CASE REPORT
A 47-year-old Chinese man with severe leukocytopenia and thrombocytopenia due to hypersplenism secondary to liver cirrhosis was admitted to our hospital (180th Hospital of the People’s Liberation Army, Quanzhou, China). Physical examination revealed palmar erythema, mild anemia, and moderate splenomegaly.
He had a history of severe upper gastrointestinal bleeding once three years ago. The patient was diagnosed with hepatitis B-related decompensated liver cirrhosis, portal hypertension, hemorrhage from ruptured esophageal varices, and splenomegaly with hypersplenism. The vasopressin analog, terlipressin, was administered to reduce the portal venous pressure, and the bleeding stopped. Abdominal computed tomography (CT) scan showed liver cirrhosis with portal hypertension and splenomegaly. Gastroscopy revealed the esophageal and gastric varices. Routine blood test (RBT) results were: white blood cells (WBC), 1.75 × 109 cells/L; granulocytes (G), 1.18 × 109 cells/L; red blood cells (RBC), 2.67 × 1012 cells/L; hemoglobin (HGB), 73 g/L; platelets (PLT), 32 × 109/L. Bone marrow biopsy results showed hyperplasia of bone marrow with nucleated cells, and a slight maturation disorder in granulocytes. The reticulocyte count (RC) was 3.5%. His model for end-stage liver disease (MELD) assessment score was 19.52. As there was no evidence of a toxic cause, or of an underlying hematologic disease, the most likely diagnosis was hypersplenism secondary to cirrhosis. Because the MELD assessment score was nearly 20, LSA was selected instead of transjugular intrahepatic portosystemic shunt (TIPS) treatment to avoid possible hepatic decompensation. Prior to the LSA treatment, celiac computed tomography angiogram (CTA) was performed to confirm patency of the collateral arteries. The patient underwent laparoscopic LSA and ligation of the gastric coronary vein at the end of December, 2010. Follow-up over three years showed that the WBC and PLT counts did not increase indicating that the hypersplenism had not significantly improved.
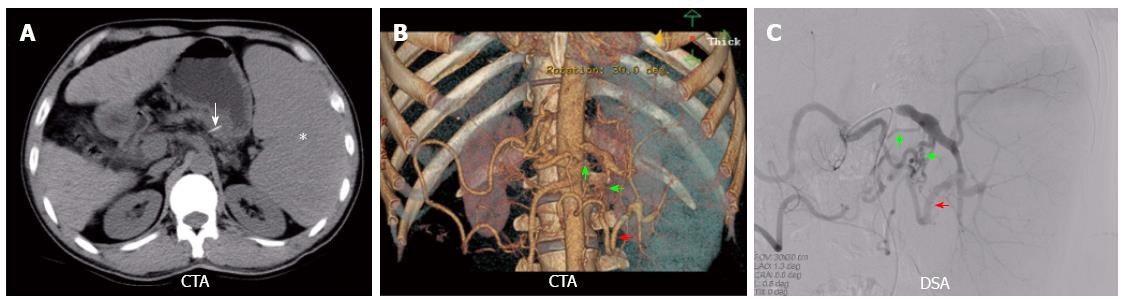
Over the following 3 years the patient remained in stable clinical condition but required a few admissions to the hospital, mainly for respiratory infections. However, RBT revealed recurrent severe leukocytopenia and thrombocytopenia. He was admitted to our hospital for further treatment. On admission, the RBT results: WBC, 1.65 × 109 cells/L; G, 1.10 × 109 cells/L; RBC, 3.67 × 1012 cells/L; HGB, 109 g/L; PLT, 30 × 109/L, PLT size was normal. The RC was 3.3%. Bone marrow biopsy results again showed hyperplasia, a slight maturation disorder in granulocytes with a slightly increased proportion of phagocytes. Serum test results were: albumin, 32.1 g/L; total bilirubin, 33.8 µmol/L; alanine aminotransferase, 43 IU/L; aspartate aminotransferase, 56 IU/L. Clotting test results were: international normalized ratio, 1.23; prothrombin time, 16.5 s; prothrombin activity, 50.3%. Abdominal ultrasound examination revealed an enlarged spleen (175 mm × 63 mm) and liver cirrhosis. The liver was shrunken, and the portal diameter was 14.5 mm with hepatic inflow at a velocity of 181 mm/s. An abdominal CT scan showed liver cirrhosis with portal hypertension and splenomegaly. Ischemic necrosis was not seen, but the bio-clamp (Figure 1A) was seen in the splenic artery area. Celiac CTA (Figure 1B) showed that the proximal splenic artery was not enlarged, but the gastroduodenal artery and the right gastro-epiploic artery were enlarged, and the right gastro-epiploic artery and the left gastro-epiploic artery were anastomosed. There were two compensatory arteries supplying the spleen. The collateral arteries were confirmed as connected to the hilar splenic artery from the left gastro-epiploic artery and from the dorsal pancreatic artery on a celiac arteriogram. The Child-Pugh score was 8 (B-class), and the MELD assessment score was 18.53. He was diagnosed with liver cirrhosis, portal hypertension, and splenomegaly with hypersplenism.
Figure 1 Imaging studies.
A: After ligation of splenic artery treatment, abdominal computed tomography showed that the size of the spleen was enlarged (white star). Ischemic necrosis was not seen in the spleen. However, the bio-clamp (white arrow) was seen in the splenic artery area; B and C: Splenic compensatory arteries: the dorsal pancreatic artery (green arrow), and the left gastro-epiploic artery (red arrow). CTA: Computed tomography angiogram; DSA: Digital subtraction angiography.
Because of the high risk of complications with splenectomy, PSE was performed through the compensatory arteries. Prior to embolization, selective angiography of the celiac trunk, the splenic artery and the superior mesenteric artery were performed in the patient through the right femoral artery using a 5 Fr diagnostic catheter. Digital subtraction angiography (Figure 1C) showed similar findings in the splenic artery and collateral circulation arteries as seen previously on CTA. PSE was performed by cannulation of the hilar splenic artery through the compensatory arteries with a Radifocus SP microcatheter (Terumo Corporation; Tokyo, Japan) (Figure 2A and B). Sixty-five percent of the spleen was embolized with fine particles of gelatin sponge. Preoperative antibiotic prophylaxis was administered 1 d prior to the procedure. Following embolization, he was monitored clinically, and antibiotics were administered after the procedure for 7 d. The patient complained of post-embolization syndrome including fever, left upper abdominal pain, nausea, vomiting, ascites, and left pleural effusion. These were effectively relieved by anti-inflammatory, antipyretic, analgesic and diuretic medications. After 2 wk, the symptoms resolved.
Figure 2 Partial splenic embolization therapy.
A: Partial splenic embolization (PSE) therapy through the collateral artery of dorsal pancreatic artery; B: PSE therapy through the left gastro-epiploic artery; C: Abdominal computed tomography (CT) revealed that ischemic infarction (black arrow) was seen in the spleen 2 wk after PSE; D-F: Follow-up abdominal CT was performed after 4 mo (D) and 9 mo (E and F), showing that the size of the spleen and infarction (black arrows) progressively decreased.
At 2 wk post-operation, an abdominal CT (Figure 2C) scan showed a shadow pattern of low density in the spleen, suggesting splenic ischemic infarction. The blood results were: WBC, 7.54 × 109 cells/L; G, 6.05 × 109 cells/L; RBC, 3.31 × 1012 cells/L; HGB, 100 g/L; and PLT, 231 × 109/L. Follow-up abdominal CT scan was performed 4 mo (Figure 2D) and 9 mo (Figure 2E and F) after the PSE, and showed that the size of both the spleen and the infarction progressively decreased. Both WBC and PLT counts increased significantly, peaked at 2 wk, then gradually fell during one year follow-up period, and remained within the normal range. There were no significant changes in RBC counts either after PSE or during the follow-up period. Follow-up at one year by abdominal ultrasound examination revealed that the portal diameter was 13.5 mm with a hepatic inflow velocity of 203 mm/s. The patient and the size of spleen remained stable.
DISCUSSION
Liver cirrhosis with hypersplenism can result in severe thrombocytopenia and/or leukocytopenia. The management of hypersplenism includes several possible approaches such as splenectomy, PSE, total splenic artery embolization (TSAE), LSA, etc. Splenectomy can cause a sustained and long-term increase in WBC and PLT counts. However, severe complications after splenectomy have been reported and range from 9.6%-26.6%[2,3,12]. In addition, splenectomy is often associated with an increased long-term risk of septic events[3,12]. PSE was first performed in the treatment of hypersplenism by Spigos et al[13] in 1979, and has gained increasing popularity as an alternative to splenectomy[7,14]. The net result of PSE is a partial splenectomy which allows for retention of splenic immune function[15]. PSE is accomplished by segmental and subsegmental arterial embolization of the spleen, and can be controlled to result in partial to complete splenic ischemic infarction[16]. The curative effect of PSE is long-term, and there are few complications, and low mortality. In PSE, the splenic infarction rate is a critical factor for the improvement of thrombocytopenia. To ensure a sustained and long-term increase in PLT and WBC counts, the extent of splenic infarction needs to be greater than 50%[6]. However, severe postoperative complications occurred more frequently in these patients[17]. In addition, quantitative control of the splenic infarction is difficult in this procedure, and is dependent on the experience of the operators. The reduction in portal hypertension reduces the risk of hemorrhage of the upper digestive tract. In addition, PSE has been suggested to be an effective method for the treatment of bleeding from gastric varices and portal hypertension[18]. TSAE has been shown to be a safe and effective method for the treatment of splenic artery aneurysms[19,20], and hypersplenism[21]. LSA is also used to treat hypersplenism, and leads to increased PLT and WBC counts in the short term[22], but there have been problems after long-term follow up. The effect of total splenic artery ligation or embolization is limited once the splenic collateral circulation is established[23], resulting in a recurrence of hypersplenism. Because the spleen has an abundant collateral circulation network, collateral circulation is frequently established within a short period of time after ligation. The main splenic collateral arteries are the left gastric artery, short gastric artery, dorsal pancreatic artery and the left gastro-epiploic artery[24,25]. Therefore, TSAE and LSA do not usually cause complete ischemic necrosis of the spleen.
PSE, LSA and TSAE are reasonably safe and effective procedure for controlling hypersplenism in patients with cirrhosis. However, hypersplenism is not significantly improved following the treatment in some cases, and there are very limited reports of retreatment required to control hypersplenism after LSA. The retreatment of hypersplenism includes several possible approaches, including splenectomy, ablation[24-26], and TIPS[27,28]. Despite of technological advances, splenectomy is still the most commonly used treatment modality. In this case, splenectomy might be an alternative treatment modality when LSA was failed. However, splenectomy is associated with high rates of morbidity and mortality. In the current case, the patient was not able to receive such treatment due to severe thrombocytopenia and leukocytopenia. These conditions presented increased risks of life-threatening hemorrhage and infection for the patient. Celiac CTA revealed two compensatory arteries supplying the spleen. Therefore, PSE was selected for our patient to treat the severe thrombocytopenia and leukocytopenia. The complications observed in the immediate post-operative period, post-embolization syndrome, included fever, left upper abdominal pain, nausea, vomiting, malaise, gastrointestinal symptoms, ascites, and left pleural effusion[29,30]. This is a self-limiting and benign adverse event. The most frequent side effect of post-embolization syndrome consists of associated fever and/or abdominal pain which have been reported to occur in 55%-100% of patients[4,31] and can last up to 40 d[4]. Serious complications of PSE such as splenic abscess and septicemia, are very rare[4,31]. Our patient developed moderate fever and left upper abdominal pain which lasted for 15 d. Potentially lethal postoperative complications of venous thrombosis, septicemia and splenic abscess were not observed in the current case. As a result, the patient achieved successful treatment of hypersplenism. Moreover, the portal venous diameter was decreased, and blood flow velocity was increased after PSE, which is also consistent with previous studies that reported a reduction of portal venous pressure and portal venous blood flow after PSE[18,32]. Because this is only a case report with a short follow-up period, we plan to increase the number of cases, and the follow-up period in future studies.
In conclusion, PSE can be an effective alternative for the management of hypersplenism in patients with cirrhosis, and is an effective therapeutic modality for the retreatment of hypersplenism when other modalities have failed.
COMMENTS
Case characteristics
A 47-year-old man with severe leukocytopenia and thrombocytopenia due to hypersplenism secondary to liver cirrhosis.
Clinical diagnosis
Palmar erythema, mild anemic and moderate splenomegaly.
Differential diagnosis
Aplastic anemia, myelodysplastic syndrome, low proliferative leukemia, thrombocytopenia.
Laboratory diagnosis
White blood cells: 1.65 × 109 cells/L; granulocytes: 1.10 × 109 cells/L; red blood cells: 3.67 × 1012 cells/L; hemoglobin: 109 g/L; platelets: 30 × 109/L; reticulocyte count: 3.3%; international normalized ratio: 1.23; prothrombin time: 16.5 s; prothrombin activity: 50.3%; Child-Pugh score: 8 and model for end-stage liver disease score: 18.53. Bone marrow biopsy results showed hyperplasia, a slight maturation disorder in granulocytes with a slightly increased proportion of phagocytes.
Imaging diagnosis
Abdominal computed tomography scan showed liver cirrhosis with portal hypertension and splenomegaly. Celiac computed tomography angiogram/digital subtraction angiography showed that there were two compensatory arteries supplying the spleen. The collateral arteries were confirmed as connected to the hilar splenic artery from the left gastro-epiploic artery and from the dorsal pancreatic artery on a celiac arteriogram.
Treatment
Partial splenic embolization (PSE) was performed through the compensatory arteries of the spleen.
Related reports
Very few cases about PSE for the treatment of hypersplenism after unsuccessful splenic artery ligation have been reported in the literature.
Term explanation
Both ligation of splenic artery (LSA) and PSE are minimally invasive surgical techniques. Special catheters, guide wires, and precision instruments are introduced with the guidance of medical imaging equipment to obtain a histological diagnosis and achieve local treatment.
Experiences and lessons
This case report not only describes a very rare presentation of hypersplenism after unsuccessful LSA, but also describes the successful retreatment of hypersplenism. PSE was performed through the compensatory arteries resulting in increased leukocyte and thrombocyte counts that remained within the normal range.
Peer review
This article reports successful management of hypersplenism after unsuccessful splenic artery ligation. PSE is an effective therapeutic modality for the retreatment of hypersplenism when other modalities have failed.