Published online Jan 28, 2015. doi: 10.3748/wjg.v21.i4.1334
Peer-review started: June 22, 2014
First decision: July 9, 2014
Revised: July 29, 2014
Accepted: September 5, 2014
Article in press: September 5, 2014
Published online: January 28, 2015
Processing time: 220 Days and 21.5 Hours
Immunoglobulin G4-related sclerosing cholangitis (IgG4-SC) is frequently associated with type 1 autoimmune pancreatitis (AIP). Association with AIP can be utilized in the diagnosis of IgG4-SC. However, some cases of IgG4-SC are isolated from AIP, which complicates the diagnosis. Most of the reported cases of isolated IgG4-SC displayed hilar biliary strictures, whereas isolated IgG4-SC with intrapancreatic biliary stricture is very rare. Recently, we have encountered 5 isolated intrapancreatic IgG4-SC cases that were not associated with AIP, three of which were pathologically investigated after surgical operation. They all were males with a mean age of 74.2 years. The pancreas was not enlarged in any of these cases. No irregular narrowing of the main pancreatic duct was found. Bile duct wall thickening in lesions without luminal stenosis was detected by abdominal computed tomography in all five cases, by endoscopic ultrasonography in two out of four cases and by intraductal ultrasonography in all three cases. In three cases, serum IgG4 levels were within the normal limits. The mean serum IgG4 level measured before surgery was 202.1 mg/dL (4 cases). Isolated intrapancreatic IgG4-SC is difficult to diagnose, especially if the IgG4 level remains normal. Thus, this type of IgG4-SC should be suspected in addition to cholangiocarcinoma and pancreatic cancer if stenosis of intrapancreatic bile duct is present.
Core tip: If stenosis of intrapancreatic bile duct is present and no abnormal findings of pancreas are detected, cholangiocarcinoma is suspected. Recently, we have encountered 5 isolated intrapancreatic immunoglobulin G4-related sclerosing cholangitis (IgG4-SC) cases that were not associated with autoimmune pancreatitis, three of which were pathologically investigated after surgical operation. Isolated intrapancreatic IgG4-SC is difficult to diagnose, especially if the IgG4 level remains normal. Thus, this type of IgG4-SC should be suspected in addition to cholangiocarcinoma and pancreatic cancer if stenosis of intrapancreatic bile duct is present.
- Citation: Nakazawa T, Ikeda Y, Kawaguchi Y, Kitagawa H, Takada H, Takeda Y, Makino I, Makino N, Naitoh I, Tanaka A. Isolated intrapancreatic IgG4-related sclerosing cholangitis. World J Gastroenterol 2015; 21(4): 1334-1343
- URL: https://www.wjgnet.com/1007-9327/full/v21/i4/1334.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i4.1334
Immunoglobulin G4-related sclerosing cholangitis (IgG4-SC) is frequently associated with type 1 autoimmune pancreatitis (AIP). Association with AIP can be utilized in the diagnosis of IgG4-SC[1]. However, some cases of IgG4-SC are isolated from AIP, which complicates the diagnosis[2,3].
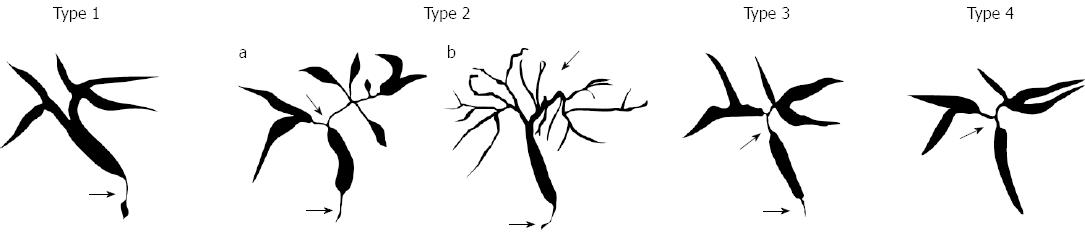
IgG4-SC displays various cholangiographic features similar to those of pancreatic cancer, primary sclerosing cholangitis (PSC), and cholangiocarcinoma (CC). The characteristic features of IgG4-SC can be classified into 4 types based on the stricture regions revealed by cholangiography and differential diagnosis (Figure 1)[4].
Most of the reported cases of isolated IgG4-SC displayed hilar biliary strictures, whereas isolated IgG4-SC with intrapancreatic biliary stricture is very rare[2,3]. Recently, we have encountered 5 isolated intrapancreatic IgG4-SC cases that were not associated with AIP, three of which were pathologically investigated after surgical operation. In the present study, we examined this series to clarify the clinical profiles of isolated intrapancreatic IgG4-SC.
We report 5 cases of isolated type1 IgG4-SC. One case was retrieved from 87 IgG4-SC case records in Nagoya City University. Two cases were presented during the 49th annual meeting of Japan Biliary Association. The other two cases were retrieved from the nationwide survey for PSC and IgG4-SC conducted in Japan. Informed consents were obtained from all the patients.
Below, we will first describe the details of these five cases. We will then characterize them in terms of serum IgG4 levels, pancreatic features, bile duct features, diagnosis, and treatment.
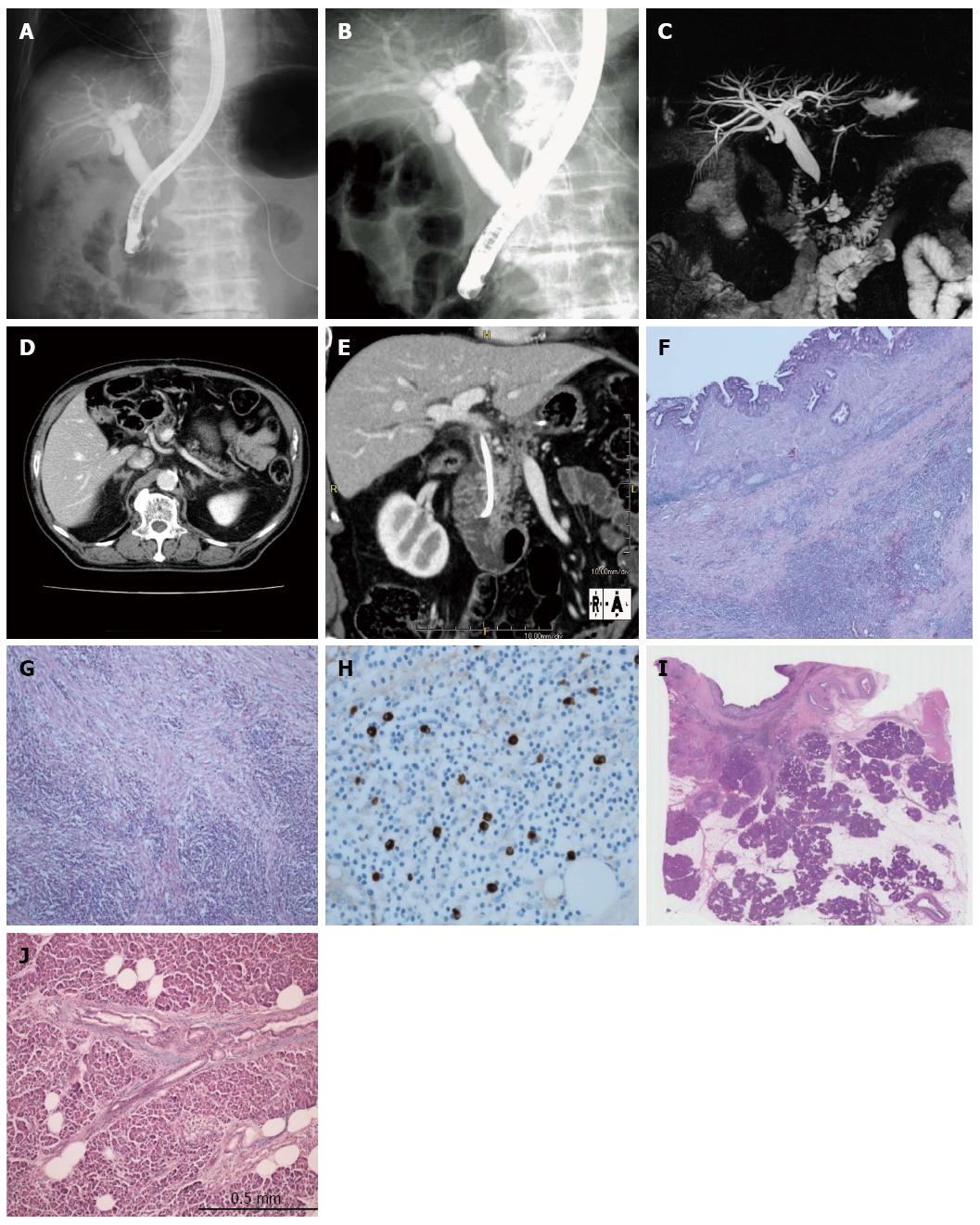
An 82-year-old man was diagnosed with intrahepatic bile duct dilation during a follow-up after gastrectomy for gastric cancer. For further evaluation, he was referred to Kansai Rosai Hospital. On admission, serum hepatobiliary enzymes were elevated, but serum IgG4 was within the normal range. Endoscopic retrograde cholangiopancreatography (ERCP) revealed stenosis of the intrapancreatic bile duct but did not show irregular narrowing of the main pancreatic duct (MPD) (Figure 2A-C). Computed tomography (CT) showed thickening of the extrahepatic bile duct wall without enlargement of the pancreas (Figure 2D and E). Positron emission tomography-CT (PET-CT) imaging with 18F-fluorodeoxyglucose (FDG) detected intense 18F-FDG uptake in the intrapancreatic duct. Bile duct cytology and endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) revealed no malignant features. With a tentative diagnosis of bile duct cancer at the intrapancreatic duct, the patient underwent pancreaticoduodenectomy. Histological examination showed no malignant cells, but infiltration of lymphocytes and IgG4-positive plasma cells, storiform fibrosis, and obstructive phlebitis in the bile duct wall were present (Figure 2F-H). Examination of the adjacent pancreatic tissue did not reveal any signs of AIP (Figure 2I and J). On the basis of these findings, the disease was diagnosed as isolated intrapancreatic IgG4-SC.
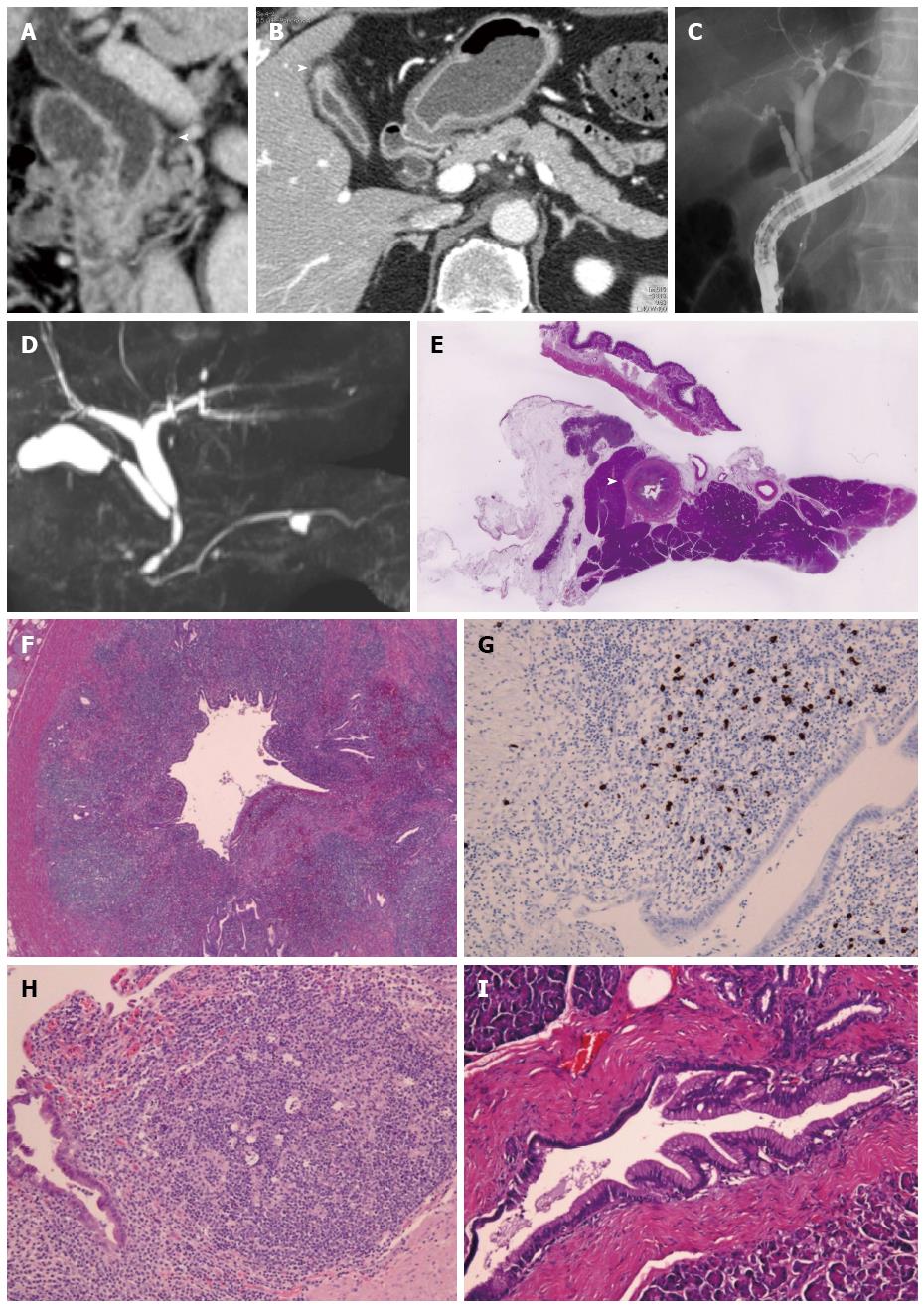
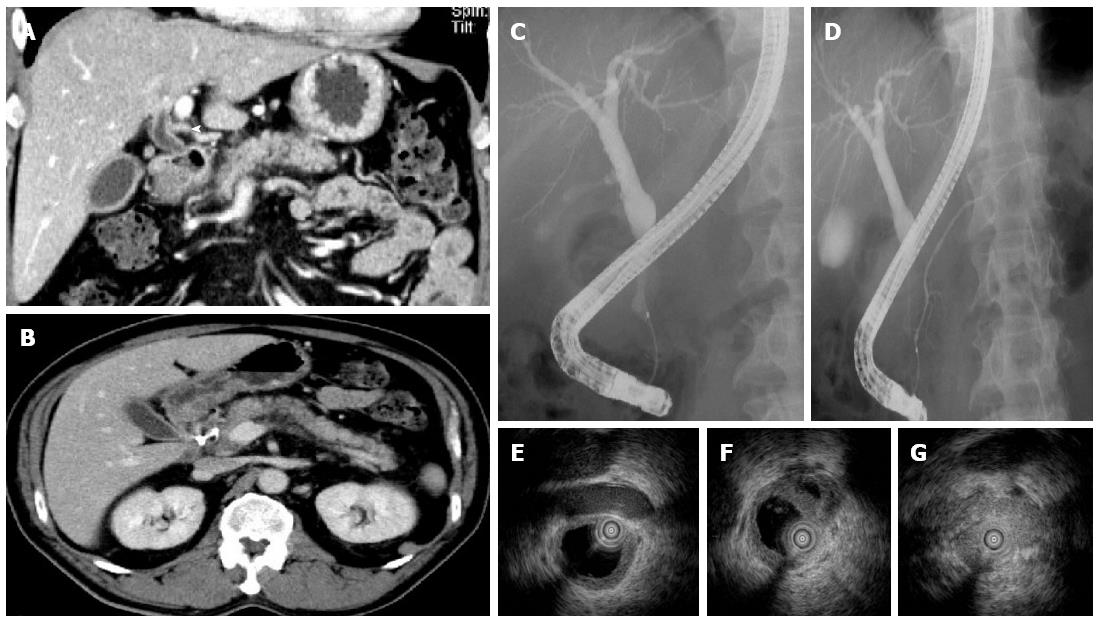
A 60-year-old man was initially admitted to an affiliate hospital for the evaluation of a gall bladder tumor. Under the suspicion of bile duct cancer he was then referred to Kanazawa University Hospital. The serum IgG4 level was within the normal range. Abdominal CT detected long segmental wall thickening in the middle and lower extrahepatic bile duct and cystic duct, but the pancreas was of normal size (Figure 3A and B). Wall thickening of the fundus of the gallbladder was also detected (Figure 3B). ERCP and magnetic resonance cholangiopancreatography (MRCP) revealed long segmental stenosis in the middle and lower extrahepatic bile duct and normal MPD with an exception of a cyst (Figure 3C and D). Transpapillary bile duct biopsy did not provide enough tissue for histopathological evaluation. Cytology of the bile duct showed Class IV, whereas PET-CT detected intense 18F-FDG uptake in the middle and lower extrahepatic bile duct. With a tentative diagnosis of cholangiocarcinoma in the middle and lower extrahepatic bile duct and adenomyomatosis of gallbladder, the patient underwent pylorus-preserving pancreaticoduodenectomy.
Histological examination revealed characteristic findings of IgG4-SC (Figure 3E-G) and IgG4-related cholecystitis (Figure 3H). The adjacent pancreatic tissue was found to be normal (Figure 3E and I). On the basis of these findings, the disease was diagnosed as isolated intrapancreatic IgG4-SC and IgG4-related cholecystitis.
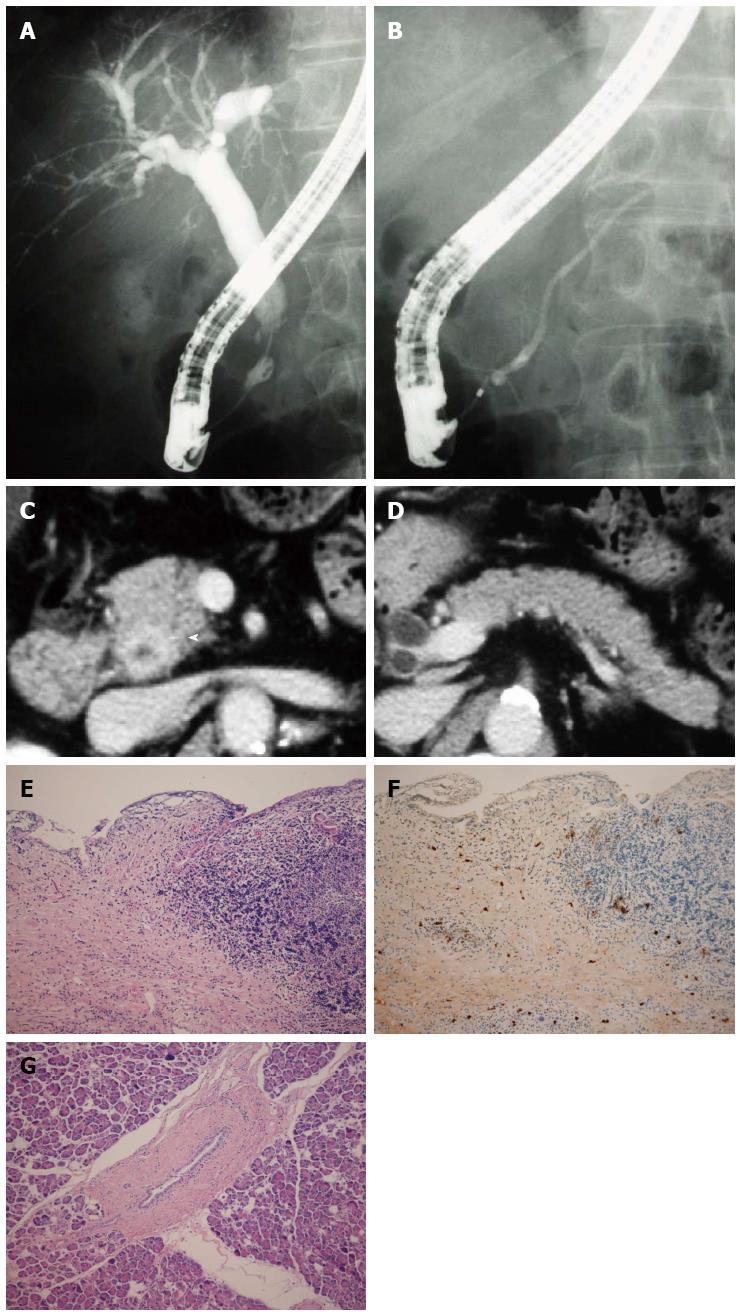
An 81-year-old man was admitted to an affiliate hospital for the treatment of common bile duct stones. ERCP revealed a stricture in the intrapancreatic duct. The patient was then referred to Kasugai Municipal Hospital for further evaluation. ERCP and MRCP showed a short stenosis in the intrapancreatic bile duct and normal MPD (Figure 4A and B). Abdominal CT detected wall thickening in the middle and lower extrahepatic bile duct and the pancreas of normal size (Figure 4C and D). The patient underwent pylorus-preserving pancreaticoduodenectomy based on the diagnosis of cholangiocarcinoma.
Histological examination revealed characteristic findings of IgG4-SC (Figure 4E and F). Examination of the adjacent pancreatic tissue indicated normal pancreas (Figure 4G). Based on these findings, the diagnosis of isolated intrapancreatic IgG4-SC was made. The level of serum IgG4 measured after the surgery was within the normal range.
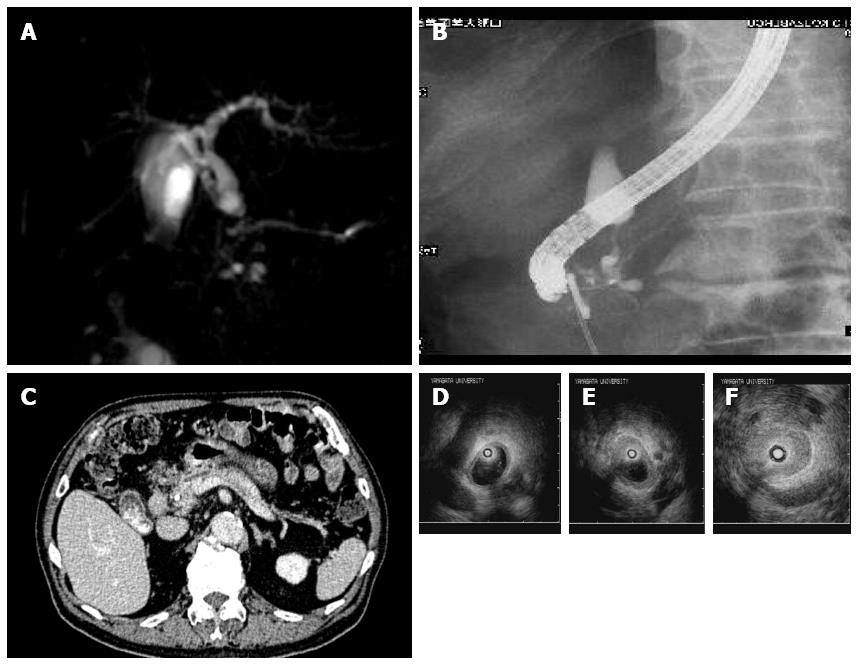
A 61-year-old man was admitted with jaundice. ERCP showed a stenosis of intrapancreatic duct. He was then referred to Tokai University Hospital for further evaluation. As in previous cases, abdominal CT detected wall thickening in the intrapancreatic bile duct, but the pancreas was of normal size (Figure 5A and B). ERCP revealed a stenosis of intrapancreatic duct and normal MPD (Figure 5C and D), whereas intraductal ultrasonography (IDUS) showed symmetric and smooth thickening of the inner hypoechoic layer of the bile ducts spreading from the intrapancreatic duct to the intrahepatic ducts (Figure 5E-G). Transpapillary bile duct biopsy showed infiltration of lymphocytes and 23 IgG4-positive plasma cells/high power field. The ratio of IgG4/IgG-positive plasma cells was 43.5%. The serum IgG4 level was 509 mg/dL. In accordance with these findings, the disease was diagnosed as isolated intrapancreatic IgG4-SC. Steroid therapy was administered with the initial dose of 30 mg. ERCP showed an improvement of the stenosis 3 mo later. He underwent maintenance steroid therapy and IgG4-SC has not recurred until now.
An 87-year-old man was admitted with jaundice. ERCP and MRCP detected a slight dilation of the MPD (Figure 6A and B). A stenosis in the intrapancreatic common bile duct was revealed with cholangiography (Figure 6A and B). Abdominal CT showed a normal-sized pancreas (Figure 6C). IDUS showed thickening of the inner hypoechoic layer of the bile ducts spreading from the intrapancreatic common bile duct to the middle common bile duct (Figure 6D-F). Transpapillary biopsy of the stenotic area of the bile duct did not reveal malignant cells. The level of serum IgG4 was 262 mg/dL. The patient was associated with IgG4-related sialadenitis and retroperitoneal fibrosis. He was diagnosed with isolated intrapancreatic IgG4-SC. Endoscopic biliary drainage was performed and followed up.
The clinical characteristics of the 5 patients are summarized in Table 1. They all were males with a mean age of 74.2 years. The pancreas was not enlarged in any of these cases. No irregular narrowing of the MPD was found. Bile duct wall thickening in lesions without luminal stenosis, which is typical of IgG4-SC, was detected by abdominal CT in all five cases, by endoscopic ultrasonography (EUS) in two out of four cases and by IDUS in all three cases. In three cases, serum IgG4 levels were within the normal limits. The mean serum IgG4 level measured before surgery was 202.1 mg/dL (4 cases).
| Case No. | Age | Sex | Year | SerumIgG4 | Pancreaticenlargement | Narrowingof MPD | Bile duct biopsy/cytology | IDUS/EUS | Wall thickeningIn lesions without luminal stenosis | Other modalities | Firstdiagnosis | Treatment |
| (1) Kansai RosaiHospital | 82 | M | 2012 | 22.8 | Atrophic | Normal(MRCP) | Negative(cytology) | Symmetric, smooth wall thickening(EUS) | Yes(CT) | Intenseuptake(PET-CT) | Cholangiocarcinoma | PD |
| (2) KanazawaUniversity | 60 | M | 2012 | 14.6 | Normal | Normal | InadequateSample(biopsy)Suspicious of adenocarcinoma(cytology) | Symmetric, smooth wall thickening(EUS) | Yes(CT) | Intenseuptake(PET-CT) | CholangiocarcinomaCholangiocarcinomaAdenomyomatosis of gall bladder | PPPD |
| (3) Kasugai municipalHospital | 81 | M | 2009 | 76(afteroperation) | Normal | Normal | Suspicious of adenocarcinoma(biopsy) | Symmetric,smooth wall thickening(IDUS) | Yes(CT, IDUS) | Cholangiocarcinoma | PPPD | |
| (4) TokaiUniversity | 61 | M | 2010 | 509 | Normal | Normal | IgG4-SC(biopsy) | Symmetric,smooth wall thickening(IDUS, EUS) | Yes(CT, IDUS, EUS) | IgG4-SC | Predonisolone | |
| (5) YamagataUniversity | 87 | M | 2009 | 262 | Normal | Dilation | No malignancy(biopsy) | Symmetric,smooth wall thickening(IDUS, EUS) | Yes(CT,IDUS, EUS) | IgG4-SC | Endoscopicbiliary drainage |
Three out of 5 cases (1, 2, and 3) were not diagnosed with IgG4-SC until the surgery. In the remaining two cases, however, the diagnosis was established without operation. Among those two, one (case 4) received steroid therapy, whereas the other (case 5) was treated with endoscopic biliary drainage only. Two out of three cases that were surgically treated (case 1 and 2) showed low serum IgG4 levels (22.8 mg/dL and 14.6 mg/dL, respectively) before the surgery, whereas in the remaining case (case 3) this parameter was not measured at that time. Only one case with high serum IgG4 (case 5) was associated with other organ involvements. The reasons for surgical treatment were as follows. Adenocarcinoma was suspected based on the results of brush cytology in two cases (case 2 and 3). The possibility of cholangiocarcinoma could not be ruled out in spite of the negative results of brush cytology and EUS-FNA in the remaining case (case1).
In three cases (case 1, 2, and 3), pathological findings in the surgical specimens of the bile duct showed severe infiltration of lymphocytes and IgG4-positive plasmacytes as well as prominent fibrosis in the bile duct. These findings were compatible with the diagnosis of IgG4-SC. However, no inflammatory changes compatible with AIP in the adjacent pancreas tissue were found in any of these three cases.
Based on the Japanese clinical diagnostic criteria of 2012[5], all five cases were diagnosed as definite IgG4-SC.
IgG4-SC is often associated with AIP, and the frequency of isolated IgG4-SC is low. Most of the reported cases of isolated IgG4-SC showed type 3 or type 4 cholangiogram (Figure 1), and a series of isolated type 1 (intrapancreatic) IgG4-SC cases has not been reported until now[2,3]. In the present study we have described 5 such cases and evaluated their clinical features. Recently, two Japanese studies have evaluated the frequency of IgG4-SC in large population samples. First, a multi-institutional study has utilized our cholangiographic classification system to reveal that out of the total of 349 IgG4-SC cases, 334 (95.7%) were associated with AIP[6]. Specifically, 244/246 (99.2%), 51/56 (91.1%), 28/29 (96.5%), and 11/18 (61.0%) cases of types 1, 2, 3, and 4 IgG4-SC, respectively, were found to be associated with AIP. Of note, type 4 IgG4-SC cases showed a lower frequency of association. Second, a nationwide survey for primary and IgG4-related sclerosing cholangitis conducted by the Japanese Biliary Association has revealed that type 1, 2, 3, and 4 IgG4-SC was not associated with AIP in 7%, 7%, 9%, and 51% of the cases, respectively, whereas this percentage was 26 for the unclassified type[7]. These results indicate that only type 4 IgG4-SC is frequently found in the isolated form. Thus, the cases of isolated type 1 IgG4-SC are very rare.
The feasibility of including type 1 IgG4-SC into the IgG4-SC category has been disputed. Some researchers suggest that the stricture of the lower common bile duct, which is observed in type 1 IgG4-SC, is caused by compression due to AIP. This claim is based on the fact that the frequency of type 1 IgG4-SC was low (16%) in AIP without pancreatic head lesion[8]. However, we believe that type 1 IgG4-SC should be classified as one of the IgG4-SC types because of the following reasons. First, pathological examination of the bile duct wall obtained from surgically resected samples showed abundant IgG4-positive plasma cell infiltration, storiform fibrosis, and obstructive phlebitis, which are characteristics of IgG4-SC-associated inflammation[9]. Second, the results of an IDUS study showed continuous thickening of the bile duct wall from the intrapancreatic to the extrapancreatic bile duct[10]. In addition, this paper revealed that some cases showed inflammation of only the bile duct wall and not of the pancreas. In fact, it is difficult to identify which factor is the main contributor to the thickening of the bile duct wall, inflammation of the bile duct or compression due to AIP. However, we believe that type 1 IgG4-SC should be included into our cholangiographic classification system as an independent type because the purpose of this system is to facilitate clinical awareness of these conditions in order to avoid unnecessary surgical resection under the diagnosis of cholangiocarcinoma or liver transplantation under the diagnosis of PSC.
Some cases of IgG4-SC isolated from AIP are difficult to diagnose[2,3]. There seem to be several reasons for this. Bile duct biopsy may not be able to provide a large enough sample that would allow identification of characteristic pathological features of IgG4-SC[10], whereas the results of cytology, which has been performed in two cases presented in the current study, suggested adenocarcinoma. PET-CT showed intense 18F-FDG uptake in two cases. In addition, two studies reported that the serum IgG4 level is often normal in IgG4-SC[2,3]. In agreement with this observation, serum IgG4 levels were found to be within the normal limits in 2 out of 4 cases presented here for which the analysis was done prior to surgery.
We summarized the key findings of isolated intrapancreatic IgG4-SC in Table 2.
| Isolated intrapancreatic IgG4-SC is rare among isolated IgG4-SC |
| Isolated intrapancreatic IgG4-SC is misdiagnosed as cholangiocarcinoma of intrapancreatic duct |
| Frequency of cases with higher serum IgG4 level is low in isolated intrapancreatic IgG4-SC cases |
| Bile duct wall thickening in lesions without luminal stenosis detected by abdominal CT, EUS and IDUS is useful finding in the diagnosis of isolated intrapancreatic IgG4-SC |
Frequency of cases with higher serum IgG4 level is low in isolated intrapancreatic IgG4-SC cases. However, bile duct wall thickening in lesions without luminal stenosis detected by abdominal CT, EUS and IDUS is useful finding in the diagnosis of isolated intrapancreatic IgG4-SC.
In conclusion, isolated intrapancreatic IgG4-SC is difficult to diagnose, especially if the IgG4 level remains normal. Thus, this type of IgG4-SC should be suspected in addition to cholangiocarcinoma and pancreatic cancer if stenosis of intrapancreatic bile duct is present.
Five male patients with isolated intrapancreatic IgG4-related sclerosing cholangitis.
Three patients were misdiagnosed as cholangiocarcinoma and two patents were correctly diagnosed as isolated intrapancreatic IgG4-related sclerosing cholangitis.
Intrapancreatic cholangiocarcinoma.
In three cases, serum IgG4 levels were within the normal limits.
Stenosis and wall thickness of intrapancreatic bile duct. Bile duct wall thickening in lesions without luminal stenosis detected by abdominal computed tomography, endoscopic ultrasonography and intraductal ultrasonography is useful finding in the diagnosis.
Three surgical specimen and one bile duct biopsy showed infiltration of abundant IgG4-positive plasma cells
Three patients were surgically treated. Another underwent steroid therapy and the other endoscopic biliary drainage.
There are no case reports with isolated intrapancreatic IgG4-related sclerosing cholangitis.
Isolated intrapancreatic IgG4-related sclerosing cholangitis is type 1 IgG4-related sclerosing cholangitis without autoimmune pancreatitis.
Isolated intrapancreatic immunoglobulin G4-related sclerosing cholangitis (IgG4-SC) is difficult to diagnose, especially if the IgG4 level remains normal. Thus, this type of IgG4-SC should be suspected in addition to cholangiocarcinoma and pancreatic cancer if stenosis of intrapancreatic bile duct is present.
In the manuscript, the authors described the clinical findings of 5 cases of isolated IgG4-SC, the manuscript is well written, and the cases are detailed introduced. The current manuscript enriches the knowledge of isolated IgG4-SC.
P- Reviewer: Dietrich CF, Kamisawa T, Podda M S- Editor: Ma YJ L- Editor: A E- Editor: Wang CH
| 1. | Nakazawa T, Naitoh I, Hayashi K, Okumura F, Miyabe K, Yoshida M, Yamashita H, Ohara H, Joh T. Diagnostic criteria for IgG4-related sclerosing cholangitis based on cholangiographic classification. J Gastroenterol. 2012;47:79-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 2. | Hamano H, Kawa S, Uehara T, Ochi Y, Takayama M, Komatsu K, Muraki T, Umino J, Kiyosawa K, Miyagawa S. Immunoglobulin G4-related lymphoplasmacytic sclerosing cholangitis that mimics infiltrating hilar cholangiocarcinoma: part of a spectrum of autoimmune pancreatitis? Gastrointest Endosc. 2005;62:152-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 130] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 3. | Hayashi K, Nakazawa T, Ohara H, Ando T, Takada H, Tanaka H, Sasaki M, Kataoka H, Nakao H, Joh T. Autoimmune sclerosing cholangiopancreatitis with little pancreatic involvements by imaging findings. Hepatogastroenterology. 2007;54:2146-2151. [PubMed] |
| 4. | Nakazawa T, Ohara H, Sano H, Ando T, Joh T. Schematic classification of sclerosing cholangitis with autoimmune pancreatitis by cholangiography. Pancreas. 2006;32:229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 128] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 5. | Ohara H, Okazaki K, Tsubouchi H, Inui K, Kawa S, Kamisawa T, Tazuma S, Uchida K, Hirano K, Yoshida H. Clinical diagnostic criteria of IgG4-related sclerosing cholangitis 2012. J Hepatobiliary Pancreat Sci. 2012;19:536-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 252] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 6. | Ohara H, Nakazawa T, Kawa S, Kamisawa T, Shimosegawa T, Uchida K, Hirano K, Nishino T, Hamano H, Kanno A. Establishment of a serum IgG4 cut-off value for the differential diagnosis of IgG4-related sclerosing cholangitis: a Japanese cohort. J Gastroenterol Hepatol. 2013;28:1247-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Tanaka A, Tazuma S, Okazaki K, Tsubouchi H, Inui K, Takikawa H. Nationwide survey for primary sclerosing cholangitis and IgG4-related sclerosing cholangitis in Japan. J Hepatobiliary Pancreat Sci. 2014;21:43-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 8. | Hirano K, Tada M, Isayama H, Yamamoto K, Mizuno S, Yagioka H, Yashima Y, Sasaki T, Kogure H, Togawa O. Endoscopic evaluation of factors contributing to intrapancreatic biliary stricture in autoimmune pancreatitis. Gastrointest Endosc. 2010;71:85-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 9. | Zen Y, Harada K, Sasaki M, Sato Y, Tsuneyama K, Haratake J, Kurumaya H, Katayanagi K, Masuda S, Niwa H. IgG4-related sclerosing cholangitis with and without hepatic inflammatory pseudotumor, and sclerosing pancreatitis-associated sclerosing cholangitis: do they belong to a spectrum of sclerosing pancreatitis? Am J Surg Pathol. 2004;28:1193-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 389] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 10. | Naitoh I, Nakazawa T, Ohara H, Ando T, Hayashi K, Tanaka H, Okumura F, Takahashi S, Joh T. Endoscopic transpapillary intraductal ultrasonography and biopsy in the diagnosis of IgG4-related sclerosing cholangitis. J Gastroenterol. 2009;44:1147-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 110] [Article Influence: 7.3] [Reference Citation Analysis (0)] |