Patients With HCV-1 Treated With Boceprevir Plus Peginterferon Alfa-2b/Ribavirin. Berlin: European Association for Study of Liver 2011; 481. |
| 21. | Lim SG, Yu ML, Piratvisuth T, Hu KQ, Poordad F, Bronowicki JP, Bognar FA, Deng W, Wahl J, Helmond FA. Treatment-week 4 response with peginterferon/ribavirin predicts treatment outcome to boceprevir: implications for Asian patients with chronic hepatitis C genotype 1. Singapore: Asia Pacific Association for Study of Liver 2013; Abstract 1476. |
| 22. | Bacon B, Bruno S, Schiff E, Kwo P, Buti M, Pedicone L, Deng W, Burroughs M, Brass C, Albrecht J. Predictors of Sustained Virologic Response Among Poor Interferon Responders When Boceprevir is Added to Peginterferon alfa-2b/Ribavirin. Massachusetts: American Association for Study of Liver Disease 2011; . |
| 23. | Vitrellis package insert. Whitehouse Station: Merck Sharpe and Dohm 2011; . |
| 24. | Lawitz E, Poordad F, Bronowicki JP, Marcellin P, Feinman SV, Kwo P, Guyader D, Davis M, Harrison S, Pedicone L. The Effect of Using Lower Limit of Quantitation (LLQ) vs Lower Limit of Detection (LLD) for the Definition of Undetectable HCV RNA: Data from the RESPOND-2 and SPRINT-2 trials. Boston: American Association for Study of Liver Disease 2011; Abstract 167. |
| 25. | Cheng WS, Roberts SK, McCaughan G, Sievert W, Weltman M, Crawford D, Rawlinson W, Marks PS, Thommes J, Rizkalla B. Low virological response and high relapse rates in hepatitis C genotype 1 patients with advanced fibrosis despite adequate therapeutic dosing. J Hepatol. 2010;53:616-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Bruno S, Shiffman ML, Roberts SK, Gane EJ, Messinger D, Hadziyannis SJ, Marcellin P. Efficacy and safety of peginterferon alfa-2a (40KD) plus ribavirin in hepatitis C patients with advanced fibrosis and cirrhosis. Hepatology. 2010;51:388-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 120] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 27. | Zeuzem S, Berg T, Gane E, Ferenci P, Foster GR, Fried MW, Hezode C, Hirschfield GM, Jacobson I, Nikitin I. Simeprevir increases rate of sustained virologic response among treatment-experienced patients with HCV genotype-1 infection: a phase IIb trial. Gastroenterology. 2014;146:430-41.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 174] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 28. | Kowdley KV, Lawitz E, Poordad F, Cohen DE, Nelson D, Zeuzem S, Everson GT, Kwo P, Foster GR, Sulkowski M. Safety and Efficacy of Interferon-free Regimens of ABT-450/R, ABT-267, ABT-333 /- Ribavirin In Patients with Chronic HCV GT1 Infection: Results from the AVIATOR Study. Amsterdam: European Association for Study of Liver 2013; . |
| 29. | Gane EJ, Stedman CA, Hyland RH, Ding X, Pang PS, Symonds WT, McHutchison JG. ELECTRON: All-Oral Sofosbuvir-Based 12-Week Regimens for the Treatment of Chronic HCV GT 1 Infection. Amsterdam: European Association of Study of the Liver 2013; . |
| 30. | Jacobson IM, Ghalib RH, Rodriguez-Torres M, Younossi ZM, Corregidor A, Sulkowski MS, DeJesus E, Pearlman B, Rabinovitz M, Gitlin N. SVR results of a once-daily regimen of simeprevir (TMC435) plus sofosbuvir (GS-7977) with or without ribavirin in cirrhotic and non-cirrhotic HCV genotype 1 treatment-naïve and prior null responder patients: The COSMOS study. Washington: American Association for Study of Liver Disease 2013; . |
| 31. | Hebner C, Pang PS, Hyland RH, Miller MD, Mo H, Lawitz E, Poordad F, Membreno FE. Once Daily Sofosbuvir/Ledipasvir Fixed Dose Combination is Highly Effective in Subjects with Baseline NS5A Inhibitor and NS3 Protease Inhibitor Resistance-Associated Variants: The Lonestar Trial. Washington: American Association for Study of Liver Disease 2013; 1844. |
| 32. | Hézode C, Fontaine H, Dorival C, Larrey D, Zoulim F, Canva V, de Ledinghen V, Poynard T, Samuel D, Bourlière M. Triple therapy in treatment-experienced patients with HCV-cirrhosis in a multicentre cohort of the French Early Access Programme (ANRS CO20-CUPIC) - NCT01514890. J Hepatol. 2013;59:434-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 362] [Article Influence: 30.2] [Reference Citation Analysis (0)] |