Published online Jan 28, 2015. doi: 10.3748/wjg.v21.i4.1349
Peer-review started: May 26, 2014
First decision: July 9, 2014
Revised: July 18, 2014
Accepted: September 12, 2014
Article in press: September 16, 2014
Published online: January 28, 2015
Processing time: 249 Days and 3.7 Hours
A 4-mo history of both epigastralgia and back pain was presented in a 39-year-old male. Computed tomography showed right lung nodule and abdominal mass attached to the gastric wall, measuring approximately 30 mm and 70 mm in diameter. Since biopsy samples from the lung and abdomen revealed poorly differentiated adenocarcinoma and malignant tumor, clinicians first interpreted the abdominal mass as metastatic carcinoma, and a right lower lobectomy with following resection of the mass was performed. Gross examination of both lesions displayed gray-whitish to yellow-whitish cut surfaces with hemorrhagic and necrotic foci, and the mass attached to the serosa of the lesser curvature on the gastric body. On microscopic examination, the lung tumor was composed of a proliferation of highly atypical epithelial cells having abundant eosinophilic cytoplasm, predominantly arranged in an acinar or solid growth pattern with vessel permeation, while the abdominal tumor consisted of sheets or nests with markedly atypical epithelioid cells having pleomorphic nuclei and abundant eosinophilic to clear cytoplasm focally in a radial perivascular or infiltrative growth pattern. Immunohistochemically, the latter cells were positive for HMB45 or α-smooth muscle actin, but the former ones not. Therefore, we finally made a diagnosis of malignant perivascular epithelioid cell tumor (PEComa) arising in the gastric serosa, combined with primary lung adenocarcinoma. Furthermore, small papillary carcinoma of the thyroid gland was identified. The current case describes the coincidence of malignant PEComa with other carcinomas, posing a challenge in distinction from metastatic tumor disease.
Core tip: We reported the first single-case of malignant perivascular epithelioid cell tumor (PEComa) arising in the gastric serosa, combined with primary lung adenocarcinoma of poorly differentiated type. It is likely that the present malignant PEComa might pose a challenge in distinction from metastatic lung carcinoma on the examination of the small inadequate biopsy specimen. Pathologists should be aware that its characteristic features could lead to a misdiagnosis especially in this case. Furthermore, we suggest that a large panel of antibodies including various melanocytic, muscle or epithelial markers in immunohistochemistry should be useful and critical aids for reaching the correct diagnosis of malignant PEComa.
- Citation: Yamada S, Nabeshima A, Noguchi H, Nawata A, Nishii H, Guo X, Wang KY, Hisaoka M, Nakayama T. Coincidence between malignant perivascular epithelioid cell tumor arising in the gastric serosa and lung adenocarcinoma. World J Gastroenterol 2015; 21(4): 1349-1356
- URL: https://www.wjgnet.com/1007-9327/full/v21/i4/1349.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i4.1349
Perivascular epithelioid cell (PEC) was first introduced by Pea et al[1] and Bonetti et al[2] in the early 1990s, in order to present the concept of a family of tumor, i.e., perivascular epithelioid cell tumor (PEComa), characterized by a proliferation of peculiar muscle cells having a specific expression of melanoma-associated antigens, such as HMB45[1,2]. In 1996, Zamboni et al[3] subsequently described the term PEComa to introduce this rare family of mesenchymal tumors containing characteristic epithelioid cells with a close association with blood vessels. PEComa family tumors include angiomyolipoma of the kidney and liver, pulmonary lymphangioleiomyomatosis, clear cell “sugar” tumor (CCST) of the lung, extrapulmonary CCST, clear cell myo melanocytic tumor of the falciform ligament/ligamentum teres, and abdominopelvic sarcoma of PECs[1-4]. In fact, the World Health Organization have already accepted the designation of PEComa as a distinct mesenchymal neoplasm predominantly composed of histopathologically unique PECs since 2002[5]. PEComas have been reported in various organs, such as the uterus and adnexa, pancreas, small and large intestine, mesentery, breast, skull base, soft tissue and so on[3-15]. Until now, the case number reported as PEComas of the digestive tract in the English literatures is small, less than 50, within our thorough investigation, as previously described in stomach, jejunum, ileum, cecum, descending colon, and rectum[5,9-11,16,17]. The most common site of involvement with gastrointestinal PEComas is the colon, followed by the small intestine, as more recently reported[17]. Although PEComas show a wide spectrum of biological behavior, classified into “benign”, of uncertain “malignant potential”, and “malignant” categories[4,5], the histopathological criteria for the diagnosis of malignant PEComa have not been clearly established to date, due to its rarity in part. Indeed, there have been 6 histopathological features suggestive of high risk factors of malignancy: (1) tumor size > 5 cm or 8 cm; (2) infiltrative growth pattern; (3) high nuclear grade and hypercellularity; (4) a high rate of mitosis, more than 1 per 50 high-power fields; (5) coagulative necrosis; and (6) vascular invasion[4,5,11,12], even though ”true“ malignant PEComas are extremely rare and its histogenesis and cytogenesis remain to be elucidated. Large PEComas (> 5 cm) without any above features have uncertain malignant potential, whereas any PEComas with the 2 or more high-risk features might be considered as malignant[4,5,11,12]. In contrast, ”benign“ PEComas lacking all these features only rarely metastasize[5]. Nevertheless, those above criteria have not yet been validated in larger series. However, it would be critical to establish an accurate initial diagnosis, including “benign”, “of uncertain malignant potential”, or “malignant” PEComas, even by small biopsy specimens.
We report an extremely rare case of malignant PEComa arising in the gastric serosa combined with primary lung adenocarcinoma of poorly differentiated type and thyroid papillary carcinoma, likely confused with metastatic carcinoma in the gastric wall, based on an inadequate volume of biopsy sample.
The patient was a 39-year-old middle-aged Japanese male. The surgical tumor specimens after fixation in 10% neutral buffered formalin were embedded in paraffin for histological or immunohistochemical examinations. All immunohistochemical stainings were carried out using Dako Envision kit (Dako, Glostrup, Denmark) according to the manufacturer’s instructions, and using commercially available pre-diluted monoclonal or polyclonal antibodies against the following antigens: cytokeratins (Cam5.2; Becton Dickinson Immunocytometry Systems, San Jose, CA, diluted 1:1, and AE1/AE3; Dako, diluted 1:5000), epithelial membrane antigen (EMA; Dako, diluted 1:100), thyroid transcription factor 1 (TTF-1; Dako, diluted 1:100), Napsin A (Nichirei Bioscience, Tokyo, Japan, diluted 1:1), CD10 (NOVOCASTRA laboratories Ltd., Newcastle, United Kingdom, diluted 1:20), CD34 (Immuno Tech. Co., Ltd., Osaka, Japan, diluted 1:150), CD45 (Dako, diluted 1:400), CD56 (NICHIREI, Tokyo, Japan, diluted 1:1), CD68 (KP-1; Dako, diluted 1:100), CD117 (c-Kit; IBL, Gunma, Japan, diluted 1:15), synaptophysin (Dako, diluted 1:20), chromogranin A (Dako, diluted 1:200), S-100 protein (Dako, diluted 1:900), HMB45 (Enzo Life Sciences Ltd., New York, diluted 1:100), Melan A (NOVOCASTRA, 1:50), microphthalmia transcription factor (MiTF; NOVOCASTRA, diluted 1:10), TFE3 (Santa Cruz Biotechnology, Santa Cruz, CA, United States, 1:600), α-smooth muscle actin (α-SMA; Dako, diluted 1:150), pan-muscle actin (HHF-35; Enzo, New York, United States, diluted 1:20), desmin (Dako, diluted 1:300), h-caldesmon (Dako, diluted 1:50), and Ki-67 (MIB-1; Dako, diluted 1:50). However, no chromosome studies have been performed.
The patient was admitted to hospital due to a 4-mo history of both epigastralgia and back pain. The patient had neither signs of tuberous sclerosis complex nor any family history of it. He was a non-smoker. There was no history of malignancy, immunosuppressive disorders, use of immunosuppressive medications, or unusual infections.
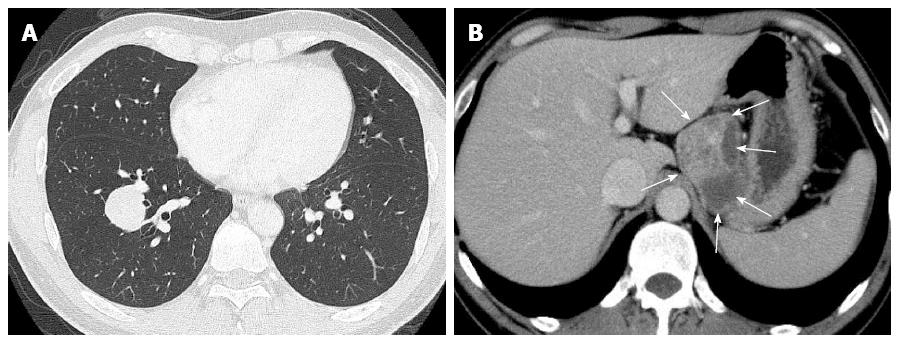
Laboratory data, including blood cell count, chemistry and tumor markers, were almost within normal limits, except for slightly high levels of carcinoembryonic antigen (3.7 ng/mL). A chest computed tomography (CT) scan revealed a relatively well-demarcated nodule, measuring approximately 30 mm × 30 mm, in the right lower lobe, S9 (Figure 1A). Moreover, an abdominal CT scan showed a relatively well-defined huge mass with heterogeneously enhancement, measuring approximately 70 mm × 60 mm, attached to the gastric wall and separated from the left kidney and adrenal gland (Figure 1B). Besides, a view of neck ultrasound revealed a well-demarcated nodule, measuring approximately 9 mm, in the right lobe of the thyroid gland. CT scans of the head, chest and abdomen disclosed no definite evidence of neoplastic foci or other metastases in the lymph nodes or other organs, including the bilateral kidney or adrenal gland. The patient had neither recurrence nor metastases of malignant PEComa, lung carcinoma, and thyroid carcinoma, respectively, and was alive and well at 6 mo after the operation.
The first bronchial brushing and washing cytology specimens were predominantly consisted of clusters of cohesive and three-dimensional tumor cells having large hyperchromatic nuclei and prominent nucleoli with necrotic backgrounds. Based on that, we first interpreted it as poorly differentiated adenocarcinoma, confirmed by following transbronchial lung biopsy from the pulmonary nodule. On the other hand, the percutaneous biopsy specimen from the abdominal mass showed extensively necrotic and hemorrhagic tissue, admixed with quite tiny fragments of tumor lesion, composed of a solid proliferation of highly atypical cells having hyperchromatic pleomorphic nuclei and abundant eosinophilic to clear cytoplasm (data not shown). Clinicians first interpreted the gastric serosal mass as metastatic carcinoma of the lung carcinoma, but we pathologists tentatively made a diagnosis of malignant tumor. Therefore, the surgeons performed an ordinary right lower lobectomy with following laparoscopic combined resection of the gastric serosal mass and one part of the gastric wall. Finally, the fine needle aspiration cytology from the thyroid small (less than 1 cm) tumor revealed papillary carcinoma, however, careful follow-up but not thyroidectomy was done.
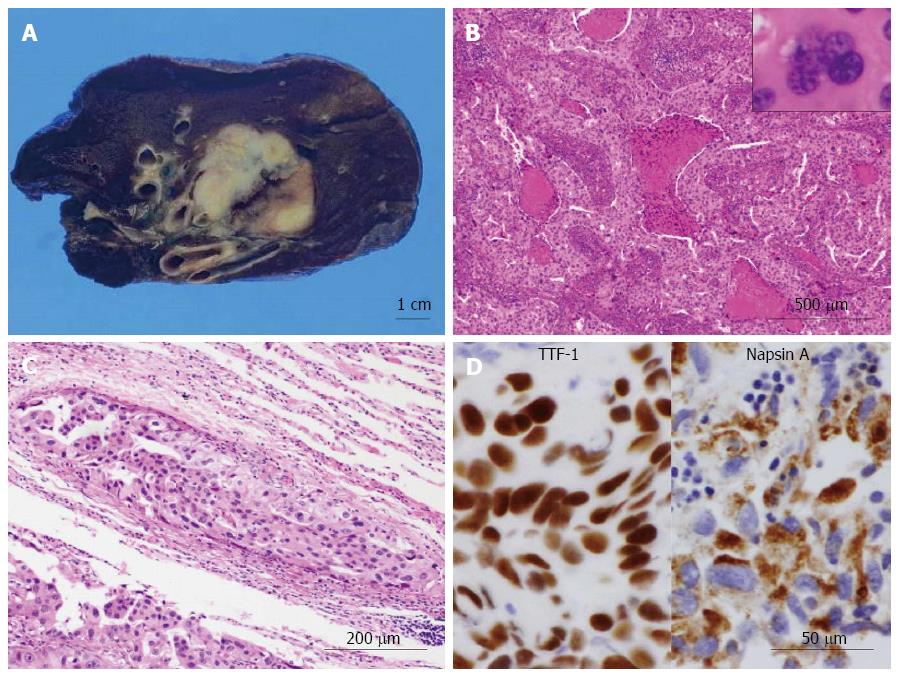
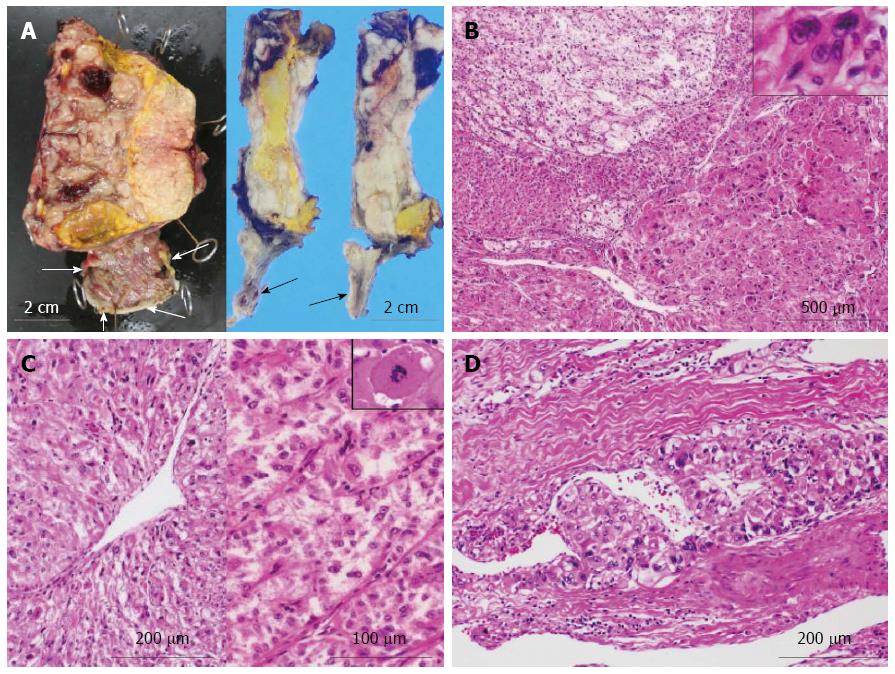
On gross examination, the cut surface of the lung tumor showed a solid firm and lobulated mass, measuring 32 mm × 30 mm × 29 mm, which looked from gray-whitish to yellow-whitish in color, accompanied with focal necrosis and hemorrhage (Figure 2A). The background of the lung had no remarkable change, e.g., not emphysematous (Figure 2A). Microscopic findings revealed a proliferation of medium-sized to large atypical epithelial cells having hyperchromatic pleomorphic nuclei and abundant eosinophilic cytoplasm, predominantly arranged in an acinar or solid fashion with frequent necrotic foci (Figure 3B), involving the adjacent bronchial wall with vessel permeation (Figure 2C). On high-power view, mitotic counts were high (more than 10 per 50 high-power fields) and multi-nucleated giant tumor cells were readily encountered (Figure 2B). Immunohistochemically, these adenocarcinoma cells were positive for TTF-1 and Napsin A (Figure 2D). Based on all these features, we indicated that these carcinoma cells were characteristic of glandular differentiation, and finally made a diagnosis of moderately to poorly differentiated adenocarcinoma of the lung, further classified as invasive adenocarcinoma, acinar predominant, based on the histological classification system from the International Association for the Study of Lung Cancer (IASLC)/American Thoracic Society/European Respiratory Society International Multidisciplinary Classification of Lung Adenocarcinoma[18]. Final pathological stage was determined as pT2aN0M0, stage IB, according to the IASLC classification[19].
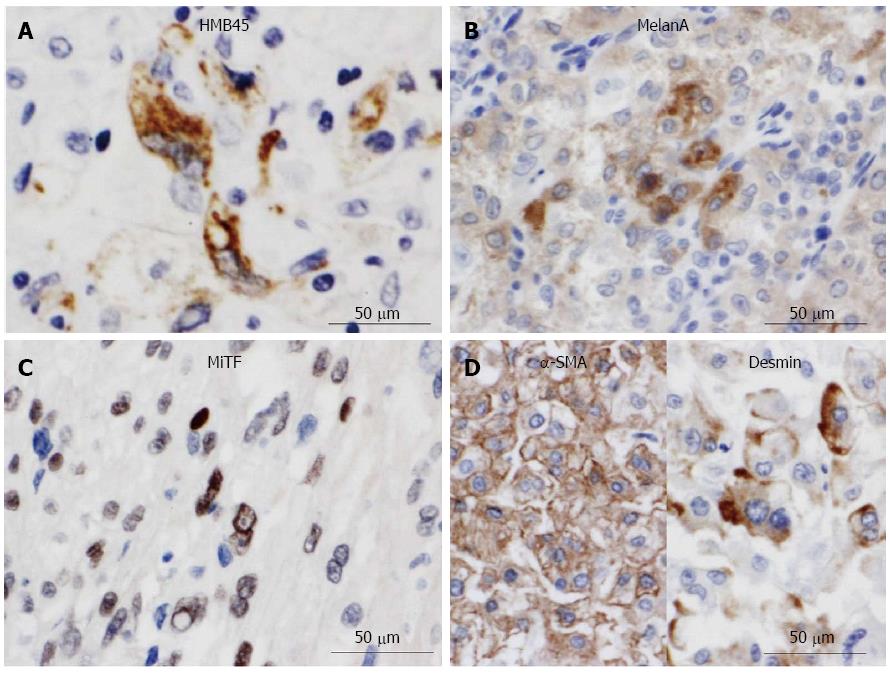
Next, gross examination of the surgical specimen from the abdominal mass showed that the huge tumor, measuring 73 mm × 65 mm × 61 mm, had gray-whitish to yellow-whitish cut surfaces with hemorrhagic and yellowish necrotic foci, attached to the serosa of the lesser curvature on the gastric body (Figure 3A) and separated from the left kidney and adrenal gland. Microscopically, the abdominal tumor consisted of sheets or nests of markedly atypical epithelioid cells having hyperchromatic pleomorphic nuclei and abundant eosinophilic to clear cytoplasm, admixed with a large number of multi-nucleated giant cells, supported by delicate fibrovascular septa (Figure 3B). Spindle cell-predominant components were very few. These tumor nests predominantly showed an alveolar or trabecular growth pattern with coagulative necrotic foci, occasionally and characteristically displaying a radial perivascular fashion (Figure 3C). On high-power view, the large tumor cells sometimes showed atypical mitosis with relatively high mitotic rates (more than 2 per 10 high-power fields) (Figure 3C). On the other hand, vascular permeation of the infiltrative tumor nests was partly noted in the peripheral areas (Figure 3D). In immunohistochemistry, these epithelioid cells were specifically positive for melanocytic markers, such as HMB45 (Figure 4A), Melan A (Figure 4B), and MiTF (Figure 4C), and muscle markers, such as α-SMA (Figure 4D), desmin (Figure 4D), HHF-35, and h-caldesmon, and focally positive for CD10, whereas negative for TTF-1, Napsin A, epithelial markers, including EMA, Cam 5.2, and AE1/AE3, neuroendocrine markers, such as CD56, chromogranin A, and synaptophysin, S-100 protein, TFE3, CD34, c-Kit, CD45, and CD68. Moreover, relatively higher MIB-1 labeling index, 3% to 5%, was found within the gastric serosal tumor cells. Based on all the clinicopathological features, we made a final diagnosis of malignant PEComa arising in the gastric serosa. All immunohistochemical profiles of malignant PEComa arising in the gastric serosa and primary lung adenocarcinoma are summarized in Table 1.
| Antibodies | Malignant PEComa | Lung adenocarcinoma |
| EMA | - | + |
| AE1/AE3 | - | + |
| Cam5.2 | - | + |
| TTF-1 | - | + |
| Napsin A | - | + |
| Synaptophysin | - | - |
| Chromogranin A | - | - |
| CD56 | - | - |
| NSE | - | - |
| S-100 protein | - | - |
| CD34 | - | - |
| c-Kit | - | - |
| TFE3 | - | - |
| α-SMA | + | - |
| h-caldesmon | + | - |
| HHF-35 | + | - |
| Desmin | + | - |
| MelanA | + | - |
| HMB-45 | + | - |
| MiTF | + | - |
| CD10 | + | - |
The most important clinical differential diagnosis in the present malignant PEComa case is with metastatic lung adenocarcinoma of poorly differentiated type. Immunohistochemical analyses can resolve the distinction from metastatic carcinoma very easily, since the PEComa cells were specifically positive for melanoma-associated antigens, representing as HMB45, and muscle markers, such as α-SMA and desmin, whereas completely negative for lung adenocarcinoma markers, TTF-1 and Napsin A, but in striking contrast, the lung carcinoma cells were not. However, the adenocarcinoma cells in our case microscopically shares with malignant PEComa not only a solid sheet-like growth pattern but markedly cellular atypia displaying hyperchromatic pleomorphic nuclei, abundant eosinophilic cytoplasm, and occasionally multi-nucleated giant cells, admixed with a number of mitotic figures. Thus, we pathologists should be aware that its features possibly make us misinterpret as a metastatic focus only on small or inadequate biopsy specimens. On the other hand, among malignant tumors, histopathologically differential diagnoses include epithelioid extra-gastrointestinal stromal tumor (extra-GIST), malignant melanoma, epithelioid leiomyosarcoma or metastatic clear cell renal cell carcinoma (RCC)[4-17]. Although malignant PEComa and above each neoplasm share variable histological features, immunohistochemical profiles (Table 1) of malignant PEComa also can readily distinguish from epithelioid extra-GIST, epithelioid leiomyosarcoma, and metastatic RCC mainly by positive staining for melanocytic markers and negative staining for c-Kit, CD34, and epithelial markers including cytokeratin, and differentiate from melanoma chiefly by negative staining for S-100 protein and positive staining for muscle markers, respectively[1-17].
It is very likely that the present case is clinicopathologically remarkable for two reasons at least: first, to the best of our knowledge, this is the first single-case report of malignant PEComa arising in the gastric serosa, and the fourth occurrence of gastric PEComa[4,5,9-11,16,17]. Actually, to date, the number of “true” cases reported as PEComa of the digestive tract in the English literatures is not large, and the most recent reference of single-case paper (in fact, gastric “benign” PEComa) is from 2010[16]. According to those previous papers, the criteria of “benign”, “of uncertain malignant potential” to “malignant” for PEComa have not been clearly established[4,5,11], and intriguingly, there has been no known normal counterpart of PEComa. Fu et al[12] have recently proposed that infiltrating appearance (e.g., vascular invasion) and extensive coagulative necrosis should be much more pivotal factors to be used for the evaluation of “malignant” PEComa, corresponding to our case, rather than hypercellularity or numerous mitotic figures. By contrast, more recently, the relatively larger series of PEComa especially arising in the gastrointestinal tract have revealed that, similar to us, the presence of marked nuclear atypia, diffuse pleomorphism, and more than 2 mitoses per 10 high-power fields have a significantly close relationship with the development of metastatic disease, manifesting as malignant PEComa[17]. Malignant PEComas are still extremely rare, and thus, it is interesting and critical to study this topic with regard to histopathological criteria of “malignancy” after further collecting and investigating a substantial number of surgical cases of PEComas in the future.
Second, this middle-aged (i.e., relatively young) male patient suffered from multifocal malignancy: (1) malignant PEComa arising in the gastric serosa; (2) primary lung adenocarcinoma of poorly differentiated type in the right lower lobe; and (3) thyroid papillary carcinoma of the right lobe. Within our thorough investigation, the present case is the first report of PEComa combined with multiple malignancy, as well. We might provide the possible evidence for the first time that one part of malignant PEComas have a predilection for multifocal growth fashion, including other malignant neoplasms, rather than metastasis, even though it is known that the most common metastatic sites of PEComas include the lungs, liver, lymph nodes, peritoneum, bone, brain, and adrenal gland[5,17]. According to the first series of gastrointestinal PEComas[17], of 13 patients who developed metastatic foci, 3 patients (23%) showed lung metastases. Nevertheless, future convincing data will be further required to determine whether our speculation is significant or not.
In summary, we reported an extremely rare case of malignant PEComa arising in the gastric serosa, combined with primary lung adenocarcinoma of poorly differentiated type and papillary carcinoma of the thyroid gland. It is likely that the current malignant PEComa might pose a challenge in distinction from metastatic lung carcinoma on the examination of the small biopsy specimen, since its section contained tiny foci of viable tumor epithelioid cells in the background of extensively necrosis and hemorrhage. All pathologists should be aware that its characteristic features could lead to a misdiagnosis especially in case of inadequate specimens. Furthermore, we suggest that a large panel of antibodies including various melanocytic, muscle or epithelial markers in immunohistochemistry should be useful and critical aids for reaching the correct diagnosis of malignant PEComa. PEComa arising in digestive tract may be more common than generally considered.
A 39-year-old male with a 4-mo history of both epigastralgia and back pain.
The patient had neither signs of tuberous sclerosis complex nor any family history of it. There was no history of malignancy, immunosuppressive disorders, use of immunosuppressive medications, or unusual infections.
Metastatic carcinoma of the gastric serosa from primary lung cancer.
Laboratory data, including blood cell count, chemistry and tumor markers, were almost within normal limits, except for slightly high levels of carcinoembryonic antigen (3.7 ng/mL).
A chest computed tomography (CT) scan revealed a relatively well-demarcated nodule, measuring approximately 30 mm × 30 mm, in the right lower lobe, S9. Moreover, an abdominal CT scan showed a relatively well-defined huge mass with heterogeneously enhancement, measuring approximately 70 mm × 60 mm, attached to the gastric wall and separated from the left kidney and adrenal gland.
The authors made a final diagnosis of malignant perivascular epithelioid cell tumor (PEComa) arising in the gastric serosa, combined with primary lung adenocarcinoma of poorly differentiated type.
The surgeons performed an ordinary right lower lobectomy with following laparoscopic combined resection of the gastric serosal mass and one part of the gastric wall.
Until now, the case number reported as PEComas of the digestive tract in the English literatures is small, less than 50, within our thorough investigation, as previously described in stomach, jejunum, ileum, cecum, descending colon, and rectum. The most common site of involvement with gastrointestinal PEComas is the colon, followed by the small intestine.
Perivascular epithelioid cell was first introduced by Pea et al in the early 1990s, in order to present the concept of a family of tumor, i.e., PEComa, characterized by a proliferation of peculiar muscle cells having a specific expression of melanoma-associated antigens, such as HMB45.
To the best of our knowledge, this is the first single-case report of malignant PEComa arising in the gastric serosa, and the fourth occurrence of gastric PEComa.
This article reports an extremely rare case of malignant PEComa arising in the gastric serosa combined with primary lung adenocarcinoma of poorly differentiated type and thyroid papillary carcinoma, likely confused with metastatic carcinoma in the gastric wall.
P- Reviewer: Jafari A, Scheppach W, Xiao EH S- Editor: Gou SX L- Editor: A E- Editor: Zhang DN
| 1. | Pea M, Bonetti F, Zamboni G, Martignoni G, Fiore-Donati L, Doglioni C. Clear cell tumor and angiomyolipoma. Am J Surg Pathol. 1991;15:199-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 87] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 2. | Bonetti F, Pea M, Martignoni G, Doglioni C, Zamboni G, Capelli P, Rimondi P, Andrion A. Clear cell (“sugar”) tumor of the lung is a lesion strictly related to angiomyolipoma--the concept of a family of lesions characterized by the presence of the perivascular epithelioid cells (PEC). Pathology. 1994;26:230-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 202] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 3. | Zamboni G, Pea M, Martignoni G, Zancanaro C, Faccioli G, Gilioli E, Pederzoli P, Bonetti F. Clear cell “sugar” tumor of the pancreas. A novel member of the family of lesions characterized by the presence of perivascular epithelioid cells. Am J Surg Pathol. 1996;20:722-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 272] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 4. | Folpe AL, Kwiatkowski DJ. Perivascular epithelioid cell neoplasms: pathology and pathogenesis. Hum Pathol. 2010;41:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 264] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 5. | Hornick AL, Pan CC. PEComa. World Health Organization Classification of Tumours of Soft tissue and Bone. Lyon, France: IARC Press 2013; 230-231. |
| 6. | Vang R, Kempson RL. Perivascular epithelioid cell tumor (‘PEComa’) of the uterus: a subset of HMB-45-positive epithelioid mesenchymal neoplasms with an uncertain relationship to pure smooth muscle tumors. Am J Surg Pathol. 2002;26:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 207] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 7. | Fink D, Marsden DE, Edwards L, Camaris C, Hacker NF. Malignant perivascular epithelioid cell tumor (PEComa) arising in the broad ligament. Int J Gynecol Cancer. 2004;14:1036-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Yamada Y, Yamamoto H, Ohishi Y, Nishiyama K, Fukuhara M, Saitou T, Tsuneyoshi M, Oda Y. Sclerosing variant of perivascular epithelioid cell tumor in the female genital organs. Pathol Int. 2011;61:768-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Yanai H, Matsuura H, Sonobe H, Shiozaki S, Kawabata K. Perivascular epithelioid cell tumor of the jejunum. Pathol Res Pract. 2003;199:47-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Evert M, Wardelmann E, Nestler G, Schulz HU, Roessner A, Röcken C. Abdominopelvic perivascular epithelioid cell sarcoma (malignant PEComa) mimicking gastrointestinal stromal tumour of the rectum. Histopathology. 2005;46:115-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Yamamoto H, Oda Y, Yao T, Oiwa T, Kobayashi C, Tamiya S, Kawaguchi K, Hino O, Tsuneyoshi M. Malignant perivascular epithelioid cell tumor of the colon: report of a case with molecular analysis. Pathol Int. 2006;56:46-50. [PubMed] |
| 12. | Fu X, Jiang JH, Gu X, Li Z. Malignant perivascular epithelioid cell tumor of mesentery with lymph node involvement: a case report and review of literature. Diagn Pathol. 2013;8:60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Govender D, Sabaratnam RM, Essa AS. Clear cell ‘sugar’ tumor of the breast: another extrapulmonary site and review of the literature. Am J Surg Pathol. 2002;26:670-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 57] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Lehman NL. Malignant PEComa of the skull base. Am J Surg Pathol. 2004;28:1230-1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Harris GC, McCulloch TA, Perks G, Fisher C. Malignant perivascular epithelioid cell tumour (“PEComa”) of soft tissue: a unique case. Am J Surg Pathol. 2004;28:1655-1658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 44] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Mitteldorf CA, Birolini D, da Camara-Lopes LH. A perivascular epithelioid cell tumor of the stomach: an unsuspected diagnosis. World J Gastroenterol. 2010;16:522-525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Doyle LA, Wang WL, Dal Cin P, Lopez-Terrada D, Mertens F, Lazar AJ, Fletcher CD, Hornick JL. MUC4 is a sensitive and extremely useful marker for sclerosing epithelioid fibrosarcoma: association with FUS gene rearrangement. Am J Surg Pathol. 2012;36:1444-1451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 175] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 18. | Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger KR, Yatabe Y, Beer DG, Powell CA, Riely GJ, Van Schil PE. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6:244-285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3651] [Cited by in RCA: 3627] [Article Influence: 259.1] [Reference Citation Analysis (0)] |
| 19. | Groome PA, Bolejack V, Crowley JJ, Kennedy C, Krasnik M, Sobin LH, Goldstraw P. The IASLC Lung Cancer Staging Project: validation of the proposals for revision of the T, N, and M descriptors and consequent stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol. 2007;2:694-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 463] [Article Influence: 25.7] [Reference Citation Analysis (1)] |