Published online Dec 21, 2015. doi: 10.3748/wjg.v21.i47.13268
Peer-review started: January 10, 2015
First decision: March 10, 2015
Revised: May 3, 2015
Accepted: July 3, 2015
Article in press: July 3, 2015
Published online: December 21, 2015
Processing time: 338 Days and 14.2 Hours
AIM: To investigate the potential roles of enhancer of zeste homolog2 (EZH2), Bmi-1 and miR-203 in cell proliferation and invasion in hepatocellular carcinoma (HCC) cell line Hep3B.
METHODS: A total of 73 patients who underwent surgical resection at Fuzong Clinical Medical College of Fujian Medical University were enrolled in this study. Hep3B cells were cultivated in RPMI 1640 medium supplemented with 10% fetal bovine serum at 37 °C. Vectors that containing cDNA of the EZH2 gene or miR-203 targeted shRNA plasmid were constructed, and then transfected into Hep3B cells. The mRNA expression of miR-203, EZH2, and Bmi-1 was analyzed using quantitative real-time polymerase chain reaction analysis, and the protein levels of EZH2 and Bmi-1 were detected by Western blot analysis. Effect of EZH2 or miR-203 on cell proliferation was observed by methyl thiazolyl tetrazolium assay, and cell apoptosis was assessed using flow cytometry. Besides, effect of EZH2 or miR-203 on tumor cell invasion was detected using Transwell assay.
RESULTS: The mRNA levels of EZH2 and Bmi-1 in HCC tissues and in Hep3B cells were significantly higher compared with those in normal samples (P < 0.01), while miR-203 level was significantly lower in HCC tissues (P < 0.01). Hep3B cells transfected with EZH2-shRNA or miR-203-shRNA showed lower expression levels of EZH2 and Bmi-1 (P < 0.05). Compared with controls, Hep3B cells transfected with EZH2-shRNA had relative slow cell proliferation, indicating that low expression of EZH2 and Bmi-1 and overexpression of miR-203 could inhibit Hep3B cell proliferation (P < 0.05). The average apoptosis rate of Hep3B cells transfected with EZH2-shRNA vector was about 18.631%, while that of Hep3B cells transfected with shRNA vector was about 5.33%, suggesting that EZH2 was down-regulated by transfecting with EZH2-shRNA, and the down-regulated EZH2 contributed to the cell apoptosis. Low expression of EZH2 and Bmi-1 and overexpression of miR-203 could reduce Hep3B cell invasion (P < 0.05).
CONCLUSION: Our study suggests that EZH2 and Bmi-1 are up-regulated while miR-203 is down-regulated in Hep3B cells. MiR-203 may contribute to the metastasis and enhance apoptosis of HCC cells by regulating EZH2 and Bmi-1. Our study may provide a theoretical basis for metastasis of HCC and targeted therapy of HCC.
Core tip: In this study, we analyzed the expression levels of Bmi-1, EZH2, and miR-203 in hepatocellular carcinoma (HCC) tissues and in Hep3B cell line. Comprehensive experimental methods were used to investigate the roles of Bmi-1, EZH2, and miR-203 in Hep3B cell proliferation, invasion and apoptosis. This study aimed to investigate the potential collaborate regulation mechanism of EZH2, Bmi-1, and miR-203 in metastasis and invasion of HCC.
- Citation: Yang F, Lv LZ, Cai QC, Jiang Y. Potential roles of EZH2, Bmi-1 and miR-203 in cell proliferation and invasion in hepatocellular carcinoma cell line Hep3B. World J Gastroenterol 2015; 21(47): 13268-13276
- URL: https://www.wjgnet.com/1007-9327/full/v21/i47/13268.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i47.13268
Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related deaths worldwide, with increasing incidence in many countries[1]. Due to the easy metastasis and easy recurrence of HCC, hepatic resection is the main treatment method for patients suffering HCC[2]. However, liver transplantation provides the best chance for such patients and offers long-term survival[3]. Therefore, to identify some reliable biomarkers for the prediction of metastasis and recurrence of HCC will be of great significance.
Polycomb group (PcG) is a group of proteins that control the transcriptional memory of cells by maintaining the stable silencing of specific sets of genes through chromatin modifications[4]. The polycomb repressive complex 1 (PRC1) and PRC2 are two distinct PcG complexes for PcG protein[5]. Increasing studies prove that enhancer of zeste homolog2 (EZH2) is a polycomb group protein and that EZH2 is overexpressed in HCC[6,7]. Also, B cell-specific moloney murine leukaemia virus insertion site 1 (Bmi-1) represses the transcription of their target genes via an epigenetic mechanism[8]. Effendi et al[9] proves that overexpression of Bmi-1 in early-stage HCC is correlated with ATP-binding cassette transporter B1 expression. Also, up-regulated Bmi-1 enhances the invasion and metastasis of HCC[10]. Besides, Fu et al[11] prove that Bmi-1 and EZH2 are associated with the progression and aggressive biological behavior of HCC.
MicroRNAs (miRNAs) are some endogenous non-coding small molecules that regulate the gene expression at the posttranscriptional level. Numerous miRNAs play crucial roles in HCC, and miR-203 has been suggested to be a predictor for HCC after liver transplantation[12]. Also, miR-203 induces cell apoptosis and represses cell growth by targeting Bmi-1 in HCC[13]. EZH2 could regulate the expression of some miRNAs, although the mechanism is still unclear[14]. Although many studies have devoted to elucidating the roles of Bmi-1 and EZH2 in HCC progression, collaborate regulation mechanism of EZH2, Bmi-1, and miR-203 in proliferation and invasion of HCC remains incompletely described.
In this study, we analyzed the expression levels of Bmi-1, EZH2, and miR-203 in HCC tissues and in Hep3B cell line. Comprehensive experimental methods were used to investigate the roles of Bmi-1, EZH2, and miR-203 in Hep3B cell proliferation, invasion and apoptosis. This study aimed to investigate the potential collaborate regulation mechanism of EZH2, Bmi-1, and miR-203 in metastasis and invasion of HCC.
A total of 73 patients who underwent surgical resection at Department of Hepatobiliary Surgery, Fuzong Clinical Medical College of Fujian Medical University from January 2007 to January 2014 were enrolled in this study. Informed consent was obtained from all cases for research use of the specimens, and all study protocols were approved by the Ethics Committee for Clinical Research of Fuzhou General Hospital. All the patients have received no radiotherapy or chemotherapy before routine surgery.
The HCC cell line Hep3B was given as a gift by Shanghai Institute of Biochemistry and Cell Biology (China) and was cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS) (Hyclone, United States) in a humidified chamber with 95% air and 5% CO2 at 37 °C.
The cDNA corresponding to the full-length human EZH2 gene was amplified by sequencing and subcloned into the lentivirus vector pLKO.1-TRC cloning vector (10878). The specific primers for EZH2 were: sense, 5’-CCGGGCTAGGTTAATTGGGACCAAACTCGAGTTTGGTCCCAATTAACCTAGCTTTTTG-3’ and antisense, 5’-AAT-TCAAAAAGCTAGGTTAATTGGGACCAAACTCGAGTTTGGTCCCAATTAACCTAG-3’.
In addition, to construct the overexpression vector of miR-203, the cultivated Hep3B cells at logarithmic phase were digested using 0.25% trypsin to make the cell concentration to 5 × 106/mL. The digested Hep3B cells (100 μL) were injected into the cell incubator (10 cm2). After being incubated at 37 °C with 5% of CO2 until cell confluence reached 50%-60%, shRNA (10 μL) and opti-MEM (490 μL) were mixed with the Hep3B cells. The total mixture was incubated in 10 mL medium with 10% FBS.
Finally, Hep3B cells transfected with EZH2-shRNA/miR-203-shRNA were seeded in 6-well plates using Deofect EU transfection reagent (Roche, Germany) according to the manufacturer’s protocol. Clones with stable transfection of EZH2-shRNA/miR-203-shRNA or empty vector were selected using 3 μg/mL puromycin dihydrochloride (Sigma, United States).
Total RNA from HCC tissues and Hep3B cells was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, United States) according to the manufacturer’s instructions. Expression of miR-203 was determined using the Taqman microRNA assay kit (Taqman microRNA assay kit), and U6 snRNA was used for the normalization of relative abundance of miR-203. Total RNA was reverse-transcribed into cDNA using the primescript RT reagent kit (Takara, Japan), and 1 μL of cDNA was used for each PCR reaction.
Hep3B cells transfected with the EZH2-shRNA or empty vector were lysed with RIPA and total proteins were separated by SDS-PAGE after the concentration was determined, and transferred to PVDF membranes. Primary antibodies against EZH2 and BMI-1 were added and incubated overnight in TBST with 5% bovine serum albumin. After washing with TBST, the membranes were further incubated for 1 h at room temperature with a secondary antibody conjugated with horseradish peroxidase. Finally, the immunoreactive protein bands were visualized with a chemiluminescence kit (NEN Life Science Products).
To investigate the role of miR-203 in HCC cell proliferation, cells (100 μL, about 5000 cells) transfected with miR-203 were injected into 96-well plates and then incubated at 37 °C with 5% CO2. Methyl thiazolyl tetrazolium (10 μg/mL) was added into the cells to determine the cell viability and the cells were further incubated at 37 °C for 4 h. Cell proliferation reagent WST-1 (10 μL; Roche, United States) was added into the cultivated cells. The absorbance of cells was read at 450 nm using a microplate reader (Bio-Rad, 3550, United States).
Hep3B cells were transfected with 50 μL EZH2-shRNA/miR-203 shRNA or control vector, and cell apoptosis assay was performed using Annexin V FITC apoptosis Detection Kit I (BD Pharmingen, United States) at 72 h after transfection according to the manufacture’s protocol. Cell suspension (100 μL) was incubated with 5 μL of annexin-V and 1 μL of propidium iodide at room temperature for 10 min. Total stained cells were analyzed using flow cytometry (BD-bio, San Diego, CA, United States).
To determine the effect of EZH2 or miR-203 on HCC cell invasion, clones of Hep3B cells transfected with different vectors were injected into the 24-well Transwell cell culture chambers (8-μm pores; Millipore, United States) and Matrigel (BD, United States). After serum starvation for 24 h, Hep3B cells in 350 μL serum-free DMEM were transferred to the upper chamber. The lower chamber was added with DMEM with 15% FBS as a chemoattractant. The number of invasive cells that remained adherent to the outside of the membrane were fixed and stained.
All the data are expressed as the mean ± SD. Correlations between clinicopathological variables and EZH2, BMI-1 and miR-203 expression were analyzed with Pearson’s tests. Variance analysis between groups was performed by one-way ANOVA and the significance of differences between control and treatment groups was tested using Dummett’s multiple comparisons test. All statistical analyses were performed using the SPSS software package (SPSS, Chicago, IL, United States). P < 0.05 was considered statistically significant.
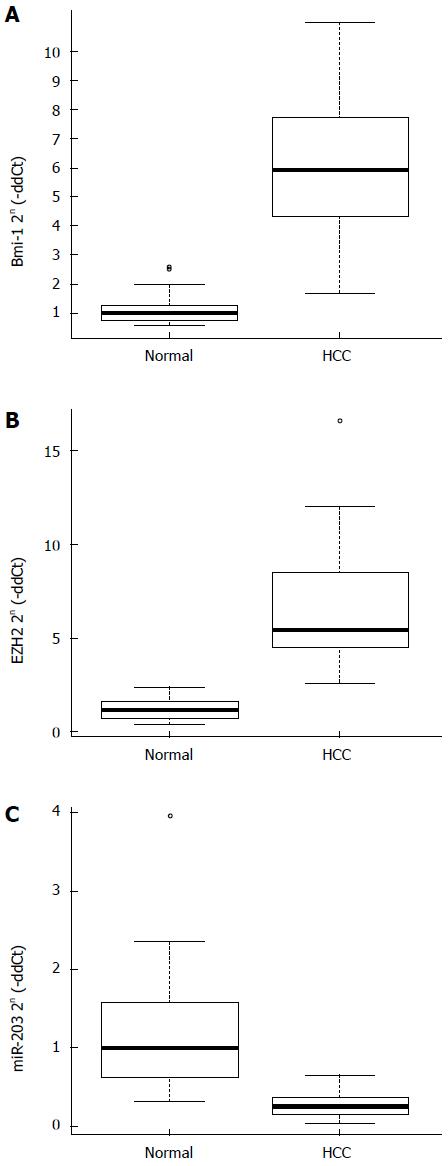
The results of quantitative real-time PCR analysis are shown in Figure 1. The mRNA levels of both EZH2 and Bmi-1 were significantly higher in HCC tissues compared with normal tissues (P < 0.01; Figure 1A and B). Besides, miR-203 level was lower in HCC tissue samples than in normal tissues (P < 0.01; Figure 1C).
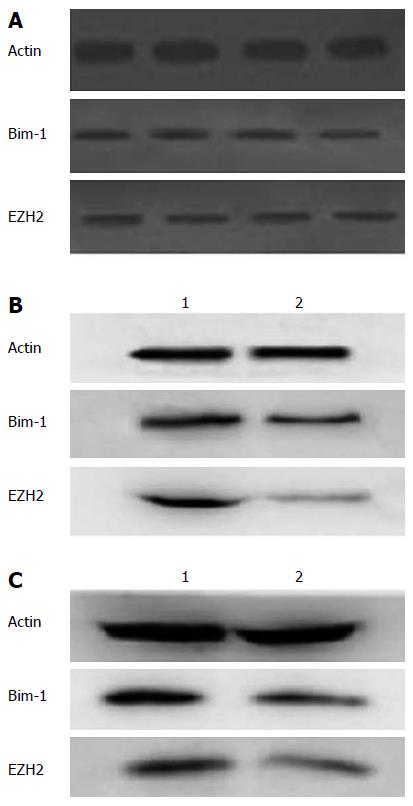
To further investigate the expression protein levels of EZH2 and Bmi-1, Western bot analysis was conducted (Figure 2). The results showed that the expression levels of both EZH2 and Bmi-1 proteins were higher in HCC tissues compared with control tissues (P < 0.05; Figure 2A).
Besides, Western blot analysis showed that the expression levels of Bmi-1 and EZH2 in Hep3B cells transfected with EZH2-shRNA declined compared with control cells (Figure 2B). Meanwhile, expression levels of Bmi-1 and EZH2 in Hep3B cells overexpressing miR-203 were lower than those in Hep3B cells transfected with control vector (Figure 2C).
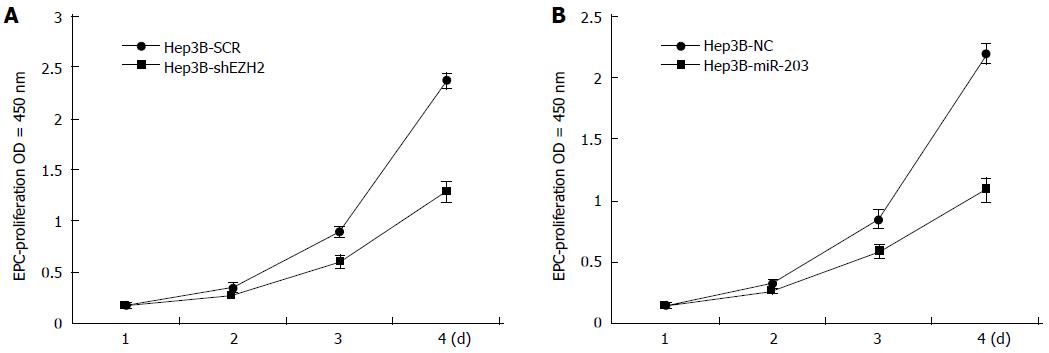
After being cultivated for 4 d in the 96-well plates, the cell proliferation ability of Hep3B cells transfected with EZH2-shRNA/miR-203-shRNA or control vector was detected (Figure 3). Compared with the controls, Hep3B cells transfected with EZH2-shRNA had relative slow cell proliferation (Figure 3A), and the same tendency was observed in cells transfected with miR-203-shRNA (Figure 3B), suggesting that low expression of EZH2 and high expression of miR-203 both could inhibit Hep3B cell proliferation.
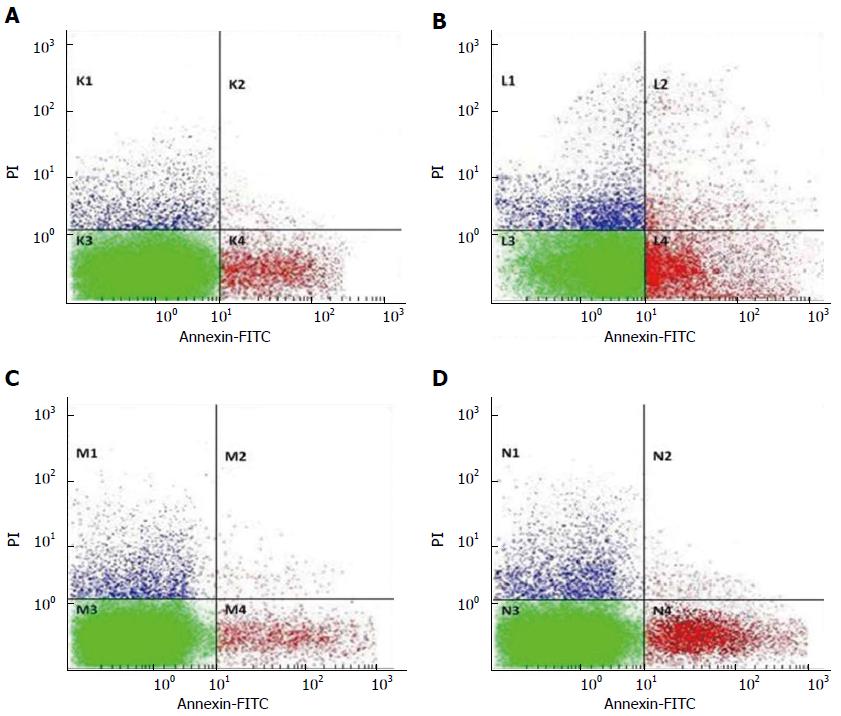
Flow cytometry was used to investigate the role of EZH2 and miR-203 in apoptosis of HCC cells (Figure 4). The results showed that the average apoptosis rate of Hep3B cells transfected with EZH2-shRNA vector was about 18.631%, while that of Hep3B cells transfected with control shRNA vector was about 5.33%, suggesting that EZH2 was down-regulated by transfecting with EZH2-shRNA, and the down-regulated EZH2 contributed to the cell apoptosis (Figure 4A and B).
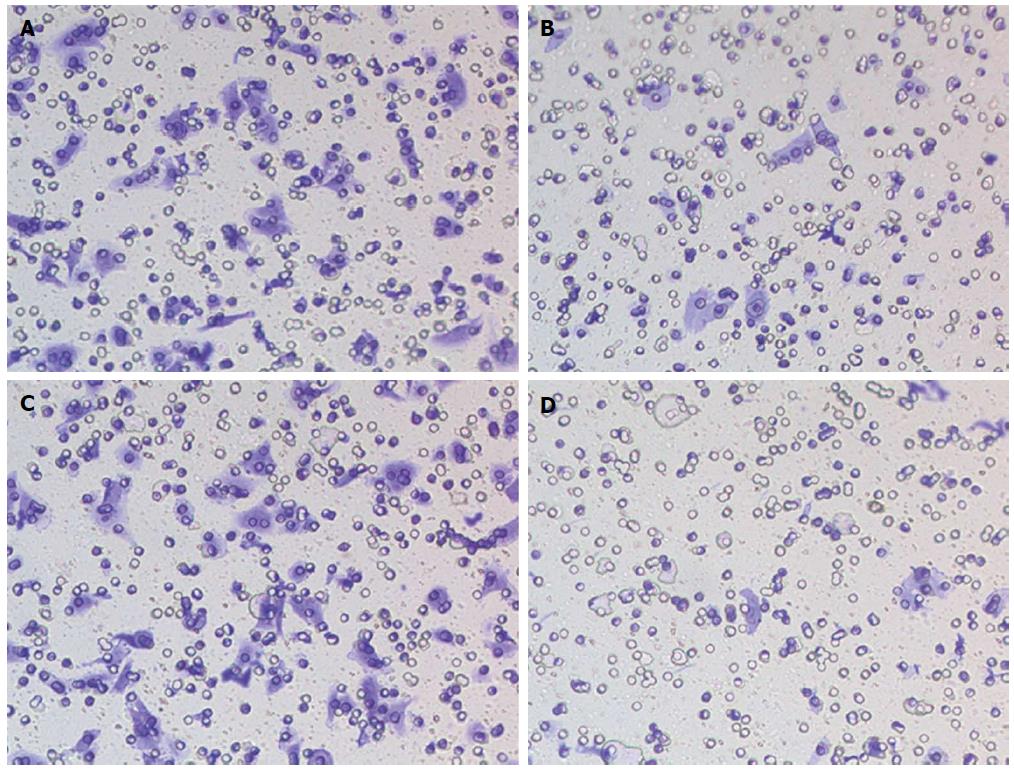
The roles of EZH2 and miR-203 on HCC tumor cells invasion were shown in Figure 5. The results showed that violet crystals in Hep3B cells transfected with EZH2-shRNA were less than that in control group, indicating that the cell invasion ability of Hep3B cells transfected with EZH2-shRNA was obviously abated compared with that of Hep3B cells transfected with shRNA vector (Figure 5A and B).
Besides, Transwell invasion assay displayed that violet crystals in Hep3B cells with miR-203 overexpression were less than that in control group, suggesting that overexpression of miR-203 inhibiting the invasion of HCC cells (Figure 5C and D).
HCC is the third leading cause of cancer-related deaths worldwide, with increasing incidence in many countries[1]. Due to the easy metastasis and recurrence of HCC, hepatic resection is the main treatment method for patients with HCC[2]. High recurrence rate of intra-hepatic and distant metastases is the major obstacle to improving the survival of patients with HCC. Clinical data showed that PcG proteins such as EZH2 and Bmi-1, and miR-203 play key roles in HCC metastasis and recurrence. Clarifying the mechanisms and identifying key factors underlying invasion and metastasis that could reflect the metastasis and recurrence of HCC will be necessary. In the present study, we found that PcG proteins Bmi-1 and EZH2, and miRNA-203 coordinately regulated HCC, which might play an important role in HCC recurrence and metastasis.
Our data showed that the two PcG proteins EZH2 and Bmi-1 had high expression levels both in HCC tissues and in Hep3B cells, suggesting their crucial roles in HCC development. PcG proteins are important in maintaining cell identity and regulation of the cell cycle[15]. EZH2 was found to be associated with adverse pathological characteristics by some investigators[16]. Overexpression of EZH2 and Bmi-1 is associated with a poor prognosis in several types of human tumors[17,18,19]. Sasaki et al[20] proved that high levels of EZH2 and Bmi-1 were related to HCC progression. Besides, qRT-PCR analysis showed that miR-203 is down-regulated in HCC tissues and in Hep3B cells. It has been demonstrated that miR-203 plays key roles in controlling proliferation, migration, and invasion of cancer cells, such as prostate cancer cells[21]. Recent studies show that miR-203 is epigenetically silenced in HCC[22,23]. Therefore, our results are consistent with those of the former studies.
Our study showed that proliferation ability of Hep3B cells transfected with EZH2-shRNA or miR-203-shRNA was lower compared with Hep3B cells transfected with control vector. The polycomb group protein EZH2 was up-regulated in proliferating HCC cells[24] and EZH2 could be inhibited by the tumor suppressor miR-124 in HCC[25]. Besides, Wang et al[26] proved that down-regulation of Bmi-1 could retard the cell proliferation in HCC, and Chiba et al[27] proved that Bmi-1 enhanced the proliferation of HCC cells. Thus, high levels of Bmi-1 and EZH2 may contribute to cell proliferation of HCC. Meanwhile, bioinformatics analysis showed that miR-203 was related to the cell growth of HCC[28]. Our results are consistent with those of Sasaki et al[20], who found that the expression of Bmi-1 and EZH2 was heterogeneous and associated with vascular infiltration, histological grade and cell proliferation in HCC. Based on our results, we speculate that high expression of Bmi-1 and EZH2 or low expression of miR-203 could contribute to Hep3B cell proliferation.
On the other hand, our data showed that cell apoptosis of Hep3B cells transfected with EZH2-shRNA was lower than that in Hep3B cells transfected with control vector, and the same tendency was observed in Hep3B cells transfected with miR-203 shRNA. This finding indicates the crucial role of EZH2 and Bmi-1 in regulating HCC cell apoptosis and invasion. Su et al[29] proved that EZH2 could be silenced by the down-regulated miR-101, which contributed to HCC cell apoptosis. Also, overexpression of Bmi-1 leads to increased self-renewal and tumorigenesis of liver stem cells in mice[30], and high expression level of Bmi-1 in HCC was significantly correlated with recurrence of HCC via increasing the cell apoptosis[31]. Yonemitsu et al[17] proved that cell proliferation was apparently inhibited in HepG2 cells with EZH2 and Bmi-1 knockdown. Thus, high levels of EZH2 and Bmi-1 may be the contributors to HCC metastasis and recurrence. Based on our results, we speculate that EZH2 may collaborate with Bmi-1 in HCC development and progression by regulating Hep3B cell apoptosis and invasion. Meanwhile, miR-203 is the first skin-specific miRNA discovered in recent years[12]. It is involved not only in the regulation of embryonic epidermal differentiation, building a protective layer of skin for psoriasis and other skin diseases, but also in proliferation, differentiation, invasion, metastasis and apoptosis of tumor cells as a tumor suppressor or oncogenic factor[32]. The abnormal expression of miR-203 can alter the expression of many targeted genes, leading to breast, prostate, liver and other tumors[22]. Abnormal expression of miR-203 caused by promotor methylation resulted in aberrant expression of many target genes including Bmi-1, thus leading to the occurrence of breast cancer and liver cancer[33,34]. Knockdown of EZH2 both in breast cancer and in breast cancer cell line resulted in the down-regulation of specific miRNAs such as miR-203 and miR-200 to enhance the expression of Bmi-1[35], indicating that miR-203 may function by regulating many target genes.
In conclusion, our study suggests that EZH2 and Bmi-1, together with miR-203, may form a regulatory axis (EZH2-Bmi-1-miR-203) to regulate Hep3B cell proliferation and invasion. Down-regulating EZH2 causes the down-regulation of Bmi-1 and the up-regulation of miR-203. The overexpression of miR-203 and down-regulation of EZH2 and Bmi-1 may decrease the invasion and proliferation but increase the apoptosis of Hep3B cells. miR-203 might play an important role in the link of EZH2 and Bmi-1. Our study may provide evidence of coordinated regulation of PcG proteins EZH2 and Bmi-1 through miR-203, which regulates the invasion and proliferation of Hep3B cells. However, further studies that focus on the role of miR-203 are still needed.
To clarify the mechanisms and identify the key factors of Bmi-1/EZH2/miR-203 underlying invasion and metastasis that could reflect the metastasis and recurrence of hepatocellular carcinoma.
This study found that PcG proteins of Bmi-1 and EZH2, and miR-203 coordinately regulated hepatocellular carcinoma, which may play crucial roles in hepatocellular carcinoma recurrence and metastasis.
This study found that two PcG proteins EZH2 and Bmi-1 had high expression levels in hepatocellular carcinoma tissues and in Hep3B cells, suggesting their crucial roles in hepatocellular carcinoma development. Low expression of miR-203 or high expression of EZH2 and Bmi-1 contribute to Hep3B cell proliferation. The present study suggests that EZH2 and Bmi-1, together with miR-203, may form a regulatory axis to regulate Hep3B cell proliferation and invasion.
This study may provide evidence of coordinated regulation of PcG proteins EZH2, Bmi-1 through miR-203, which regulated invasion and proliferation of Hep3B cells.
The authors detected the expression of PcG proteins EZH2 and Bmi-1 both in hepatocellular carcinoma tissues and in Hep3B cells. SiRNA of miR-203 was constructed and transfected into Hep3B cells.
This study investigated the coordinated role between PcG proteins EZH2 and Bmi-1, and miR-203 in regulating hepatocellular carcinoma Hep3B cell proliferation, invasion, and cell apoptosis, and the study suggested that EZH2-Bmi-1-miR-203 may be a regulatory axis to regulate Hep3B cell proliferation and invasion.
P- Reviewer: Deepak P, Siddiqui I S- Editor: Yu J L- Editor: Wang TQ E- Editor: Zhang DN
| 1. | Tung-Ping Poon R, Fan ST, Wong J. Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann Surg. 2000;232:10-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 2. | Zhou Y, Zhao Y, Li B, Xu D, Yin Z, Xie F, Yang J. Meta-analysis of radiofrequency ablation versus hepatic resection for small hepatocellular carcinoma. BMC Gastroenterol. 2010;10:78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 141] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 3. | Cheng YC, Chen TW, Fan HL, Yu CY, Chang HC, Hsieh CB. Transarterial chemoembolization for intrahepatic multiple recurrent HCC after liver resection or transplantation. Ann Transplant. 2014;19:309-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Deleris A, Stroud H, Bernatavichute Y, Johnson E, Klein G, Schubert D, Jacobsen SE. Loss of the DNA methyltransferase MET1 Induces H3K9 hypermethylation at PcG target genes and redistribution of H3K27 trimethylation to transposons in Arabidopsis thaliana. PLoS Genet. 2012;8:e1003062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 118] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 5. | Raaphorst FM, van Kemenade FJ, Blokzijl T, Fieret E, Hamer KM, Satijn DP, Otte AP, Meijer CJ. Coexpression of BMI-1 and EZH2 polycomb group genes in Reed-Sternberg cells of Hodgkin’s disease. Am J Pathol. 2000;157:709-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 132] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 6. | Gong Y, Wang X, Liu J, Shi L, Yin B, Peng X, Qiang B, Yuan J. NSPc1, a mainly nuclear localized protein of novel PcG family members, has a transcription repression activity related to its PKC phosphorylation site at S183. FEBS Lett. 2005;579:115-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Ciarapica R, De Salvo M, Carcarino E, Bracaglia G, Adesso L, Leoncini PP, Dall’Agnese A, Walters ZS, Verginelli F, De Sio L. The Polycomb group (PcG) protein EZH2 supports the survival of PAX3-FOXO1 alveolar rhabdomyosarcoma by repressing FBXO32 (Atrogin1/MAFbx). Oncogene. 2014;33:4173-4184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 8. | Kim HG, Kim DJ, Li S, Lee KY, Li X, Bode AM, Dong Z. Polycomb (PcG) proteins, BMI1 and SUZ12, regulate arsenic-induced cell transformation. J Biol Chem. 2012;287:31920-31928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Effendi K, Mori T, Komuta M, Masugi Y, Du W, Sakamoto M. Bmi-1 gene is upregulated in early-stage hepatocellular carcinoma and correlates with ATP-binding cassette transporter B1 expression. Cancer Sci. 2010;101:666-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 10. | Li X, Yang Z, Song W, Zhou L, Li Q, Tao K, Zhou J, Wang X, Zheng Z, You N. Overexpression of Bmi-1 contributes to the invasion and metastasis of hepatocellular carcinoma by increasing the expression of matrix metalloproteinase (MMP)-2, MMP-9 and vascular endothelial growth factor via the PTEN/PI3K/Akt pathway. Int J Oncol. 2013;43:793-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 11. | Fu WM, Tang LP, Zhu X, Lu YF, Zhang YL, Lee WY, Wang H, Yu Y, Liang WC, Ko CH. MiR-218-targeting-Bmi-1 mediates the suppressive effect of 1,6,7-trihydroxyxanthone on liver cancer cells. Apoptosis. 2015;20:75-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Chen HY, Han ZB, Fan JW, Xia J, Wu JY, Qiu GQ, Tang HM, Peng ZH. miR-203 expression predicts outcome after liver transplantation for hepatocellular carcinoma in cirrhotic liver. Med Oncol. 2012;29:1859-1865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 13. | Sato F, Tsuchiya S, Meltzer SJ, Shimizu K. MicroRNAs and epigenetics. FEBS J. 2011;278:1598-1609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 430] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 14. | Thomas MB, Zhu AX. Hepatocellular carcinoma: the need for progress. J Clin Oncol. 2005;23:2892-2899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 305] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 15. | Furuyama T, Tie F, Harte PJ. Polycomb group proteins ESC and E(Z) are present in multiple distinct complexes that undergo dynamic changes during development. Genesis. 2003;35:114-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Mihic-Probst D, Kuster A, Kilgus S, Bode-Lesniewska B, Ingold-Heppner B, Leung C, Storz M, Seifert B, Marino S, Schraml P. Consistent expression of the stem cell renewal factor BMI-1 in primary and metastatic melanoma. Int J Cancer. 2007;121:1764-1770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 83] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 17. | Yonemitsu Y, Imazeki F, Chiba T, Fukai K, Nagai Y, Miyagi S, Arai M, Aoki R, Miyazaki M, Nakatani Y. Distinct expression of polycomb group proteins EZH2 and BMI1 in hepatocellular carcinoma. Hum Pathol. 2009;40:1304-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 18. | Breuer RH, Snijders PJ, Smit EF, Sutedja TG, Sewalt RG, Otte AP, van Kemenade FJ, Postmus PE, Meijer CJ, Raaphorst FM. Increased expression of the EZH2 polycomb group gene in BMI-1-positive neoplastic cells during bronchial carcinogenesis. Neoplasia. 2004;6:736-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 92] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 19. | Liu S, Dontu G, Mantle ID, Patel S, Ahn NS, Jackson KW, Suri P, Wicha MS. Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res. 2006;66:6063-6071. [PubMed] |
| 20. | Sasaki M, Ikeda H, Itatsu K, Yamaguchi J, Sawada S, Minato H, Ohta T, Nakanuma Y. The overexpression of polycomb group proteins Bmi1 and EZH2 is associated with the progression and aggressive biological behavior of hepatocellular carcinoma. Lab Invest. 2008;88:873-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 110] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 21. | Viticchiè G, Lena AM, Latina A, Formosa A, Gregersen LH, Lund AH, Bernardini S, Mauriello A, Miano R, Spagnoli LG. MiR-203 controls proliferation, migration and invasive potential of prostate cancer cell lines. Cell Cycle. 2011;10:1121-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 171] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 22. | Saini S, Majid S, Yamamura S, Tabatabai L, Suh SO, Shahryari V, Chen Y, Deng G, Tanaka Y, Dahiya R. Regulatory Role of mir-203 in Prostate Cancer Progression and Metastasis. Clin Cancer Res. 2011;17:5287-5298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 218] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 23. | Furuta M, Kozaki KI, Tanaka S, Arii S, Imoto I, Inazawa J. miR-124 and miR-203 are epigenetically silenced tumor-suppressive microRNAs in hepatocellular carcinoma. Carcinogenesis. 2010;31:766-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 462] [Cited by in RCA: 464] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 24. | Sudo T, Utsunomiya T, Mimori K, Nagahara H, Ogawa K, Inoue H, Wakiyama S, Fujita H, Shirouzu K, Mori M. Clinicopathological significance of EZH2 mRNA expression in patients with hepatocellular carcinoma. Br J Cancer. 2005;92:1754-1758. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 170] [Cited by in RCA: 188] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 25. | Zheng F, Liao YJ, Cai MY, Liu YH, Liu TH, Chen SP, Bian XW, Guan XY, Lin MC, Zeng YX. The putative tumour suppressor microRNA-124 modulates hepatocellular carcinoma cell aggressiveness by repressing ROCK2 and EZH2. Gut. 2012;61:278-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 321] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 26. | Wang H, Pan K, Zhang HK, Weng DS, Zhou J, Li JJ, Huang W, Song HF, Chen MS, Xia JC. Increased polycomb-group oncogene Bmi-1 expression correlates with poor prognosis in hepatocellular carcinoma. J Cancer Res Clin Oncol. 2008;134:535-541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 82] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 27. | Chiba T, Miyagi S, Saraya A, Aoki R, Seki A, Morita Y, Yonemitsu Y, Yokosuka O, Taniguchi H, Nakauchi H. The polycomb gene product BMI1 contributes to the maintenance of tumor-initiating side population cells in hepatocellular carcinoma. Cancer Res. 2008;68:7742-7749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 758] [Reference Citation Analysis (0)] |
| 28. | Ladeiro Y, Couchy G, Balabaud C, Bioulac-Sage P, Pelletier L, Rebouissou S, Zucman-Rossi J. MicroRNA profiling in hepatocellular tumors is associated with clinical features and oncogene/tumor suppressor gene mutations. Hepatology. 2008;47:1955-1963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 527] [Cited by in RCA: 545] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 29. | Su H, Yang JR, Xu T, Huang J, Xu L, Yuan Y, Zhuang SM. MicroRNA-101, down-regulated in hepatocellular carcinoma, promotes apoptosis and suppresses tumorigenicity. Cancer Res. 2009;69:1135-1142. [PubMed] |
| 30. | Lessard J, Sauvageau G. Bmi-1 determines the proliferative capacity of normal and leukaemic stem cells. Nature. 2003;423:255-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1131] [Cited by in RCA: 1115] [Article Influence: 50.7] [Reference Citation Analysis (0)] |
| 31. | Lee TK, Cheung VC, Ng IO. Liver tumor-initiating cells as a therapeutic target for hepatocellular carcinoma. Cancer Lett. 2013;338:101-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 32. | Wei W, Wanjun L, Hui S, Dongyue C, Xinjun Y, Jisheng Z. miR-203 inhibits proliferation of HCC cells by targeting survivin. Cell Biochem Funct. 2013;31:82-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 33. | Karakatsanis A, Papaconstantinou I, Gazouli M, Lyberopoulou A, Polymeneas G, Voros D. Expression of microRNAs, miR-21, miR-31, miR-122, miR-145, miR-146a, miR-200c, miR-221, miR-222, and miR-223 in patients with hepatocellular carcinoma or intrahepatic cholangiocarcinoma and its prognostic significance. Mol Carcinog. 2013;52:297-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 277] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 34. | Jin J, Deng J, Wang F, Xia X, Qiu T, Lu W, Li X, Zhang H, Gu X, Liu Y. The expression and function of microRNA-203 in lung cancer. Tumour Biol. 2013;34:349-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 35. | Varambally S, Cao Q, Mani RS, Shankar S, Wang X, Ateeq B, Laxman B, Cao X, Jing X, Ramnarayanan K. Genomic loss of microRNA-101 leads to overexpression of histone methyltransferase EZH2 in cancer. Science. 2008;322:1695-1699. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 853] [Cited by in RCA: 832] [Article Influence: 48.9] [Reference Citation Analysis (0)] |