Published online Jan 28, 2015. doi: 10.3748/wjg.v21.i4.1305
Peer-review started: March 11, 2014
First decision: March 27, 2014
Revised: May 19, 2014
Accepted: June 14, 2014
Article in press: June 17, 2014
Published online: January 28, 2015
Processing time: 322 Days and 7.8 Hours
AIM: To quantitatively summarize and appraise the available evidence of urea breath test (UBT) use to diagnose Helicobacter pylori (H. pylori) infection in patients with dyspepsia and provide pooled diagnostic accuracy measures.
METHODS: We searched MEDLINE, EMBASE, Cochrane library and other databases for studies addressing the value of UBT in the diagnosis of H. pylori infection. We included cross-sectional studies that evaluated the diagnostic accuracy of UBT in adult patients with dyspeptic symptoms. Risk of bias was assessed using QUADAS (Quality Assessment of Diagnostic Accuracy Studies)-2 tool. Diagnostic accuracy measures were pooled using the random-effects model. Subgroup analysis was conducted by UBT type (13C vs14C) and by measurement technique (Infrared spectrometry vs Isotope Ratio Mass Spectrometry).
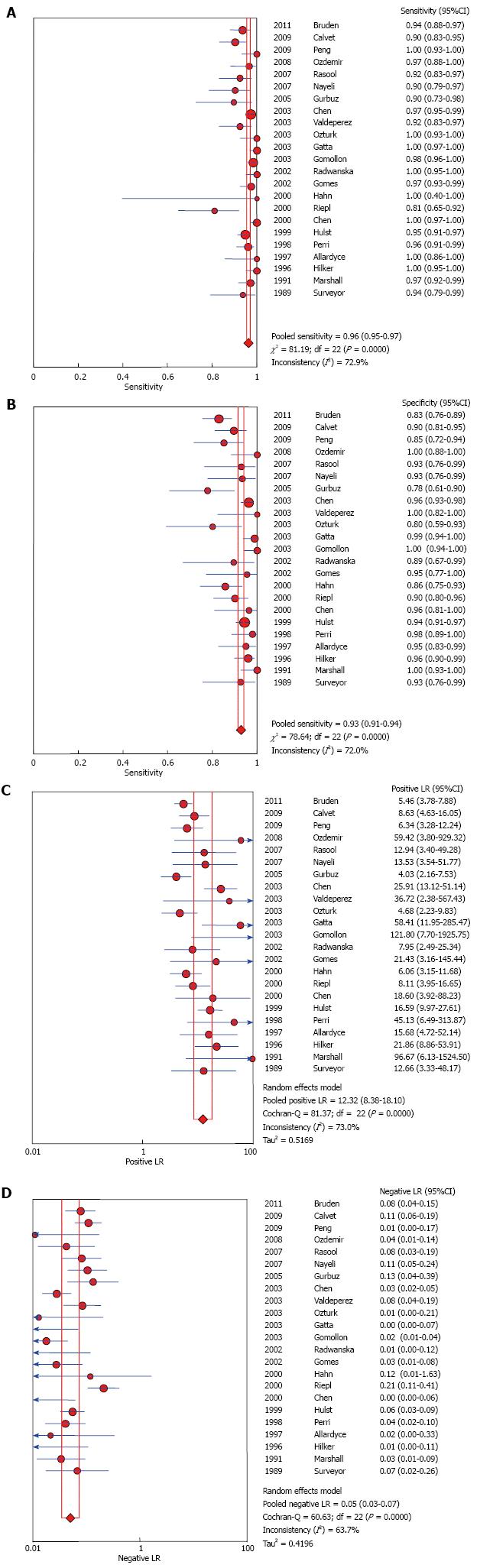
RESULTS: Out of 1380 studies identified, only 23 met the eligibility criteria. Fourteen studies (61%) evaluated 13C UBT and 9 studies (39%) evaluated 14C UBT. There was significant variation in the type of reference standard tests used across studies.Pooled sensitivity was 0.96 (95%CI: 0.95-0.97) andpooled specificity was 0.93 (95%CI: 0.91-0.94). Likelihood ratio for a positive test was 12 and for a negative test was 0.05 with an area under thecurve of 0.985. Meta-analyses were associated with a significant statistical heterogeneity that remained unexplained after subgroup analysis. The included studies had a moderate risk of bias.
CONCLUSION: UBT has high diagnostic accuracy for detecting H. pylori infection in patients with dyspepsia. The reliability of diagnostic meta-analytic estimates however is limited by significant heterogeneity.
Core tip: Urea breath test (UBT) is a commonly used non-invasive test to diagnose Helicobacter pylori (H. pylori) infection in patients with dyspepsia. Multiple trials are available in literature, but they reported different diagnostic accuracy estimates. We conducted systemic review and meta-analysis to explore the available evidence and provide pooled diagnostic accuracy measures. Our meta-analysis showed that UBT has high diagnostic accuracy for detecting H. pylori infection in patients with dyspepsia. Given the potentially preventable diseases associated with chronic, untreated H. pylori infection, more widespread adoption of UBT testing may be indicated.
-
Citation: Ferwana M, Abdulmajeed I, Alhajiahmed A, Madani W, Firwana B, Hasan R, Altayar O, Limburg PJ, Murad MH, Knawy B. Accuracy of urea breath test in
Helicobacter pylori infection: Meta-analysis. World J Gastroenterol 2015; 21(4): 1305-1314 - URL: https://www.wjgnet.com/1007-9327/full/v21/i4/1305.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i4.1305
Helicobacter pylori (H. pylori) is a gram-negative bacterium found on the luminal surface of the gastric epithelium. It was first isolated by Warren and Marshall in 1983. It induces chronic inflammation of the underlying mucosa. The infection is usually contracted in the first few years of life and tends to persist indefinitely unless treated. At least 50% of the world’s population is thought to carry H. pylori. The organism can survive in the acidic environment of the stomach partly owing to its remarkably high urease activity. Urease converts the urea present in gastric juice to alkaline ammonia and carbon dioxide[1].
Although the full spectrum of pathogenesis is currently unknown, H. pylori has been linked to a variety of upper gastrointestinal disorders. Reported symptoms of H. pylori infection are relatively non-specific, such as epigastric pain, postprandial fullness, bloating, nausea, and vomiting, along with signs of acid hypersecretion and delayed gastric emptying[2,3]. In addition, infection with H. pylori is linked to three important upper gastrointestinal diseases: duodenal or gastric ulcers, gastric cancer, and gastric mucosa-associated lymphoid-tissue lymphoma.
Many invasive and non-invasive methods can be used to diagnose H. pylori infection, including endoscopy with biopsy, serology for immunoglobulin titers, stool antigen analysis, and the urea breath test (UBT). Given the user-friendly, non-invasive features of UBT, this detection method may be preferred in many clinical settings. However, to date, the performance characteristics of UBT have been inconsistently described and remain incompletely defined.
UBT can play a useful role in the diagnostic evaluation of dyspeptic patients who have comorbidities that increase their risk of upper endoscopy, are intolerant to upper endoscopy, or have known or suspected gastric atrophy. Stool antigen testing can also be used to non-invasively detect active H. pylori infection, and the choice of diagnostic modality depends on factors such as cost, laboratory infrastructure, and concomitant use of medications such as proton pump inhibitors or antibiotics that may influence test results. Serum antibody test results can vary by geographic region, and may stay positive for a prolonged period following H. pylori eradication, thereby limiting the clinical utility for determining the presence or absence of current infection[4].
There are two UBTs available and gained Food and Drug Administration approval: 13C and 14C tests. Both tests are affordable and can provide real-time results. Some physicians may prefer the 13C test as it is non-radioactive compared to 14C which uses a radioactive isotope, especially in young children and pregnant women, though dose of radianis very minimal (about 1 microCi)[5]; the dose of radiation is the dose of 14C-UBT with the mini dose equals to 1 microCi (37 kbq) which has a high diagnostic accuracy[6]. UBT is indicated to confirm H. pylori colonization and to monitor its eradication. Positive UBT indicates an active H. pylori infection which require treatment or further confirmation with invasive procedures. Initial treatment for H. Pylori consist of either triple, quadruple, or sequential therapy regimens, which all of them includes a proton pump inhibitor plus various antibiotic regimen; treatment periods generally varied from 7 to 14 d[4].
In this systematic review and meta-analysis, we aimed at summarizing data and appraising the relevant articles of UBT for diagnosis of H. pylori infection in dyspeptic patients and provide pooled diagnostic accuracy measures.
Search and analysis methods, eligibility criteria, and the outcomes of interest were specified in advance in a protocol developed by study investigators.
We included cross-sectional studies with consecutive patients that evaluated the diagnostic accuracy of UBT in adult patients with dyspeptic symptoms. We included articles that compare 13C-UBT or 14C-UBT H. pylori test with a reference standard which is H. pylori (culture and/or histological examination) and/or not (serologic test either blood or stool).
We excluded studies that enrolled children or adolescents under 18 year of age, subjects who presented for reasons other than dyspeptic symptoms, bleeding peptic ulcer, complicated dyspeptic cases that need surgery, those who received previous therapy for H. pylori within the last 3 mo, or long term use of corticosteroids and immunosuppressant drugs and screening studies. Only articles presenting true positive, true negative, false positive and false negative data were included in the present study. Studies where data was missing and studies with high risk of bias were excluded.
There was no inclusion restriction on the type of UBT performed. Both 13C and 14C types where included. Studies where the UBT was performed through an invasive method were excluded.
A librarian searched electronic databases for published and in-press studies from 1990 (the date where UBT became available) through November 2013 including PubMed, EMBASE, LILACS and Cochrane databases. The search terms used were “H. pylori”, “Helicobacter pylori”, “Helicobacter infection”, “gastritis”, “dyspepsia”, “breath test”, “urea breath test”, “UBT”, “13C-UBT” and “14C-UBT” with its MeSH terms (Medical Subject Headings) and keywords. We used Boolean operator (OR) to combine synonyms and (AND) to combine the cases with tests. No language restriction was applied. Reference lists were also scanned.
Two authors (MF, WM) screened titles and abstracts for inclusion criteria. Full text articles were retrieved for relevant articles. An abstraction format developed by authors that includes: study citation, author name and year of publication, patients’ mean age and other baseline characteristics, UBT variant, gold standard used, time between the test and gold standard, description of the cases, and diagnostic study data (numbers of true positive, false positive, false negative, and true negative test results). Disagreement was resolved by consensus.
Two reviewers (MF and IY) independently assessed the quality of the included studies using the QUADAS (Quality Assessment of Diagnostic Accuracy Studies)-2 instrument[7]. This tool is designed to assess the quality of primary diagnostic accuracy studies for inclusion in the systematic review. It consists of four key domains covering patient selection, index test, reference standard, and flow of patients through the study and timing of the index test(s) and reference standard. Each domain is assessed in terms of the risk of bias and the first three are also assessed in terms of concerns regarding applicability.
Risk of bias is judged as “low”, “high”, or “unclear”. If all signaling questions for a domain are answered “yes” then risk of bias can be judged “low”. If any signaling question is answered “no” this flags the potential for bias.
We considered low risk of bias in different domains as follows: Patient selection if non-complicated dyspeptic patients were enrolled in consecutively. Index test, where it was interpreted independent from the reference standard. Reference standard, when it correctly classifies H. pylori and non-H. pylori. Flow and time, the appropriate interval between index test and the reference standard is within 7 d, and breathing samples were collected within 30 min.
The meta-analysis was conducted using Meta-Disc 1.4[8]. Random effect model was followed in all analyses. The diagnostic accuracy measures used in the analysis were sensitivity, specificity, likelihood ratio for positive and negative test (LR+ and LR-), receiver operating characteristics (ROC) curve, and diagnostic odds ratio. We assessed heterogeneity using the I-squared statistic and Q test. Publication bias was conducted using the Deeks’ funnel plot asymmetry test, with P-value < 0.05 for the slope coefficient indicating significant asymmetry[9].
To explore the robustness of our results and evaluate for potential causes of heterogeneity, we conducted several a priori determined analyses. We tested the bivariate mixed effects regression model to determine if results were robust to the correlation between sensitivity and specificity. Bivariate analysis were conducted as implemented in STATA version 12.0 (StataCorp, College Station, TX, United States)[10].
We also conducted subgroup analyses based on the risk of bias in the included studies as it pertains to the various domains of QUADAS-2 tool (such as for the index test and the gold standard test). We evaluated if the type of UBT test (13C vs14C) or measurement technique (isotope ration mass spectrometry vs infrared mass spectrometry) affected the pooled estimates. We conducted an interaction test for subgroup analyses as suggested by Altman and Bland[11] and there was no statistically significant difference to suggest a subgroup effect.
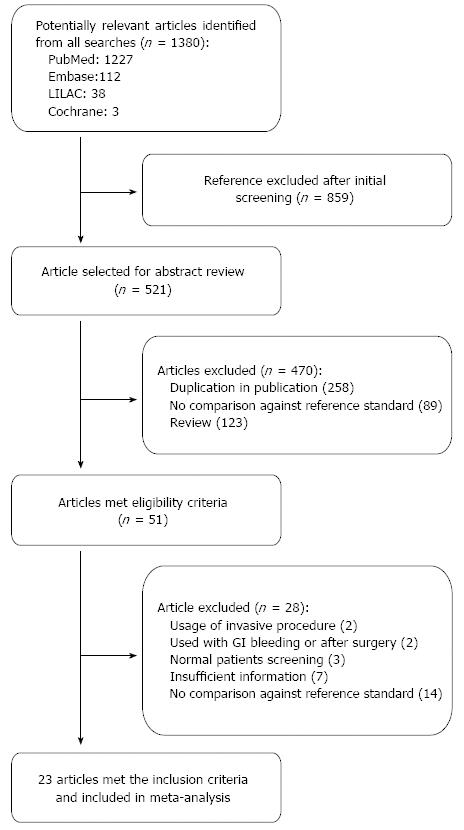
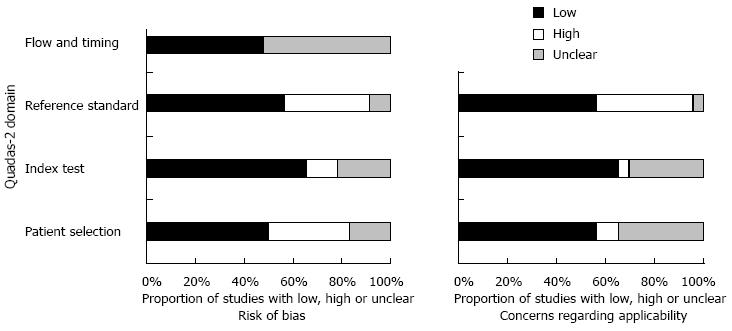
The initial search yielded 1380 studies that were potentially relevant; of which, 23 studies that enrolled a total of 3999 participants were finally included. The study selection process is depicted in Figure 1 including causes of exclusion. More than 50% of quality assessment items articles have low risk of bias of all domains. The agreement between risk of bias assessment between reviewers were 70%, disagreement was resolved by discussion and consensus. Figure 2 visually summarizes the risk of biasin the included studies.
Table 1 shows the characteristics of all included studies. Of the 23 studies, 14 studies (61%) compared 13C UBT with a reference standard, while 9 studies (39%) used 14C UBT. The included studies were conducted in 16 countries, however all but one were published in English (Spanish)[12]. The mean age across studies was (40-59 year) and female gender distribution was (13%-74%).
| Ref. | Country | Year | No. of patients | Study design | UBT (13C/14C) | Infrared assisted | Reference standard | Mean age (mean ± SD) | Females | UBT threshold | Time | |
| Allardyce et al[13] | New Zealand | 1997 | 63 | Cross-sectional | 14C | No | Histo or (Biopsy and rapid urea test) | 56.5 | 26 | 41% | 82% DPM | 30 min and 60 min post ingestion |
| Bruden et al[16] | Estonia | 2011 | 280 | Cross-sectional | 13C | No | Culture or (Histo and RUT) | 53.5 | 185 | 66% | ≥ 5% | NA |
| Calvet et al[27] | Spain | 2009 | 199 | Cross-sectional | 13C | Yes | Any two positive (Histopathology, RUT, UBT, and fecal serology) | 48.2 ± 14.2 | 107 | 53% | 8.5% | 20 min after drinking solution |
| Chen et al[29] | Taiwan | 2003 | 586 | Cross-sectional | 13C | Yes | Culture alone or RUT | 45.7 ± 13.3 | 280 | 46.6% | ≥ 2% | 20 min after drinking solution |
| Chen et al[25] | Japan | 2000 | 169 | Cross-sectional | 13C | No | Combined (Histo and serology) | 53.9 ± 15.7 | 68 | 40% | 2.5% | 20 min after normal respiration |
| Gatta et al[30] | Italy | 2003 | 200 | Cross-sectional | 13C | No | Combined (Histology and rapid urease) and/or culture | 53 ± 13 | 113 | 56% | NA | 30 min post ingestion |
| Gomes et al[22] | Brazil | 2002 | 137 | Cross-sectional | 14C | No | Combined (Histo and RUT) | 46.7 ± 16.6 | 67 | 45% | 1000-2000 CPM | 30 min post ingestion |
| Gomollon et al[17] | Spain | 2003 | 314 | Cross-sectional | 13C | No | Culture and/or Combined (Histo and RUT) | 54.1 ± 18 | 168 | 53.5% | ≥ 5% | 30 min post ingestion |
| Gurbuz et al[23] | Turkey | 2005 | 65 | Cross-sectional | 14C | No | Combind tests (Histo and RUT) | 42.4 ± 15.5 | 46 | 67.7% | > 50 CPM | 10 min after drinking solution |
| Hahn et al[31] | United States | 2000 | 100 | Cross-sectional | 13C | No | Combined (Histo, UBT and serology) | 58.8 ± 14 | 9 | 13.4% | > 2.3% | 30 min after administration |
| Hilker et al[14] | Germany | 1996 | 174 | Cross-sectional | 13C | No | Histo | 46 | 106 | 60.9% | > 250 | 30 min after administration |
| van der Hulst et al[26] | Italy | 1999 | 544 | Cross-sectional | 13C | Yes | Histo and culture | 46.5 | 379 | 62.7% | > 5% | 30 min after administration |
| Marshall et al[32] | United States | 1990 | 153 | Cross-sectional | 14C | No | Combined (Culture, RUT and histo) | -- | 77 | 50% | > 6% | 30 min after administration |
| Ortiz-Olvera Nayeli et al[18] | Mexico | 2007 | 88 | Cross-sectional | 13C | No | Culture and/or combined (Histo and RUT) | 45 ± 15 | 49 | 55.6% | > 4.22% | 30 min after administration |
| Ozdemir et al[28] | Turkey | 2008 | 89 | Cross-sectional | 14C | No | Combined; any 2 positive ( RUT, PCR and histo) | 45 ± 13 | 59 | 66% | > 25 CPMas Heliprobe | 10 min after drinking solution |
| Oztürk et al[15] | Turkey | 2003 | 75 | Cross-sectional | 14C | No | Histology | 41 ± 14 | 56 | 74.6% | 100 DPM | NA |
| Peng et al[19] | Taiwan | 2009 | 100 | Cross-sectional | 13C | Yes | Culture or combind (Histo and RUT) | 55 | 44 | 55% | 4.8% | 15 min after drinking solution |
| Perri et al[20] | Belgium | 1998 | 172 | Cross-sectional | 13C | No | Histo and/or culture | 39.7 ± 14.1 | 81 | 47% | 3.3% | Every 15 min for 1 h after ingestion ofthe urea solution |
| Kopański et al[3] | Poland | 2002 | 92 | Cross-sectional | 14C | No | Combined (Culture, serology, UBT and urine test for C-urea) | 45.5 | 36 | 39% | > 5% | 30 min after administration |
| Rasool et al[24] | Pakistan | 2007 | 94 | Cross-sectional | 14C | No | Two reference tests. Patient did both separately: (1) Histo; (2) RUT | 40.8 ± 12.8 | 34 | 36% | > 50 CPM | After 10 min |
| Riepl et al[33] | Austria | 2000 | 100 | Cross-sectional | 13C | Yes | Combined 3 tests (Histo, UAT and culture) | 51.6 ± 1.4 | 49 | 49% | > 4% | NA |
| Surveyor et al[21] | Australia | 1989 | 63 | Cross-sectional | 14C | No | Histo and/or culture | 58.8 ± 14.5 | 30 | 47% | NA | Every 5 min for 30 min |
| Valdeperez et al[12] | Spain | 2003 | 85 | Cross-sectional | 13C | No | Histo and RUT | 41.6 | 44 | 50.5% | NA | 30 min after administration |
There was variation (10 folds) in the type of reference standard tests used by different studies (Table 2). Seven studies (30.4%) used one reference standard starting with either histopathology or culture at first and only used subsequent tests if the first test was negative (histopathology in three studies[13-15], and culture in four studies[16-19]). Two studies (8.7%)[20,21] used histopathology or culture, nine studies (39.1%)[12,22-24] used two combined tests (histopathology and rapid urease test “RUT” in four studies, histopathology and serology in one study[25], histopathology and culture in one study[26], and any two tests in three studies[27-29]. Four studies (17.4%)[30-33] used three combined tests, and one study (4.3%)[3] used four combined tests as reference standard. Histopathology is the most common approach when combined tests were used. In three studies[3,27,31], UBT was part of combined reference standards.
| Ref. | TP | FP | FN | TN | Total |
| Allardyce et al[13] | 24 | 2 | 0 | 37 | 63 |
| Bruden et al[16] | 131 | 24 | 9 | 116 | 280 |
| Calvet et al[27] | 102 | 9 | 11 | 77 | 199 |
| Chen et al[29] | 361 | 8 | 10 | 205 | 584 |
| Chen et al[25] | 135 | 1 | 0 | 26 | 162 |
| Gatta et al[30] | 113 | 1 | 0 | 86 | 200 |
| Gomes et al[22] | 112 | 1 | 3 | 21 | 137 |
| Gomollon et al[17] | 249 | 0 | 4 | 61 | 314 |
| Gurbuz et al[23] | 26 | 8 | 3 | 28 | 65 |
| Hahn et al[31] | 4 | 9 | 0 | 54 | 67 |
| Hilker et al[14] | 76 | 4 | 0 | 94 | 174 |
| van der Hulst et al[26] part 1 | 255 | 14 | 14 | 231 | 514 |
| van der Hulst et al[26] part 2 | 161 | 3 | 12 | 72 | 248 |
| Marshall et al[32] | 101 | 0 | 3 | 49 | 153 |
| Ortiz-Olvera Nayeli et al[18] | 46 | 2 | 5 | 28 | 81 |
| Ozdemir et al[28] | 57 | 0 | 2 | 30 | 89 |
| Oztürk et al[15] | 48 | 5 | 0 | 20 | 73 |
| Peng et al[19] | 53 | 7 | 0 | 40 | 100 |
| Perri et al[20] | 121 | 1 | 5 | 46 | 173 |
| Kopański et al[3] | 75 | 2 | 0 | 17 | 94 |
| Rasool et al[24] | 61 | 2 | 5 | 26 | 94 |
| Riepl et al[33] | 30 | 7 | 7 | 63 | 107 |
| Surveyor et al[21] | 30 | 2 | 2 | 25 | 59 |
| Valdeperez et al[12] | 61 | 0 | 5 | 19 | 85 |
UBT had high sensitivity and specificity 0.96 (95%CI: 0.95-0.97) and 0.93 (95%CI: 0.91-0.94); respectively. LR+ and LR- were 12.32 (95%CI: 8.38-18.1) and 0.05 (95%CI: 0.03-0.07) respectively. The AUC was 0.985. Forest plots are depicted in Figure 3. There was no evidence of publication bias (P > 0.05 using Deeks’ asymmetry test).
Inconsistency between results for sensitivity and specificity among studies were 72.9% and 72% respectively with statistically significant Q test (P < 0.05). Heterogeneity could be explained by either clinical or methodological variation; the performed subgroup analyses could not explain the difference.
13C UBT vs 14C UBT: Of the total studies recruited in this systematic review, 14 were conducted using 13C UBT vs 9 using 14C UBT (Table 3). Both versions of the test showed high performance against the Gold standard test without a significant difference. Figures are shown in online supplement materials (Figure 3). Interaction test for subgroup analyses as suggested by Altman and Bland[11] showed no statistically significant difference to suggest a subgroup effect (P = 0.87).
| Subgroup | No. of studies | Sensitivity | Specificity |
| UBT 13C | 14 | 0.96 (0.95-0.97) | 0.94 (0.92-0.95) |
| UBT 14C | 9 | 0.97 (0.95-0.98) | 0.91 (0.87-0.94) |
| Infrared assisted UBT | 5 | 0.95 (0.93-0.96) | 0.93 (0.91-0.95) |
| Infrared not assisted UBT | 18 | 0.97 (0.96-0.98) | 0.93 (0.91-0.95) |
Use of infrared in UBT: Out of total 23 studies, 6 studies used infrared technique in measuring urea level. Both methods showed high performance against the gold standard test without a significant difference. Subgroup analysis based on the risk of bias. Figures are shown in online supplement materials (Figure 2).
There was no significant difference in diagnostic accuracy measures based on the risk of bias in terms of the key domains of patient selection, index test, reference standard, and flow of patients through the study and timing of the index test and reference standard. Interaction test for subgroup analyses showed no statistically significant difference to suggest a subgroup effect (P = 0.23).
Sensitivity analysis using bivariate model: Diagnostic accuracy measures were similar under the bivariate model and meta-analysis results appeared robust to the choice of model.
UBT is a noninvasive test for diagnosis of gastric H. pylori infection. Twenty-three studies for both UBT 13C and 14C for detection of H. pylori infection in adults were included. The result of the meta-analysis showed that the test performance was high and the test has significant discrimination power between those who have the infection and those who haven’t.
The quality of this evidence is considered moderate due to the presence of heterogeneity, which may be explained by using different types of reference standards, timing between ingestion of the capsule and testand may be due to the variation in the methodological quality of the included studies It is very likely that the test performance is different across patients with varying pre-test risk although our analysis could not detect such difference. This analysis, focused on adults, shows similar diagnostic accuracy measures to those found in a different meta-analysis in children (sensitivity of 0.95 and specificity of 0.94 in children)[34].
In addition to the non-invasive nature of UBT, it offers the advantage of providing a comprehensive assessment that is not reliant upon the possible sampling error associated with endoscopic biopsy, due to patchy distribution of H. pylori[15]. Other limitations of the biopsy-based tests relate to their dependency on the pathologist skill and experience with studies documenting intern observer variability[35,36]. On the other hand, there are some limitations for UBT. For example, UBT results can be affected by exposure to H. pylori therapy such as, antibiotics, proton pump inhibitors or bismuth. It requires specialized equipment for carbon dioxide measurement and infrastructure to manage radioactive materials, and it is an expensive test.
The primary strength of this study relates to the search of electronic databases for relevant articles and the careful appraisal of study quality. The limitations mainly relate to dealing with aggregate data that limits our ability to provide estimates based on patient-level characteristics and pre-test risk level. Another significant limitation relates to heterogeneity that was unexplained despite multiple subgroup analyses. The observed heterogeneity can be attributed to several factors. The urease activity of the oral flora can affect the reading of the UBT; this can be accounted for by asking the patient to wash the mouth before conducting the test. Other authors suggested the use of Nasogastric tube. The cut off value and the time to take the reading after the meal ingestion was not clearly stated in many of the studies involved. The nature of the radioisotope meal and individual patient characteristics such as anthropometric measures, sex and age might have also contributed to within as well as between studies variability. All these factors could have contributed to the persistence of heterogeneity even after adjusting for UBT type (13C vs14C) and technique of measurement (radioisotope mass spectrometry vs infrared spectrometry) in subgroup analysis.
In conclusion, UBT has high diagnostic accuracy for detecting H. pylori infection in patients with dyspepsia. Given the clinically significant, potentially preventable diseases associated with chronic, untreated H. pylori infection (such as gastric adenocarcinoma), more widespread adoption of UBT testing may be indicated to simultaneously improve public health and reduce treatment expense. The reliability of diagnostic meta-analytic estimates however is limited by significant heterogeneity, and the findings from this study should therefore be interpreted with appropriate caution.
Helicobacter pylori (H. pylori) is a gram-negative bacterium found on the luminal surface of the gastric epithelium and induces chronic inflammation of the underlying mucosa. The organism can survive in the acidic environment of the stomach partly owing to its remarkably high urease activity. Urease converts the urea present in gastric juice to alkaline ammonia and carbon dioxide. Urea breath test (UBT) is a commonly used non-invasive test to diagnose H. pylori infection in patients with dyspepsia.
There are two UBTs available and gained Food and Drug Administration approval: 13C and 14C tests. Both tests are affordable and can provide real-time results. UBT is indicated to confirm H. pylori colonization and to monitor its eradication.
Many invasive and non-invasive methods can be used to diagnose H. pylori infection, including endoscopy with biopsy, serology for immunoglobulin titers, stool antigen analysis, and the UBT. Given the user-friendly, non-invasive features of UBT, this detection method may be preferred in many clinical settings.
UBT can play a useful role in the diagnostic evaluation of dyspeptic patients who have comorbidities that increase their risk of upper endoscopy, are intolerant to upper endoscopy, or have known or suspected gastric atrophy. The study results suggest that UBT has high diagnostic accuracy for detecting H. pylori infection in patients with dyspepsia.
This systematic review has been well performed; with a well expressed objective, precise criteria for the studies included and the relevant studies which have been selected for further evaluation. The quality of each included study has been properly evaluated. Its main drawback is the heterogeneity of the included studies; this, however, is not the fault of the authors of the meta-analysis.
P- Reviewer: Cerwenka HR, Hara K, Hoff DAL S- Editor: Nan J L- Editor: A E- Editor: Zhang DN
| 1. | McColl KE. Clinical practice. Helicobacter pylori infection. N Engl J Med. 2010;362:1597-1604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 529] [Cited by in RCA: 550] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 2. | Perri F, Clemente R, Festa V, Annese V, Quitadamo M, Rutgeerts P, Andriulli A. Patterns of symptoms in functional dyspepsia: role of Helicobacter pylori infection and delayed gastric emptying. Am J Gastroenterol. 1998;93:2082-2088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Kopański Z, Jung A, Wasilewska-Radwańska M, Kuc T, Schlegel-Zawadzka M, Witkowska B. Comparative diagnostic value of the breath test and the urine test with 14C-urea in the detection of the Helicobacter pylori infection. Nucl Med Rev Cent East Eur. 2002;5:21-24. [PubMed] |
| 4. | Chey WD, Wong BC. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol. 2007;102:1808-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 819] [Cited by in RCA: 830] [Article Influence: 46.1] [Reference Citation Analysis (3)] |
| 5. | Leide-Svegborn S, Stenström K, Olofsson M, Mattsson S, Nilsson LE, Nosslin B, Pau K, Johansson L, Erlandsson B, Hellborg R. Biokinetics and radiation doses for carbon-14 urea in adults and children undergoing the Helicobacter pylori breath test. Eur J Nucl Med. 1999;26:573-580. [PubMed] |
| 6. | Raju GS, Smith MJ, Morton D, Bardhan KD. Mini-dose (1-microCi) 14C-urea breath test for the detection of Helicobacter pylori. Am J Gastroenterol. 1994;89:1027-1031. [PubMed] |
| 7. | Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA, Bossuyt PM. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6953] [Cited by in RCA: 9582] [Article Influence: 684.4] [Reference Citation Analysis (0)] |
| 8. | Zamora J, Abraira V, Muriel A, Khan K, Coomarasamy A. Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Med Res Methodol. 2006;6:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1446] [Cited by in RCA: 1573] [Article Influence: 82.8] [Reference Citation Analysis (0)] |
| 9. | Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol. 2005;58:882-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1792] [Cited by in RCA: 2228] [Article Influence: 111.4] [Reference Citation Analysis (1)] |
| 10. | Chu H, Cole SR. Bivariate meta-analysis of sensitivity and specificity with sparse data: a generalized linear mixed model approach. J Clin Epidemiol. 2006;59:1331-132; author reply 1331-132;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 437] [Cited by in RCA: 537] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 11. | Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ. 2003;326:219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2009] [Cited by in RCA: 2388] [Article Influence: 108.5] [Reference Citation Analysis (0)] |
| 12. | Valdepérez J, Vicente R, Novella MP, Valle L, Sicilia B, Yus C, Gomollón F. [Is the breath test reliable in primary care diagnosis of Helicobacter pylori infection?]. Aten Primaria. 2003;31:93-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Allardyce RA, Chapman BA, Tie AB, Burt MJ, Yeo KJ, Keenan JI, Bagshaw PF. 37 kBq 14C-urea breath test and gastric biopsy analyses of H. pylori infection. Aust N Z J Surg. 1997;67:31-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Hilker E, Domschke W, Stoll R. 13C-urea breath test for detection of Helicobacter pylori and its correlation with endoscopic and histologic findings. J Physiol Pharmacol. 1996;47:79-90. [PubMed] |
| 15. | Oztürk E, Yeşilova Z, Ilgan S, Arslan N, Erdil A, Celasun B, Ozgüven M, Dağalp K, Ovali O, Bayhan H. A new, practical, low-dose 14C-urea breath test for the diagnosis of Helicobacter pylori infection: clinical validation and comparison with the standard method. Eur J Nucl Med Mol Imaging. 2003;30:1457-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Bruden DL, Bruce MG, Miernyk KM, Morris J, Hurlburt D, Hennessy TW, Peters H, Sacco F, Parkinson AJ, McMahon BJ. Diagnostic accuracy of tests for Helicobacter pylori in an Alaska Native population. World J Gastroenterol. 2011;17:4682-4688. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Gomollón F, Ducons JA, Santolaria S, Lera Omiste I, Guirao R, Ferrero M, Montoro M. Breath test is very reliable for diagnosis of Helicobacter pylori infection in real clinical practice. Dig Liver Dis. 2003;35:612-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Ortiz-Olvera Nayeli NX, Morán Villota S, Gallardo Wong I, Blancas Valencia JM, Cabrera Muñoz L. [Validation of a simplified 13C-urea breath test method for the diagnosis of Helicobacter pylori infection]. Rev Esp Enferm Dig. 2007;99:392-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 19. | Peng NJ, Lai KH, Lo GH, Hsu PI. Comparison of noninvasive diagnostic tests for Helicobacter pylori infection. Med Princ Pract. 2009;18:57-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Perri F, Clemente R, Pastore M, Quitadamo M, Festa V, Bisceglia M, Li Bergoli M, Lauriola G, Leandro G, Ghoos Y. The 13C-urea breath test as a predictor of intragastric bacterial load and severity of Helicobacter pylori gastritis. Scand J Clin Lab Invest. 1998;58:19-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 69] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Surveyor I, Goodwin CS, Mullan BP, Geelhoed E, Warren JR, Murray RN, Waters TE, Sanderson CR. The 14C-urea breath-test for the detection of gastric Campylobacter pylori infection. Med J Aust. 1989;151:435-439. [PubMed] |
| 22. | Gomes AT, Coelho LK, Secaf M, Módena JL, Troncon LE, Oliveira RB. Accuracy of the 14C-urea breath test for the diagnosis of Helicobacter pylori. Sao Paulo Med J. 2002;120:68-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Gurbuz AK, Ozel AM, Narin Y, Yazgan Y, Baloglu H, Demirturk L. Is the remarkable contradiction between histology and 14C urea breath test in the detection of Helicobacter pylori due to false-negative histology or false-positive 14C urea breath test? J Int Med Res. 2005;33:632-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Rasool S, Abid S, Jafri W. Validity and cost comparison of 14carbon urea breath test for diagnosis of H Pylori in dyspeptic patients. World J Gastroenterol. 2007;13:925-929. [PubMed] |
| 25. | Chen X, Haruma K, Kamada T, Mihara M, Komoto K, Yoshihara M, Sumii K, Kajiyama G. Factors that affect results of the 13C urea breath test in Japanese patients. Helicobacter. 2000;5:98-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | van der Hulst RW, Hensen EF, van der Ende A, Kruizinga SP, Homan A, Tytgat GN. [Laser-assisted 13C-urea breath test; a new noninvasive detection method for Helicobacter pylori infection]. Ned Tijdschr Geneeskd. 1999;143:400-404. [PubMed] |
| 27. | Calvet X, Sánchez-Delgado J, Montserrat A, Lario S, Ramírez-Lázaro MJ, Quesada M, Casalots A, Suárez D, Campo R, Brullet E. Accuracy of diagnostic tests for Helicobacter pylori: a reappraisal. Clin Infect Dis. 2009;48:1385-1391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 74] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 28. | Ozdemir E, Karabacak NI, Degertekin B, Cirak M, Dursun A, Engin D, Unal S, Unlü M. Could the simplified (14)C urea breath test be a new standard in noninvasive diagnosis of Helicobacter pylori infection? Ann Nucl Med. 2008;22:611-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Chen TS, Chang FY, Chen PC, Huang TW, Ou JT, Tsai MH, Wu MS, Lin JT. Simplified 13C-urea breath test with a new infrared spectrometer for diagnosis of Helicobacter pylori infection. J Gastroenterol Hepatol. 2003;18:1237-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 30. | Gatta L, Vakil N, Ricci C, Osborn JF, Tampieri A, Perna F, Miglioli M, Vaira D. A rapid, low-dose, 13C-urea tablet for the detection of Helicobacter pylori infection before and after treatment. Aliment Pharmacol Ther. 2003;17:793-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 31. | Hahn M, Fennerty MB, Corless CL, Magaret N, Lieberman DA, Faigel DO. Noninvasive tests as a substitute for histology in the diagnosis of Helicobacter pylori infection. Gastrointest Endosc. 2000;52:20-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Marshall BJ, Plankey MW, Hoffman SR, Boyd CL, Dye KR, Frierson HF, Guerrant RL, McCallum RW. A 20-minute breath test for helicobacter pylori. Am J Gastroenterol. 1991;86:438-445. [PubMed] |
| 33. | Riepl RL, Folwaczny C, Otto B, Klauser A, Blendinger C, Wiebecke B, König A, Lehnert P, Heldwein W. Accuracy of 13C-urea breath test in clinical use for diagnosis of Helicobacter pylori infection. Z Gastroenterol. 2000;38:13-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 34. | Leal YA, Flores LL, Fuentes-Pananá EM, Cedillo-Rivera R, Torres J. 13C-urea breath test for the diagnosis of Helicobacter pylori infection in children: a systematic review and meta-analysis. Helicobacter. 2011;16:327-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 35. | Andersen LP, Kiilerick S, Pedersen G, Thoreson AC, Jørgensen F, Rath J, Larsen NE, Børup O, Krogfelt K, Scheibel J. An analysis of seven different methods to diagnose Helicobacter pylori infections. Scand J Gastroenterol. 1998;33:24-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 36. | Morris A, Ali MR, Brown P, Lane M, Patton K. Campylobacter pylori infection in biopsy specimens of gastric antrum: laboratory diagnosis and estimation of sampling error. J Clin Pathol. 1989;42:727-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 76] [Article Influence: 2.1] [Reference Citation Analysis (0)] |