Published online Feb 7, 2015. doi: 10.3748/wjg.v21.i5.1518
Peer-review started: July 28, 2014
First decision: August 6, 2014
Revised: August 28, 2014
Accepted: October 21, 2014
Article in press: October 21, 2014
Published online: February 7, 2015
Processing time: 197 Days and 3 Hours
AIM: To investigate the distribution and expression of C-type natriuretic peptide (CNP)/natriuretic peptide receptor B (NPR-B) in the rectum of a rodent depression model and the interventional effect of Xiaoyaosan (XYS).
METHODS: Male rats (n = 45) of clean grade (200 ± 20 g) were divided into five groups after one week of adaptive feeding: primary control, depression model, low dose XYS, middle dose XYS, and high dose XYS. The animal experiment continued for 3 wk. Primary controls were fed normally ad libitum. The rats of all other groups were raised in solitary and exposed to classic chronic mild unpredictable stimulation each day. XYS groups were perfused intragastrically with low dose, middle dose, and high dose XYS one hour before stimulation. Primary control and depression model groups were perfused intragastrically with normal saline under similar conditions as the XYS groups. Three weeks later, all rats were sacrificed, and the expression levels of CNP and NPR-B in rectum tissues were analyzed by immunohistochemistry, real-time polymerase chain reaction, and Western blotting.
RESULTS: CNP and NPR-B were both expressed in the rectum tissues of all rats. However, the expression levels of CNP and NPR-B at both gene and protein levels in the depression model group were significantly higher when compared to the primary control group (n = 9; P < 0.01). XYS intervention markedly inhibited the expression levels of CNP and NPR-B in depressed rats. The expression levels of CNP and NPR-B in the high dose XYS group did not significantly differ from the expression levels in the primary control group. Additionally, the high and middle dose XYS groups (but not the low dose group) significantly exhibited lower CNP and NPR-B expression levels in the rectum tissues of the respectively treated rats compared to the untreated depression model cohort (n = 9; P < 0.01).
CONCLUSION: The CNP/NPR-B pathway is upregulated in the rectum of depressed rats and may be one mechanism for depression-associated digestive disorders. XYS antagonizes this pathway at least partially.
Core tip: C-type natriuretic peptide (CNP) and natriuretic peptide receptor B (NPR-B) were expressed in the mucosa, muscle, and serosa of rat rectum. Expression of CNP and NPR-B are upregulated in the rectum of depressive digestive disorder rats, with the CNP/NPR-B signal pathway possibly being involved in depressive digestive disorders. Our study shows that Xiaoyaosan inhibits the expression of CNP and NPR-B in a dose-dependent manner.
- Citation: Li P, Tang XD, Cai ZX, Qiu JJ, Lin XL, Zhu T, Owusu L, Guo HS. CNP signal pathway up-regulated in rectum of depressed rats and the interventional effect of Xiaoyaosan. World J Gastroenterol 2015; 21(5): 1518-1530
- URL: https://www.wjgnet.com/1007-9327/full/v21/i5/1518.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i5.1518
Depression is a common mental disorder that presents with depressed mood, loss of interest/pleasure, feelings of guilt/low self-worth, disturbed sleep/appetite, insomnia/hypersomnia, fatigue/loss of energy, and poor concentration/difficulty making decisions[1]. In addition to the above symptoms, depression is often associated with varying degrees of gastrointestinal motility disorders; mainly gastric smooth muscle tone reduction, stomach hunger disappearance, peristaltic dysfunction, intestinal motility hyperthyroidism, intestinal secretion, constipation, and diarrhea. Depression can also induce endocrine and immune dysfunctions[2]. Improvement of gastrointestinal motility disorder is a prerequisite for the restoration of endocrine and immune system functions to normalcy. Conversely, gastrointestinal motility disorder may aggravate depression[3]. Although depression-induced gastrointestinal motility disorder is a significant health problem, its pathogenesis and mechanisms are still not well understood.
It is generally believed that depression-induced gastrointestinal motility disorder is closely related to visceral autonomic neuropathy, genetic factors, and interactions of peptides[4], neurotransmitters[5], hormones[6], humoral factors, and cytokine secretion. In numerous mechanisms of the etiology of gastrointestinal disorders caused by depression, the effect of brain gut peptide is widely recognized[7]. Brain gut peptide is a peptide hormone that regulates gastrointestinal motility through the brain-gut axis. Brain-gut interactions play crucial roles in the regulation of digestive processes (including appetite and food intake), modulation of the gut-associated immune system, and in the coordination of the overall physical and emotional state of the organism[8]. A study has shown that a lack of orexin signaling though the brain-gut axis may trigger the development of functional gastrointestinal disorders[9]. Other studies have demonstrated that vasoactive intestinal peptides (somatostatin and 5-hydroxytryptamine) might be involved in the etiology of gastrointestinal diseases caused by depression[10].
Atrial natriuretic peptide, B-type natriuretic peptide, and C-type natriuretic peptide (CNP) are members of the natriuretic peptide family of bioactive peptides best known for their role in the regulation of blood pressure[11] and cardiovascular homeostasis[12].
CNP is found mainly in the central nervous system and endothelial cells[13], but it was recently found that CNP is a peptide hormone that coexists in the nervous system and gastrointestinal tract[14,15]. Our previous studies have indicated that CNP can relax gastric circular and longitudinal smooth muscles, and it is involved in the paracrine regulation of gastrointestinal motility via the CNP/natriuretic peptide receptor B (NPR-B)/cGMP pathway in human, rat, and guinea pig stomachs[16,17]. Xu et al[18] reported that upregulation of NPs/NPR-A, NPR-B/cGMP, and NPs/NPR-C signaling pathways may be involved in diabetes-induced loss of interstitial cells of Cajal to induce diabetic gastroparesis. However, it is still unclear whether the CNP signaling pathway is involved in gastrointestinal disorders caused by depression.
Xiaoyaosan (XYS) originated from the Song dynasty (960-1127 AD) book “Taiping Huimin Heji Jufang”. It contains eight common herbs (Bupleurum root, Chinese angelica root, white peony root, Perenniporia, bighead Atractylodes rhizome, roasted ginger, prepared licorice root, menthol, and peppermint) and has been prescribed to sooth the liver, tonify the spleen, and nourish blood. The finished product (pill, decoction, etc.) has been used for centuries in China to treat mental diseases such as depression[19-21]. Currently, it is often used for the treatment of multiple-system diseases such as mental disorders, neurological diseases[22], digestive system diseases[23], respiratory diseases, endocrine diseases, and gynecologic diseases. Liang et al[24] reported that XYS decoction regulated the symptoms of appetite and body weight loss under chronic stress. Few studies have highlighted the mechanisms underlying the intervention of XYS on gastrointestinal motility disorder induced by depression. We believe that the CNP/NPR-B signaling pathway is involved in gastrointestinal disorders caused by depression and XYS has an intervention effect on the CNP/NPR-B signaling pathway. The aim of this study, therefore, was to investigate the role of the CNP/natriuretic peptide receptor type B (NPR-B) pathway in depression-induced gastrointestinal motility disorders and the intervention effect of XYS.
RNAiso Plus, PrimeScriptTM RT reagent kit with gDNA Eraser and SYBR® Premix Ex TaqTM II were purchased from Takara Biotech, Inc. Rabbit NPR-B polyclonal antibody, rabbit CNP polyclonal antibody, and rabbit beta-actin polyclonal antibody were purchased from Beijing Biosynthesis Biotechnology Co., Ltd. Peroxidase-conjugated affini-pure goat anti-rabbit IgG was purchased from Beijing ZSGB Biotech Co., Ltd. Other reagents were obtained from standard commercial suppliers.
Male Sprague Dawley rats (License No.: SCXK (LIAO) 2008-0002; certificate No. 0003496) of clean grade (200 ± 20 g) were purchased from the Experimental Animal Center of Dalian Medical University. Animals were housed at a rearing temperature of 23 ± 2 °C and relative humidity of 55% ± 2% for one week under standard conditions with a 12 h light/dark cycle (light: 7:00 am to 7:00 pm) before experiments.
A total of 45 rats were divided into five groups: N, M, D, Z, and G (primary control, depression model, low XYS dose, middle XYS dose, and high dose XYS groups, respectively) after one week of adaptive feed. The rats were housed singly, with the exception of the primary control group, which were housed three rats per cage. Primary controls were fed normally, ad libitum. Groups M, G, Z, and D were subject to classic chronic mild unpredictable stimulation each day. Groups D, Z, and G were perfused intragastrically with low, middle, and high dose of XYS, respectively, one hour before stimulation. Groups N and M were perfused intragastrically with normal saline at the same time. The experiment continued for 3 wk. The remaining rats received one of these chronic mild unpredictable irritations daily: bondage, swimming-induced fatigue, electrical stimulation, fasting, or concussion. For bondage, the rat was bound into a narrow tube for 5 h in order to allow only very limited activity space. For swimming-induced fatigue, the rat was placed into water (20 °C, 15 cm) and then removed after 20 min. For electrical stimulation, the rat was placed into a cage connected to an electronic stimulator, and then irritated with 1.5 mA, 45 V, for 4 s at intermittent intervals of 1 min for 30 min each day. Fasting was induced for 24 h once a week. Concussion consisted of placing the rat on a horizontal oscillator and then shocking it at a frequency of once every second for 30 min. XYS gavage doses were (gavage volume 2 mL): 7.65 g/kg per day for the low dose group, 15.3 g/kg per day for the medium dose group, and 30.6 g/kg per day for the high dose group. Primary controls were fed with 2 mL of saline. All experimental protocols were approved by the local Animal Care Committee and conformed to the Guide for the Care and Use of Laboratory Animals published by the Science and Technology Commission of the People’s Republic of China (STCC Publication No. 2, revised 1988).
Conditions such as mood, fur color, irritability, eye mucosal color, and feces color were examined every day.
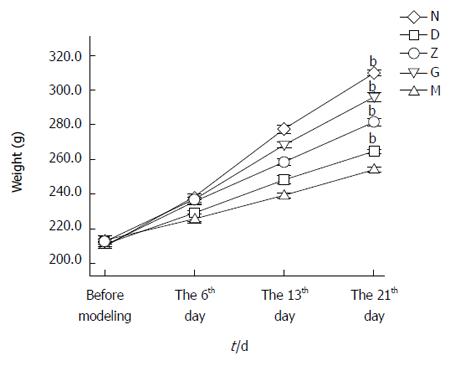
The body weight of each rat was recorded before commencing the model, on the sixth day of modeling, on the 13th day of modeling, and on the 21st day of modeling.
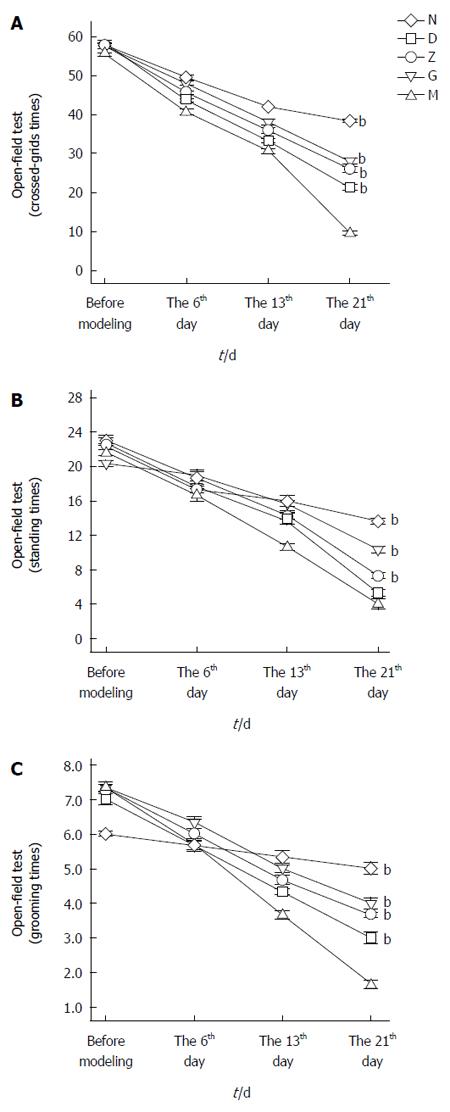
Open-field test was performed before modeling, on the 16th day of modeling, on the 13th day of modeling, and on the 21st day of modeling.
Briefly, rats were placed in the middle of an open field box and had their activity observed for 5 min. Outcome measures included: (1) The number of grids crossed (the number of times three or more paws crossing into adjacent grids); (2) Standing number (the number of times two forelimbs were lifted up from the bottom of the box to make the body upright); and (3) Grooming times (the number of times two forelimbs were used for grooming, scratching, or licking face and paws).
The rats were anesthetized by 10% chloral hydrate (350 mg/kg) by intraperitoneal injection. After sacrifice, the rectum of each rat was washed in ice-cold PBS and bisected longitudinally. The connective tissue was carefully removed from the rectum by mechanical scraping on ice. For RNA and protein extraction, sections of the rectum were flash-frozen in liquid nitrogen and stored at -80 °C. For immunohistochemistry, the rectum was fixed immediately in 4% paraformaldehyde solution at 4 °C for 12 h.
Western blotting analysis was performed to analyze protein expression in the rat rectum samples. Frozen rectum tissue was crushed in liquid nitrogen using a mortar and pestle. Each rectum sample was homogenized with lysis reagent (P0033; Beyotime Chemical Co., Jiangsu, China) according to the manufacturer’s instructions, with the protein concentration then being determined according to the Lowry method using bovine serum albumin as a standard. Total protein was separated on 10% polyacrylamide gel and then transferred onto polyvinylidene difluoride (PVDF) membrane (Millipore, Bedford, MA, United States). The membrane was blocked in 5% (w/v) skimmed milk powder with Tris Buffered Saline-Tween 20 (TBST) for 1 h at 37 °C and then incubated with the primary antibodies, rabbit polyclonal anti-rat NPR-B antibody (bs-2348R; Biosynthesis Biotechnology, Beijing, China; 1:500 dilution) and rabbit polyclonal anti-rat β-actin antibody (bs-0061R; Biosynthesis Biotechnology, Beijing, China; 1:1000 dilution) for 1 h at 37 °C. After incubation, the membrane was washed three times with TBST and then incubated with horseradish peroxidase (HRP)-linked secondary antibody, goat anti-rabbit IgG (ZB-2301; ZSGB-Biotechnology, Beijing, China; 1:5000 dilution) for NPR-B, and β-actin. Probed membrane was immersed for 1 min in enhanced chemiluminescence (ECL) detection reagent (Amersham Biosciences, Piscataway, NJ, United States). After development and image acquisition, the image from each western blotting was quantitatively analyzed using Quantity One software (Bio-Rad Laboratories, CA, United States) and normalized to their corresponding β-actin expression levels.
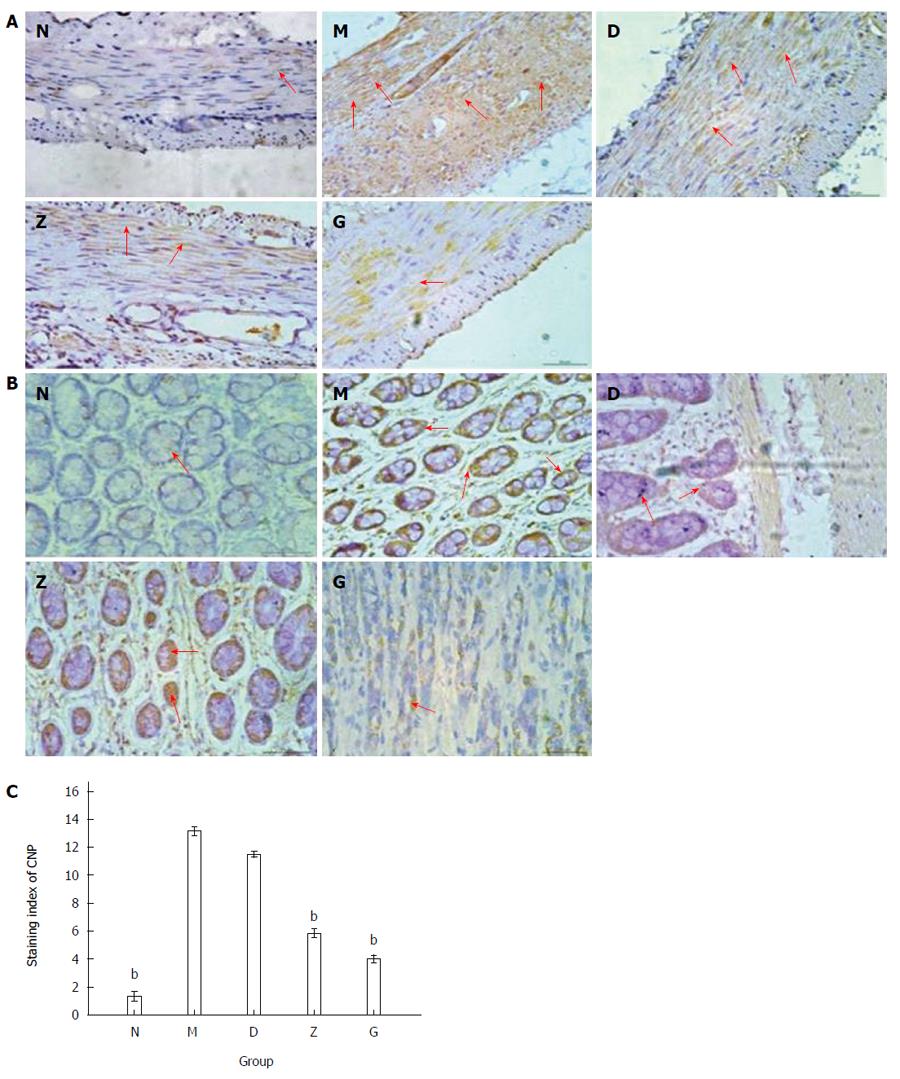
The localization and distribution of NPR-B and CNP in the rectum was investigated by immunohistochemistry. After being affused with 4% paraformaldehyde (4 °C, 12 h), rectum tissue samples were paraffin-embedded, cut into 5 μm-thick sections, and mounted serially on de-paraffinized and rehydrated glass slides. Sections were deparaffinized in three changes of xylene, hydrated in a graded ethanol series, and then washed in tap water. For antigen retrieval, sections were incubated in 10 mmol/L sodium citrate tissue antigen retrieval solution (pH = 6) that contained 0.05% Tween-20 at a high temperature and pressure for 2 min, and then cooled to room temperature. The sections were washed in distilled water, immersed in PBS, and then incubated with 0.3% H2O2 in distilled water for 20 min to block endogenous peroxides. After rinsing three times in PBS for 2 min each at room temperature, slides were incubated for 15 min at 37 °C with normal goat serum (Vector, Burlingame, CA, United States; 1:20) to block non-specific binding. The blocking serum was removed by gentle tapping, with the samples then being incubated in a humidified container with either rabbit polyclonal anti-NPR-B antibody (bs-2348R; Biosynthesis Biotechnology, Beijing, China; 1:75 dilution) or rabbit polyclonal anti-CNP antibody (bs-1000R; Biosynthesis Biotechnology, Beijing, China; 1:400 dilution) for 1 h at 37 °C, and washed with PBS 2 min × 3, followed by incubation in a humidified container with biotin-labeled goat anti-rabbit antibody (KIT-5030, Maixin Biotechnology, Fuzhou, China) for 15 min. Slides were washed with PBS 2 min × 3. The sections were stained with 3, 3’-diaminobenzidine tetrahydrochloride (DAB-0031; Maixin Biotechnology, Fuzhou, China) for approximately 2 min at room temperature and then washed with distilled water. The nuclei were stained with hematoxylin and examined using a LEICA DM 2500 light microscope (Leica Microsystem, ON, Canada). A minimum of six fields were examined for each rectum tissue, with representative tissue sections photographed with LEICA DFC450 (Leica Camera AG, ON, Canada). DAB signal examined as showing immunopositive staining was a “brown” label. The staining indices were compared among five groups and calculated from the staining intensity and percentage of cells staining per section, with three sections per group. Samples were scored from 0 to 4 according to the percentage of positive-stained cells of 0%, 1%-25%, 26%-50%, 51%-75%, and 76%-100%, respectively. Staining intensity was scored from 0 to 3 (0 = negative, 1 = weak, 2 = moderate, and 3 = intense). An histological score formula of ∑pi was used, where “i” represents the score of staining intensity and “p” represents the percentage score of the same staining intensity cells in total cells[25].
Real time fluorescence quantitative PCR (Real time-PCR) was performed to analyze the gene expression levels of NPR-B and CNP in the rat rectum samples. Total RNA was isolated from the rectum as recommended by the manufacturer of RNAiso Plus (9109, Takara Biotechnology Inc., Japan). RNA concentration was determined spectrophotometrically (Bio-photometer Eppendorf, Milan, Italy) at 260 nm and 280 nm. The ratio of readings at 260 nm and 280 nm (A260/A280) provided an estimate of the purity of RNA. The integrity and purity of total RNA was also detected by electrophoresis of samples on GelStar Stain agarose gels. Only samples that showed clear and distinct 28S and 18S ribosomal RNA bands and had spectrophotometric A260/A280 ratios of 1.9-2.1 were used. Reverse transcription was performed as recommended by the manufacturer of the PrimeScriptTM RT reagent kit with gDNA Eraser (RR047A; Takara Biotechnology Inc., Japan), which was incubated at 37 °C for 15 min, followed by incubation at 85 °C for 5 s. cDNA samples were used for analyzing specific cDNA of CNP and NPR-B. cDNA were examined by agarose gel electrophoresis to assure near equivalent levels of cDNA synthesis. Following DNase treatment, real-time PCR was performed in an optical 96-well plate using real-time monitoring of nucleic acid green dye fluorescence (SYBR Green I) with SYBR® Premix Ex TaqTM II (RR820A; Takara Biotechnology Inc., Japan) with the StepOnePlusTM System (Applied Bio-systems). Two-step standard real-time PCR procedure: Stage 1: Reps 1, 95 °C, 30 s; Stage 2: Reps 40, 95 °C, 5 s; 60 °C, 30 s. Table 1 depicts the specific primers for rat β-actin, GAPDH, CNP, and NPR-B (Bioneer, Inc., South Korea) which were used. The genes of β-actin and GAPDH were used as housekeeping gene constitutively expressed to a constant amount in cells, and gene expression of CNP and NPR-B was normalized to β-actin or GAPDH. All samples were amplified in triplicate. Ct values were analyzed and the ΔΔCt value of amplification was calculated. The relative increase/decrease of mRNA in the target genes of the experimental groups were calculated using the model group as the calibrator.
| Gene | Accession number | GenBank | |
| Forward primer sequence | Reverse primer sequence | ||
| NPR-B | NM_053838 | F: TTTACCTTGATGTCTTTGGG | R: CCTGATACTCGGGATTCG |
| CNP | NM_053750.1 | F: CATGAGCGGTCTGGGATGTTAG | R: CACATTGCGTTGGAGGTGTTTC |
| β-actin | NM_031144 | F: TCGTGCGTGACATTAAAGAG | R: TGCCACAGGATTCCATACC |
| GAPDH | NM_017008 | F: AAGTTCAACGGCACAGTCAAG | R: TACTCAGCACCAGCATCACC |
Differences between more than two independent groups were analyzed by Fisher’s test after ANOVA[26]. Where required, the data was transformed by the appropriate method to obtain a normal distribution. All experiments were repeated at least three times. The results are expressed as mean ± SE and a P value < 0.05 was considered significant.
The method of chronic mild unpredictable irritations combined with solitary raising work together to make a depression model. The state, weight, and open-field testing of rats were observed to identify whether the depression model was successful.
Performance of normal rats: Good mood, agile activity, thick and shiny hair, pale pink ear mucosa, normal eating, normal drinking, and normal stool.
Performance of depressed rats: Exhausted demeanor, dull and brown hair, dark eyes with secretions, arched and huddled together, loose stool, weight loss, reduced numbers of crossed-grids, reduced instances of standing, and reduced grooming.
On the 21st day, the average weight of the rats was 309 ± 1 g in primary control group (N), 254 ± 1 g in depression model group (M), 265 ± 1 g in low dose group (D), 281 ± 2 g in middle dose group (Z), and 296 ± 3 g in High dose group (G). The difference between the M and the N was significant (n = 9, bP < 0.01; Figure 1). The difference between the XYS groups and the depression model group was also significant (n = 9, bP < 0.01; Figure 1).
On the 21st day, the number of crossed-grids, instances of standing, and number of grooming times were compared. The differences between the depression and primary control groups was significant (n = 9, bP < 0.01; Figures 2). The differences between the XYS and depression groups were also significant (n = 9, bP < 0.01; Figures 2).
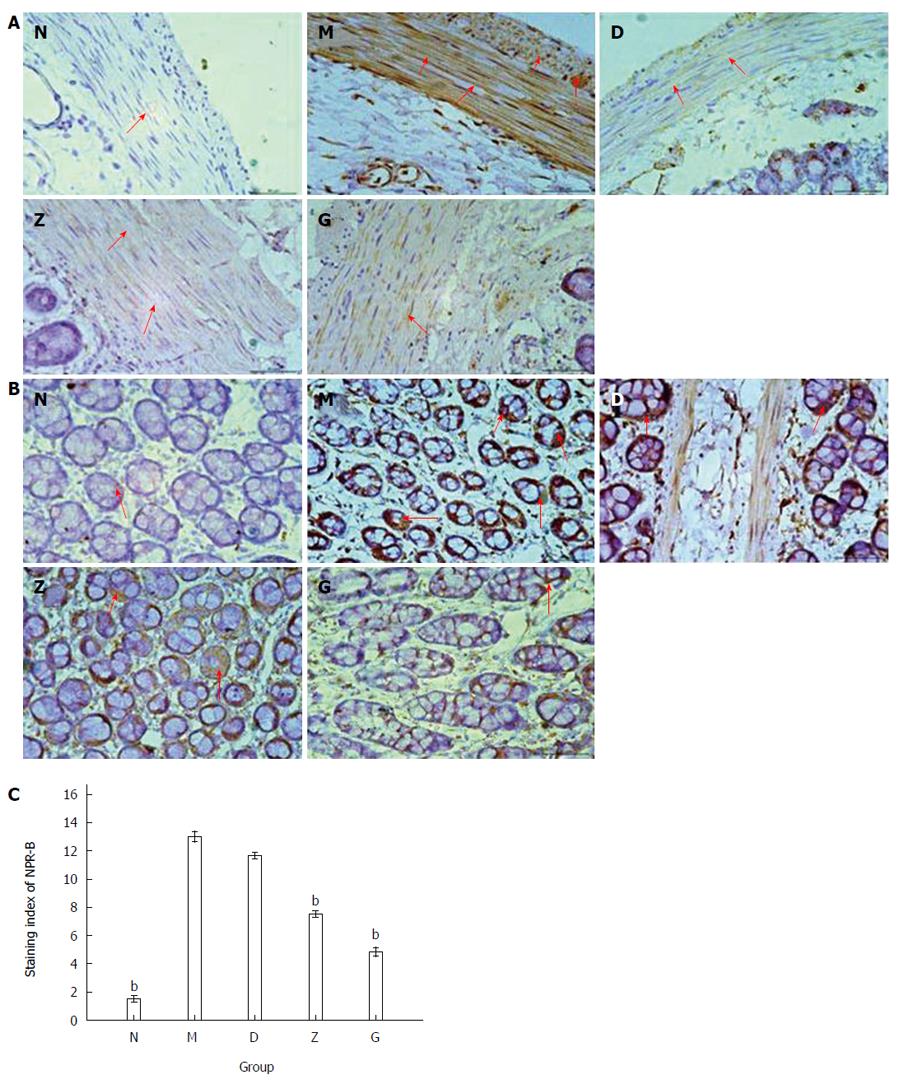
Immunohistochemistry was used to detect the expression of CNP in the rectum of depressed rats. The doses of XYS administered were as follows: 7.65 g/kg per day for D, 15.3 g/kg per day for Z, and 30.6 g/kg per day for G. CNP immunopositive brown granules were detected in the mucosa, muscle, and serosa of the rats’ rectum. CNP positive-staining was evidenced in the nucleus and cytoplasm of smooth muscle cells, basal granular cells, and stromal cells. The results showed that smooth muscle cells (n = 9; Figure 3A) and basal granular cells (n = 9; Figure 3B) in the five groups all expressed CNP, but differentially. The staining index of CNP was 1.50 ± 0.224 in N, 13.00 ± 0.365 in M, 11.67 ± 0.211 in D, 7.50 ± 0.224 in Z, and 4.83 ± 0.307 in G. The expression of CNP in the depression model group (M) was significantly higher than that in the primary control group (N). The high and middle XYS dose groups (G and Z) were not significantly different compared with the primary control group (N), but significantly decreased (n = 9; bP < 0.01; Figure 3C) compared with the depression model group (M). The low XYS dose group (D) was not significant different compared with the depression model group (M).
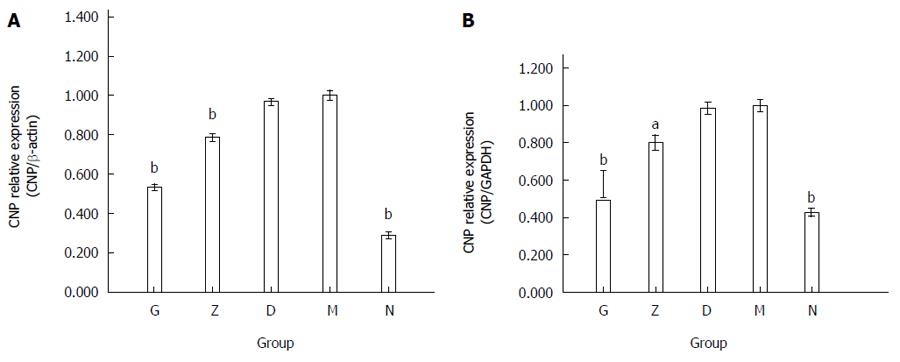
The mRNA expression levels of CNP in the various groups corroborated with the immunohistochemical data. The average ratios of CNP/β-actin between the five groups and the depression model group were 0.534 ± 0.013 in G, 0.785 ± 0.017 in Z, 0.967 ± 0.015 in D, 1.000 ± 0.025 in M, and 0.289 ± 0.017 in N (n = 9; bP < 0.01; Figure 4A). The average ratios of CNP/GAPDH between the five groups and the depression model group were 0.579 ± 0.072 in G, 0.801 ± 0.041 in Z, 0.984 ± 0.032 in D, 1.000 ± 0.031 in M, and 0.428 ± 0.022 in N (n = 9; bP < 0.01; Figure 4B). The expression of CNP in the depression model group (M) was significantly higher than that in the primary control group (N). The high and middle XYS dose groups (G and Z) were significantly decreased compared with the depression model group (M). The low XYS dose group (D) was not significant different compared with the depression model group (M).
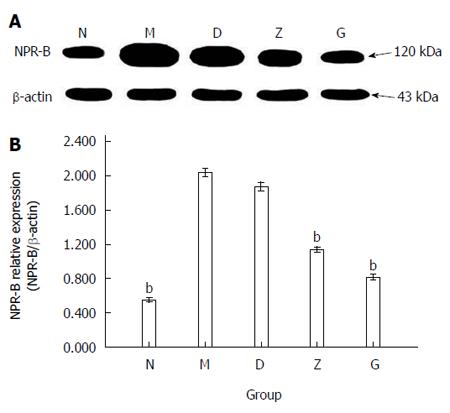
NPR-B (molecular weight: 120 kDa) expression levels in the rectum of the rats were detected using the method of western blotting. The doses were as follows: 7.65 g/kg per day for the low dose group (D), 15.3 g/kg per day for the medium dose group (Z), and 30.6 g/kg per day for the high dose group (G). The ratio of NPR-B/β-actin was 0.552 ± 0.026 in N, 2.039 ± 0.047 in M, 1.873 ± 0.048 in D, 1.136 ± 0.030 in Z, and 0.814 ± 0.033 in G (n = 9; bP < 0.01; Figure 5B).
Using immunohistochemistry evaluation, NPR-B immunopositive brown granules were expressed in the mucosa, muscle, and serosa of the rats’ rectum. NPR-B positive staining was located in the membrane of smooth muscle cells, basal granular cells, and stromal cells. The immunohistochemistry results showed that smooth muscle cells (n = 9; Figure 6A) and basal granular cells (n = 9; Figure 6B) in the five groups all expressed NPR-B, but differentially. The staining index of NPR-B was 1.33 ± 0.333 in N, 13.17 ± 0.307 in M, 11.50 ± 0.224 in D, 5.83 ± 0.307 in Z, and 4.00 ± 0.258 in G (n = 9, bP < 0.01; Figure 6C).
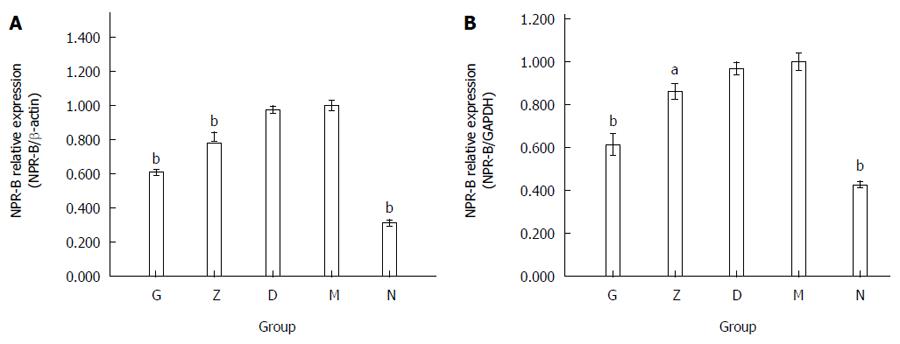
The mRNA expression level of NPR-B in the rectum of the rats corroborated with the immunohistochemistry result. The average ratio of NPR-B/β-actin between the five groups and the depression model group were 0.609 ± 0.017 in G, 0.817 ± 0.028 in Z, 0.974 ± 0.022 in D, 1.000 ± 0.030 in M, and 0.313 ± 0.020 in N (n = 9, bP < 0.01; Figure 7A). The average ratio of NPR-B/GAPDH between the five groups and the depression model group were 0.614 ± 0.051 in G, 0.862 ± 0.035 in Z, 0.969 ± 0.029 in D, 1.000 ± 0.039 in M, and 0.428 ± 0.015 in N (n = 9, bP < 0.01; Figure 7B).
NPR-B mRNA data were consistent with NRP-B IHC and western blotting data. The expression of NPR-B in the depression model group (M) was significantly higher than that in the primary control group (N). The high and middle XYS dose groups (G and Z) were not significantly different compared with the primary control group (N), but significantly decreased compared with the depression model group (M). The low XYS dose group (D) was not significant different compared with the depression model group (M).
In the present study, the expression of CNP and NPR-B in the rectum of depression rats was comparatively investigated. The results indicated that the CNP/NPR-B signal pathway is upregulated in the rectum of depressed rats, and may be contributing to the basis for depressive digestive disorders. However, XYS improved the depressive digestive disorders by downregulating the CNP signal pathway.
Gastrointestinal dysfunction is a common complication of depression, of which the symptoms include: gastrointestinal smooth muscle tone reduction, stomach hunger disappearance, and gastric peristaltic disorder[27]. Many people suffer from depression worldwide, and gastrointestinal disorders resulting from the condition seriously affect their quality of life. A large number of clinical cases have shown there to be little improvement in the condition of patients with depressive gastrointestinal disorders when stomach medicines were used alone. However, gastrointestinal disorder function improved when the patient’s mental state was managed with antidepressant drugs. Suleyman et al[28] reported that some antidepressants had gastro-protective potential in indomethacin-induced ulcers in rats. There are also studies that show that depression can cause gastrointestinal discomfort and vice versa, with mental state and the gastrointestinal tract influencing each other via the brain-gut axis. Bercik et al[29] reported that chronic gastrointestinal inflammation induced anxiety-like behavior and altered the central nervous system’s biochemistry in mice. Meanwhile, Liu et al[30] reported that transient gastric irritation in neonatal rats led to changes in their hypothalamic CRF expression, as well as depression- and anxiety-like behavior as adults.
CNP is considered to be a peptide that co-exist in the central nervous system and gastrointestinal tract that plays a bioactive role via the brain-gut axis[31]. Minamino et al[32] successfully detected CNP mRNA in various peripheral tissues, including the ileum-jejunum, colon-cecum, stomach, kidney, lung, testis, and submaxillary gland. Some studies have also demonstrated that CNP and NPR-B can be found in rat and guinea pig gastric antrum, rabbit colon, and mouse stomach pylorus and large intestine[33-35]. Del Ry et al[36] reported that the C-type natriuretic peptide signaling pathway was involved in the inhibition of myocardial contraction in heart failure, which is considered to be related to the contraction of smooth muscles. CNP signaling also has an inhibitory effect on the regulation of gastrointestinal motility, which has been experimentally demonstrated in several animal models, such as the rabbit colon[37]. Our previous study showed that CNP significantly inhibited the spontaneous contraction of smooth muscles in different species (e.g., guinea pig, rat, and humans), and that NP inhibited gastric motility via the NP-cGMP-PKG pathway[38].
Due to the several side effects associated with many synthetic chemical antidepressants, searching for new natural products for the treatment of depression from traditional Chinese medicine is drawing ever-increasing attention worldwide. A study by Dai et al[39] emphasized the antidepressant effect of XYS, and added that it could improve learning ability and memory in depressed rats. XYS, compared with typical antidepressant drugs, has many advantages such as fewer gastrointestinal reactions[40] and no dizziness, drowsiness, or palpitations. However, its action is slow, and it has complex herbal ingredients.
In spite of the previously mentioned abundant evidence that CNP/NPR-B exists in the gastrointestinal tract and can inhibit spontaneous contraction of smooth muscle, and that XYS have a therapeutic effect on depression, no study has reported the definite contact between depressive gastrointestinal motility disorders with the CNP/NPR-B signaling pathway, or the intervention effect of XYS. On the basis of this, our study investigated the possible molecular link of depressive digestive disorders through the CNP signaling pathway, as well as the impact of XYS in improving gastrointestinal motility disorders during depression.
We found that CNP and NPR-B immunopositive brown granules were expressed in the mucosa, muscle, and serosa of rat rectum. It is possible that CNP may be mainly synthesized, secreted from the mucosal layer, and act through NPR-B located on the muscular layer. Moreover, both the gene and protein expression of CNP and NPR-B were increased significantly. These may suggest upregulated activity of the CNP/NPR-B signaling pathway in the rectum of depressive digestive disorder rats. Interestingly, the expression of CNP and NPR-B decreased in depressed rats upon XYS intervention.
We also found that a higher dose of XYS produced a larger inhibitory effect, with the inhibitory effect of low dose XYS being the weakest. There was no significant difference in inhibition between medium-dose XYS (15.3 g/kg per day) and high-dose XYS (30.6 g/kg per day) administration, which may suggest that the inhibitory effect of XYS may reach a plateau of efficacy, and hence a higher dose may not necessarily translate into greater efficacy. Because the CNP/NPR-B signaling pathway has an inhibitory effect on gastrointestinal motility, its upregulation may be involved in the development of depressive digestive disorders.
It has been reported that cGMP can play an intracellular role by regulating cGMP-stimulated PDE2 or cGMP-inhibited PDE3[41]. The CNP-NPR-B/ pGC-cGMP signal pathway is modulated by PDEs in gastrointestinal smooth muscle, and therefore every stage of the CNP-NPR-B-pGC-cGMP signal pathway may be a potential target for investigating the mechanism of depressive digestive disorders.
In summary, CNP/NPR-B signaling pathway activity may be involved in digestive disorders which cause considerable morbidity in patients with the major healthcare burden that is depression. Additionally, current treatments are mainly symptomatic and frequently ineffective, as the development of new therapeutic options is hampered by a poor understanding of the underlying pathological mechanisms. XYS, however, has been demonstrated to effectively interfere with the CNP/NPR-B signaling pathway activity in the rectum of depressed rats.
A common complication of depression is depressive digestive disorders. However, its pathogenesis is not yet clear. A recent study has indicated that C-type natriuretic peptide (CNP) is secreted from the alimentary canal and plays an inhibitory role in the contraction of smooth muscles. However, whether the expression of CNP is increased in the rectum and its effect on depressive digestive disorders has not been reported.
CNP is distributed in diverse cell types such as cardiac fibroblasts, chondrocytes, glial cells, macrophages, and endothelial cells. Enterochromaffin cells in gastrointestinal mucosa and smooth muscle cells also secrete CNP. However, the other functions of the CNP signal pathway in the gastrointestinal tract in terms of physiological and pathophysiological conditions still need to be explored. In the present study, the possibility as to whether the CNP/natriuretic peptide receptor B (NPR-B) signal pathway is involved in depressive digestive disorders was investigated in depressed rats.
Recent reports have highlighted the pathogenesis of depressive digestive disorders, with previous studies focusing on the relationship between the CNP/NPR-B pathway and gastric motility. This is the first study to report that the expression of CNP and NPR-B in rectum tissue is increased in depressed rats. This study suggests that the CNP/NPR-B signal pathway may be involved in depressive digestive disorders.
By understanding that the CNP/NPR-B signal pathway may be involved in depressive digestive disorders, this study may represent a future strategy for therapeutic or preventive intervention in the treatment of patients with depression.
Depression induces many significant changes in digestive disorders, absorption, endocrine, and immune function dysfunction. However, digestive disorders improve as a precondition to the recovery of absorption, endocrine, and immune function dysfunction. At the same time, digestive disorder diseases are often accompanied by poor mood. NPR-B is the natriuretic peptide receptor for ANP, BNP, and CNP. NPR-B has a considerably higher affinity for CNP than ANP or BNP.
An interesting article pointing to a novel mechanism that may explain key factors in the CNP signaling pathway in depressive digestive disorders. The results are logical, attractive, and congruent. In many ways, the work is interesting, quite novel, and probably worthy of publication.
P- Reviewer: Del Ry S, Sandow SL S- Editor: Yu J L- Editor: Rutherford A E- Editor: Liu XM
| 1. | Cassano P, Fava M. Depression and public health: an overview. J Psychosom Res. 2002;53:849-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 322] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 2. | North CS, Hong BA, Alpers DH. Relationship of functional gastrointestinal disorders and psychiatric disorders: implications for treatment. World J Gastroenterol. 2007;13:2020-2027. [PubMed] |
| 3. | Levy RL, Olden KW, Naliboff BD, Bradley LA, Francisconi C, Drossman DA, Creed F. Psychosocial aspects of the functional gastrointestinal disorders. Gastroenterology. 2006;130:1447-1458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 342] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 4. | De Winter BY, De Man JG, Seerden TC, Depoortere I, Herman AG, Peeters TL, Pelckmans PA. Effect of ghrelin and growth hormone-releasing peptide 6 on septic ileus in mice. Neurogastroenterol Motil. 2004;16:439-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 73] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 5. | O’Mahony SM, Marchesi JR, Scully P, Codling C, Ceolho AM, Quigley EM, Cryan JF, Dinan TG. Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biol Psychiatry. 2009;65:263-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 753] [Cited by in RCA: 798] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 6. | Taché Y, Kiank C, Stengel A. A role for corticotropin-releasing factor in functional gastrointestinal disorders. Curr Gastroenterol Rep. 2009;11:270-277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 94] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 7. | Mayer EA. Gut feelings: the emerging biology of gut-brain communication. Nat Rev Neurosci. 2011;12:453-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1123] [Cited by in RCA: 1069] [Article Influence: 76.4] [Reference Citation Analysis (0)] |
| 8. | Van Oudenhove L, Vandenberghe J, Geeraerts B, Vos R, Persoons P, Fischler B, Demyttenaere K, Tack J. Determinants of symptoms in functional dyspepsia: gastric sensorimotor function, psychosocial factors or somatisation? Gut. 2008;57:1666-1673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 135] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 9. | Okumura T, Nozu T. Role of brain orexin in the pathophysiology of functional gastrointestinal disorders. J Gastroenterol Hepatol. 2011;26 Suppl 3:61-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | O’Mahony SM, Bulmer DC, Coelho AM, Fitzgerald P, Bongiovanni C, Lee K, Winchester W, Dinan TG, Cryan JF. 5-HT(2B) receptors modulate visceral hypersensitivity in a stress-sensitive animal model of brain-gut axis dysfunction. Neurogastroenterol Motil. 2010;22:573-578, e124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 11. | Woodard GE, Rosado JA, Brown J. Expression and control of C-type natriuretic peptide in rat vascular smooth muscle cells. Am J Physiol Regul Integr Comp Physiol. 2002;282:R156-R165. [PubMed] |
| 12. | Rose RA, Giles WR. Natriuretic peptide C receptor signalling in the heart and vasculature. J Physiol. 2008;586:353-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 156] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 13. | Chun TH, Itoh H, Saito T, Yamahara K, Doi K, Mori Y, Ogawa Y, Yamashita J, Tanaka T, Inoue M. Oxidative stress augments secretion of endothelium-derived relaxing peptides, C-type natriuretic peptide and adrenomedullin. J Hypertens. 2000;18:575-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Sogawa C, Wakizaka H, Aung W, Jin ZH, Tsuji AB, Furukawa T, Kunieda T, Saga T. C-type natriuretic peptide specifically acts on the pylorus and large intestine in mouse gastrointestinal tract. Am J Pathol. 2013;182:172-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Wiedemann K, Jahn H, Kellner M. Effects of natriuretic peptides upon hypothalamo-pituitary-adrenocortical system activity and anxiety behaviour. Exp Clin Endocrinol Diabetes. 2000;108:5-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Guo HS, Jin Z, Jin ZY, Li ZH, Cui YF, Wang ZY, Xu WX. Comparative study in the effect of C-type natriuretic peptide on gastric motility in various animals. World J Gastroenterol. 2003;9:547-552. [PubMed] |
| 17. | Guo HS, Cai ZX, Zheng HF, Li XL, Cui YF, Wang ZY, Xu WX, Lee SJ, Kim YC. Role of calcium-activated potassium currents in CNP-induced relaxation of gastric antral circular smooth muscle in guinea pigs. World J Gastroenterol. 2003;9:2054-2059. [PubMed] |
| 18. | Xu DY, Liu L, Cai YL, Li XL, Qiu ZX, Jin Z, Xu WX. Natriuretic peptide-dependent cGMP signal pathway potentiated the relaxation of gastric smooth muscle in streptozotocin-induced diabetic rats. Dig Dis Sci. 2010;55:589-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Zhang Y, Han M, Liu Z, Wang J, He Q, Liu J. Chinese herbal formula xiao yao san for treatment of depression: a systematic review of randomized controlled trials. Evid Based Complement Alternat Med. 2012;2012:931636. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | Meng ZZ, Chen JX, Jiang YM, Zhang HT. Effect of xiaoyaosan decoction on learning and memory deficit in rats induced by chronic immobilization stress. Evid Based Complement Alternat Med. 2013;2013:297154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 21. | Liu XJ, Zhou YZ, Li ZF, Cui J, Li ZY, Gao XX, Sun HF, Zhang LZ, Du GH, Qin XM. Anti-depressant effects of Xiaoyaosan on rat model of chronic unpredictable mild stress: a plasma metabonomics study based on NMR spectroscopy. J Pharm Pharmacol. 2012;64:578-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 22. | Li Y, Xu BY, Xiao F. [Effect of modified xiaoyao powder for improving sleep in patients with psychological stress insomnia]. Zhongguo Zhongxiyi Jiehe Zazhi. 2009;29:208-211. [PubMed] |
| 23. | Qin F, Huang X, Ren P. Chinese herbal medicine modified xiaoyao san for functional dyspepsia: meta-analysis of randomized controlled trials. J Gastroenterol Hepatol. 2009;24:1320-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Liang Y, Guo XL, Chen JX, Yue GX. Effects of the chinese traditional prescription xiaoyaosan decoction on chronic immobilization stress-induced changes in behavior and ultrastructure in rat hippocampus. Evid Based Complement Alternat Med. 2013;2013:984797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Di Martino E, Wild CP, Rotimi O, Darnton JS, Olliver RJ, Hardie LJ. IGFBP-3 and IGFBP-10 (CYR61) up-regulation during the development of Barrett’s oesophagus and associated oesophageal adenocarcinoma: potential biomarkers of disease risk. Biomarkers. 2006;11:547-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | Pilo A, Zucchelli GC, Malvano R, Masini S. Main features of computer algorhythms for RIA data reduction; comparison of some different approaches for the interpolation of the dose-response curve. J Nucl Med Allied Sci. 1982;26:235-248. [PubMed] |
| 27. | Parkman HP, Hasler WL, Fisher RS. American Gastroenterological Association technical review on the diagnosis and treatment of gastroparesis. Gastroenterology. 2004;127:1592-1622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 538] [Cited by in RCA: 508] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 28. | Suleyman H, Cadirci E, Albayrak A, Polat B, Halici Z, Koc F, Hacimuftuoglu A, Bayir Y. Comparative study on the gastroprotective potential of some antidepressants in indomethacin-induced ulcer in rats. Chem Biol Interact. 2009;180:318-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 29. | Bercik P, Verdu EF, Foster JA, Macri J, Potter M, Huang X, Malinowski P, Jackson W, Blennerhassett P, Neufeld KA. Chronic gastrointestinal inflammation induces anxiety-like behavior and alters central nervous system biochemistry in mice. Gastroenterology. 2010;139:2102-2112.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 450] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 30. | Liu L, Li Q, Sapolsky R, Liao M, Mehta K, Bhargava A, Pasricha PJ. Transient gastric irritation in the neonatal rats leads to changes in hypothalamic CRF expression, depression- and anxiety-like behavior as adults. PLoS One. 2011;6:e19498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 31. | Mayer EA, Tillisch K, Bradesi S. Review article: modulation of the brain-gut axis as a therapeutic approach in gastrointestinal disease. Aliment Pharmacol Ther. 2006;24:919-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 93] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 32. | Minamino N, Aburaya M, Kojima M, Miyamoto K, Kangawa K, Matsuo H. Distribution of C-type natriuretic peptide and its messenger RNA in rat central nervous system and peripheral tissue. Biochem Biophys Res Commun. 1993;197:326-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 103] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 33. | Itaba S, Chijiiwa Y, Matsuzaka H, Motomura Y, Nawata H. Presence of C-type natriuretic peptide (CNP) in guinea pig caecum: role and mechanisms of CNP in circular smooth muscle relaxation. Neurogastroenterol Motil. 2004;16:375-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 34. | Stepan H, Leitner E, Bader M, Walther T. Organ-specific mRNA distribution of C-type natriuretic peptide in neonatal and adult mice. Regul Pept. 2000;95:81-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 35. | Cho ES, Kim SZ, Cho KW, Park BK. Immunohistochemical localization of C-type natriuretic peptide in the rat submaxillary salivary gland. Arch Oral Biol. 2000;45:425-430. [PubMed] |
| 36. | Del Ry S, Passino C, Maltinti M, Emdin M, Giannessi D. C-type natriuretic peptide plasma levels increase in patients with chronic heart failure as a function of clinical severity. Eur J Heart Fail. 2005;7:1145-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 76] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 37. | Kim JH, Jeon GJ, Kim SZ, Cho KW, Kim SH. C-type natriuretic peptide system in rabbit colon. Peptides. 2001;22:2061-2068. [PubMed] |
| 38. | Cai CY, Cai ZX, Gu XY, Shan LJ, Wang YX, Yin XZ, Qi QH, Guo HS. Dendroaspis natriuretic peptide relaxes gastric antral circular smooth muscle of guinea-pig through the cGMP/cGMP-dependent protein kinase pathway. World J Gastroenterol. 2008;14:5461-5466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 39. | Dai Y, Li Z, Xue L, Dou C, Zhou Y, Zhang L, Qin X. Metabolomics study on the anti-depression effect of xiaoyaosan on rat model of chronic unpredictable mild stress. J Ethnopharmacol. 2010;128:482-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 94] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 40. | Varghese AK, Verdú EF, Bercik P, Khan WI, Blennerhassett PA, Szechtman H, Collins SM. Antidepressants attenuate increased susceptibility to colitis in a murine model of depression. Gastroenterology. 2006;130:1743-1753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 86] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 41. | Cai YL, Sun Q, Huang X, Jiang JZ, Zhang MH, Piao LH, Jin Z, Xu WX. cGMP-PDE3-cAMP signal pathway involved in the inhibitory effect of CNP on gastric motility in rat. Regul Pept. 2013;180:43-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |