Published online Jan 28, 2015. doi: 10.3748/wjg.v21.i4.1299
Peer-review started: June 12, 2014
First decision: June 27, 2014
Revised: July 23, 2014
Accepted: November 7, 2014
Article in press: November 11, 2014
Published online: January 28, 2015
Processing time: 230 Days and 22.4 Hours
AIM: To investigate the impact of enteral nutrition (EN) on the body composition and metabolism in patients with Crohn’s disease (CD).
METHODS: Sixty-one patients diagnosed with CD were enrolled in this study. They were given only EN (enteral nutritional suspension, TPF, non-elemental diet) support for 4 wk, without any treatment with corticosteroids, immunosuppressive drugs, infliximab or by surgical operation. Body composition statistics such as weight, body mass index, skeletal muscle mass (SMM), fat mass, protein mass and inflammation indexes such as C-reactive protein (CRP), erythrocyte sedimentation rate (ESR) and CD activity index (CDAI) were recorded before and after EN support.
RESULTS: The 61 patients were divided into three groups according to CDAI before and after EN support: A (active phase into remission via EN, n = 21), B (remained in active phase before and after EN, n = 19) and C (in remission before and after EN, n = 21). Patients in group A had a significant increase in SMM (22.11 ± 4.77 kg vs 23.23 ± 4.49 kg, P = 0.044), protein mass (8.01 ± 1.57 kg vs 8.44 ± 1.45 kg, P = 0.019) and decrease in resting energy expenditure (REE) per kilogram (27.42 ± 5.01 kcal/kg per day vs 22.62 ± 5.45 kcal/kg per day, P < 0.05). There was no significant difference between predicted and measured REE in active CD patients according to the Harris-Benedict equation. There was no linear correlation between the measured REE and CRP, ESR or CDAI in active CD patients.
CONCLUSION: EN could decrease the hypermetabolism in active CD patients by reducing the inflammatory response.
Core tip: Unlike traditional research that uses normal volunteers or ulcerative colitis patients as the control group, this study aimed to observe the same patient in different phases of Crohn’s disease (CD), and in this study, several confounding factors, such as height, age, gender and race, were removed. This study showed that enteral nutrition could decrease the hypermetabolism in active CD patients by reducing the inflammatory response.
- Citation: Zhao J, Dong JN, Gong JF, Wang HG, Li Y, Zhang L, Zuo LG, Feng Y, Gu LL, Li N, Li JS, Zhu WM. Impact of enteral nutrition on energy metabolism in patients with Crohn’s disease. World J Gastroenterol 2015; 21(4): 1299-1304
- URL: https://www.wjgnet.com/1007-9327/full/v21/i4/1299.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i4.1299
Crohn’s disease (CD) is a chronic relapsing-remitting inflammatory bowel disease (IBD) of unknown etiology[1]. Nutritional deficits are very common in IBD, particularly in CD, which are attributed to many reasons including anorexia, active inflammation, and increased intestinal nutrient losses. Some researchers have indicated that approximately 65% to 75% of CD patients are malnourished[2]. Therefore, nutritional support can be important to medical therapy in the management of CD[3]. In Western countries, the role of enteral nutrition (EN) in CD is controversial[4,5]; however, many studies in Japan have shown that EN was effective not only in maintaining remission but also in inducing remission of CD. EN can also decrease the hospitalization rate in patients with CD[6]. In Japan, EN has been advocated as the primary therapy for both active and quiescent CD in accord with the guidelines of the Japan Ministry of Health, Labor and Welfare[7-10].
Many studies have found that the disease activity has a close relationship with body composition in CD patients. However, so far, no researchers can have definitively described the relationship between them. Some researchers have proposed that CD patients in active phase had significant deficits in lean mass but preserved fat mass compared with patients in remission[11-13]. However, others shown that fat mass decreased in active phase, to the same extent as muscle mass[14].
Resting energy expenditure (REE) was also closely related to disease activity. Nevertheless, some studies that investigated REE in CD have suggested that energy expenditure is raised, particularly in the active phase[15,16], while others have suggested that REE is unchanged. The inflammatory process associated with the active disease is more than capable of increasing REE above what would be expected[17]. The exact relationship between body composition, metabolism and disease activity in CD patients requires well-designed trials in large cohorts of patients. The impact of EN support on body composition and REE in CD patients is poorly understood in the therapeutic course.
The study protocol was approved by the Ethics Committee of Jinling Hospital, and informed consent was given to each patient.
This study was aimed at finding out the impact of EN on body composition and metabolism in CD patients.
This was a prospective, single-center study undertaken at the Jinling Hospital.
Inclusion criteria for patients were (1) age between 18 and 60 years; (2) endoscopic and histological diagnosis of CD; (3) no operations over the past six months; (4) can tolerate total EN; (5) no systemic diseases that greatly influence metabolism, such as diabetes mellitus and hyperthyroidism; (6) nutritional deficiencies; or (7) no severe symptoms (such as acute strangulated intestinal obstruction). Exclusion criteria were (1) cannot tolerate EN or malabsorption syndrome; (2) medication use such as corticosteroids; and (3) surgery. Sixty-one consecutive patients (43 males and 18 females; mean age, 33.4 years) who met the criteria were included.
All of the patients included were fasted and given only EN (enteral nutritional suspension, TPF, non-elemental diet) support for 4 wk, without any treatment with corticosteroids, immunosuppressive drugs, infliximab or by surgical operation. The patients were naso-gastrically fed TPF, a type of intact-protein nutrition (bottled preparations, net content of one 500 mL bottle: 20 g of protein, 19.5 g of fat, 61.5 g of carbohydrates; 1 mL of TPF provides 1 kcal of energy) as for the quantity of daily enteral formula designed by the measured REE before EN support calculated by indirect calorimetry. An appropriate amount of exercise was also required for the patients.
The clinical disease activity was determined as CD activity index (CDAI). The active phase was defined as CDAI ≥ 150 and remission as CDAI < 150. The included patients were divided into three groups according to CDAI before and after EN support; group A (active phase in remission via EN, n = 21), group B (remained in active phase before and after EN, n = 19) and group C (in remission before and after EN, n = 21). The data collected before and after EN support were the blood inflammation indexes [hs-C-reactive protein (CRP), erythrocyte sedimentation rate (ESR) and CDAI], the body composition [weight, body mass index (BMI), skeletal muscle mass, fat mass and protein mass] and REE measured by professional and technical personnel using indirect calorimetry. The measurement of REE requires adherence to strict conditions including environmental temperature, fasting, reclining supine for a 30-min period (in our study), and rest to obtain repeatable values (the temperature difference before and after EN was less than 0.3 °C, excluding the impact of temperature on the REE). Predicted REE was commonly calculated using the Harris-Benedict equation (variables: age, weight, height and gender), the Schofield equation (variables: age, weight and gender) or just from previous experimental data (some Chinese studies have declared that 25 kcal/kg per day can be used to conveniently evaluate the REE of Chinese people). The correlations between REE and inflammation markers were also evaluated by linear analysis.
The data gathered were analyzed using SPSS 19.0. Statistical analysis between groups was performed using paired-samples t-test and independent-samples t-test, and linear analysis was used to evaluate the relations between REE and markers of inflammation markers. Significance was set at P < 0.05.
This was a prospective study. In total 61 patients were evaluated in our cohort. The majority of the patients were men (43 out of 61, mean age 36.5 ± 12.5 years) and women accounted for the rest (mean age 42.3 ± 14.2 years). Among all of the patients in our cohort, 23 had ileal CD, 17 had ileo-colonic and 21 colonic. The 61 patients were divided into the following three groups according to the efficiency of EN support for 4 wk: A (active phase into remission via EN, n = 21), B (remained in the active phase via EN, n = 19) and C (in remission before and after EN, n = 21). Table 1 shows that the ileal involvement distribution and duration of the disease among the three groups showed no statistical difference (P > 0.05).
| Group A (n = 21) | Group B (n = 19) | Group C (n = 21) | P value | |
| Male (n) | 15 | 12 | 16 | |
| Female (n) | 6 | 7 | 5 | 0.83 |
| Age (yr) | 40.3 ± 12.6 | 39.6 ± 11.7 | 40.6 ± 14.0 | 0.97 |
| BMI (kg/m2) | 16.68 ± 2.21 | 17.96 ± 3.37 | 17.74 ± 2.90 | 0.31 |
| CD type (n) | ||||
| Ileal | 8 | 6 | 9 | |
| Ileo-colonic | 5 | 6 | 6 | |
| Colonic | 8 | 7 | 6 | 0.88 |
| Duration (yr) | 2.41 ± 1.01 | 2.28 ± 1.22 | 2.33 ± 1.25 | 0.94 |
Body composition data in each group before and after EN support are shown in Table 2. The BMI of all of the enrolled patients increased significantly (P = 0.017). In group A, the protein mass (P = 0.019) and skeletal muscle mass (P = 0.044) had a statistical increase after EN support that was not observed for fat mass (P = 0.263). The minerals of patients among three groups remained the same before and after EN (P > 0.05), while the level of 25(OH)D3 of patients in groups A and B had a significant increase (P < 0.05). Nevertheless, as indicated, no significant differences were found between the body composition before and after EN support in either group B or C (P > 0.05).
| Index | Group A (n = 21) | Group B (n = 19) | Group C (n = 21) | ||||||
| Before EN | After EN | P value | Before EN | After EN | P value | Before EN | After EN | P value | |
| BMI (kg/m2) | 16.68 ± 2.61 | 17.31 ± 2.55 | 0.013 | 17.96 ± 3.37 | 18.06 ± 2.61 | 0.92 | 17.74 ± 2.90 | 18.11 ± 2.76 | 0.67 |
| SMM (kg) | 22.11 ± 4.77 | 23.23 ± 4.49 | 0.044 | 21.62 ± 4.15 | 21.79 ± 4.36 | 0.90 | 22.29 ± 5.84 | 23.38 ± 4.52 | 0.50 |
| Fat mass (kg) | 4.80 ± 4.31 | 4.14 ± 3.35 | 0.260 | 8.53 ± 3.81 | 9.64 ± 3.92 | 0.38 | 8.06 ± 1.92 | 8.14 ± 1.75 | 0.89 |
| Protein (kg) | 8.01 ± 1.57 | 8.44 ± 1.45 | 0.019 | 7.87 ± 1.35 | 7.85 ± 1.47 | 0.96 | 7.21 ± 4.72 | 8.34 ± 4.66 | 0.44 |
| Minerals(kg) | 3.56 ± 2.12 | 3.61 ± 1.98 | 0.860 | 3.39 ± 2.50 | 3.31 ± 2.01 | 0.79 | 3.43 ± 3.10 | 3.49 ± 2.44 | 0.65 |
| 25(OH)D3 (ng/mL) | 10.8 ± 4.8 | 12.3 ± 4.1 | 0.014 | 10.6 ± 3.9 | 12.0 ± 4.2 | 0.02 | 11.7 ± 4.3 | 12.2 ± 5.0 | 0.10 |
In our cohort, the rate of EN induced remission of CD was 52.5% (group A/group A + B, 21/40). The objective of the study was to assess the inflammation indexes of patients in active phase before EN support (groups A and B). When the active groups, groups A and B, were analyzed, significant differences were consistently observed in CRP (P < 0.05), ESR (P < 0.05) and CDAI (P < 0.05) after EN support for 4 wk compared to the level before EN support, all of which are explicitly demonstrated in Table 3.
| Index | Group A (n = 21) | Group B (n = 19) | Active groups (A + B, n = 40) | ||||||
| Before EN | After EN | P value | Before EN | After EN | P value | Before EN | After EN | P value | |
| CRP (mg/L) | 27.17 ± 31.60 | 10.37 ± 14.42 | 0.019 | 28.75 ± 16.29 | 16.30 ± 14.70 | 0.031 | 28.09 ± 31.21 | 10.00 ± 14.56 | 0.024 |
| ESR (mm/h) | 29.14 ± 15.12 | 16.25 ± 12.41 | 0.020 | 27.96 ± 16.88 | 20.12 ± 14.01 | 0.042 | 28.65 ± 17.35 | 18.30 ± 18.73 | 0.030 |
| CDAI | 239.21 ± 52.60 | 126.10 ± 33.21 | 0.013 | 226.18 ± 60.24 | 188.02 ± 49.33 | 0.045 | 230.93 ± 61.69 | 174.32 ± 68.52 | 0.044 |
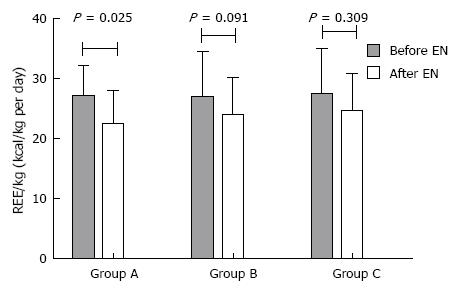
As shown in Figure 1, REE per kilogram in group A had a significant decrease via EN support (P = 0.025), different from groups B and C (P = 0.091 and 0.309, respectively).
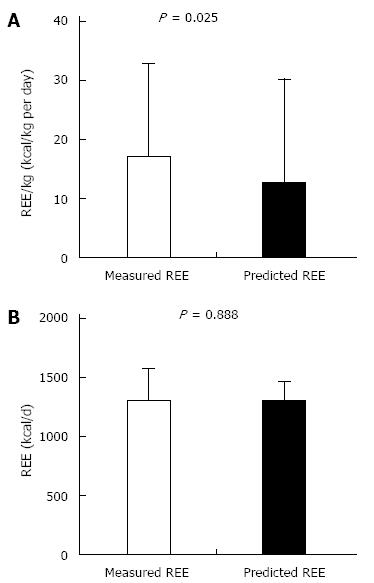
The results showed that the experimental 25 cal/kg would undoubtedly underestimate the REE of CD patients (Figure 2A; P = 0.025), but there were no significant differences between the actual REE and predicted REE using the Harris-Benedict equation (P = 0.888) (Figure 2B). In addition, no positive linear correlation between REE (baseline values) and CRP (r = -0.511, P = 0.21), ESR (r = -0.395, P = 0.085) or CDAI (r = 0.185, P = 0.435) was found, as we expected.
Some researchers have proposed that CD patients in the active phase have significant deficits in lean mass but preserved fat mass compared with patients in remission[11-13]. However, others believe that fat mass decreases in the active phase, along with muscle mass[14]. EN can improve the BMI, skeletal muscle and protein mass in active CD patients while inducing CD remission, most likely by correcting the state of negative nitrogen balance, increasing the storage and decreasing the expenditure of the muscle and protein mass. Increased REE further increases REE, most likely in response to the increase of organ mass which is more metabolically active than skeletal muscle[15,16].
Studies have shown that the measured REE in CD patients is significantly higher than that of healthy controls[17], though no linear correlation between the measured REE and CRP, ESR or CDAI has been observed. Predicting the REE with the experimental 25 cal/kg would underestimate REE of CD patients, which is shown in Figure 2A. No significant difference between the actual REE and REE predicted using the Harris-Benedict equation was found. The Harris-Benedict equation was suitable for patients in Western countries. Chinese studies have shown that predicting the REE by the Harris-Benedict equation was usually 20% to 30% higher than the measured REE in normal people. Therefore, the REE in active CD patients was much higher than that in normal people, as proved by many previous studies[18]. The REE predicted by the Harris-Benedict equation was roughly the same as the measured REE of active CD patients in this study with a restricted number of patients. The opinion that the REE likely decreases with increasing disease activity has been recently proposed[16]. Some studies have suggested that the increased REE in CD patients is related to the increased lean tissue instead of the hypermetabolism in CD patients[19,20]. However, this study demonstrated that for the same patient REE in the active phase of CD was significantly elevated compared with the REE in remission with an increase of lean tissue. The result proves that the state of hypermetabolism actually exists in active CD patients. Increased REE with unmatched dietary intake is amongst the many proposed mechanisms for the poor nutritional status of patients with CD. During nutritional therapy for CD patients, the REE variation with disease activity should be a primary consideration, but whether they had the accurate correlation still confused researchers. Some studies have examined the effect of disease activity on children with CD and have shown either no change in REE with disease activity[16,21,22] or increased REE at times of active disease[23].
Routine energy supplements for patients with active CD cannot be justified on the basis of predicted REE or just by experience. Individual management plans are essential and emphasis should be placed on the assessment of total energy needs (including the hypermetabolism and activity level) and titrating intake against weight gain to optimize energy balance and thereby promote body composition[24,25]. The IBD study group of the Japanese Ministry of Health, Labor and Welfare recommended that the total energy of TPN or EN should be 40-45 kcal/body weight/d in active CD patients. In contrast, European guidelines recommend that 25-30 kcal/body weight/d is optimal for active CD[26,27]. The energy metabolism status of active CD patients varied while they took EN in the remission induction therapy. Therefore, timing detection of the REE is recommended to appropriately assess the nutritional requirements of CD patients.
Nutritional deficits are very common in inflammatory bowel disease (IBD), particularly in Crohn’s disease (CD), in which nutritional deficits are attributed to many causes, including anorexia, active inflammation, and increased intestinal nutrient losses. Enteral nutrition (EN) was not only effective in maintaining remission but also in inducing remission of CD. EN has been advocated as a primary therapy for both active and quiescent CD. The impact of EN on body composition and metabolism in CD patients remains inconclusive when EN induced CD remission.
EN support plays an important role in the treatment of IBD, particularly CD. The quantity, time, type and appropriate use of EN for CD patients attracted much attention from IBD researchers.
Unlike traditional research that used normal volunteers or ulcerative colitis patients as the control group, this study aimed at observing the same patient in different phases of CD and eliminated several confounding factors, such as height, age, gender and race. The results showed that EN could decrease the hypermetabolism in active CD patients by reducing the inflammatory response.
This study provided new information about the proper quantity of EN for CD patients. To appropriately assess the nutritional requirements of CD patients, dynamic monitoring of resting energy expenditure (REE) is recommended.
Metabolism was measured by REE per kilogram. The inflammatory status of CD patients was evaluated with C-reactive protein, erythrocyte sedimentation rate and CD activity index.
It is an interesting study, although the number of the patients enrolled is small. I think this article is a good study about EN that is effective in remission induction of active CD and also has an impact on the body metabolism and composition of CD patients.
P- Reviewer: Green JT, Sinagra E, Yang CH S- Editor: Gou SX L- Editor: Wang TQ E- Editor: Ma S
| 1. | Yamamoto T, Nakahigashi M, Saniabadi AR, Iwata T, Maruyama Y, Umegae S, Matsumoto K. Impacts of long-term enteral nutrition on clinical and endoscopic disease activities and mucosal cytokines during remission in patients with Crohn’s disease: a prospective study. Inflamm Bowel Dis. 2007;13:1493-1501. [PubMed] |
| 2. | Vaisman N, Dotan I, Halack A, Niv E. Malabsorption is a major contributor to underweight in Crohn’s disease patients in remission. Nutrition. 2006;22:855-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 3. | Griffiths AM. Enteral nutrition in the management of Crohn’s disease. JPEN J Parenter Enteral Nutr. 2005;29:S108-S12; discussion S108-S112; discussion S112-7, S184-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Mackey AC, Green L, Liang LC, Dinndorf P, Avigan M. Hepatosplenic T cell lymphoma associated with infliximab use in young patients treated for inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2007;44:265-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 321] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 5. | Zachos M, Tondeur M, Griffiths AM. Enteral nutritional therapy for induction of remission in Crohn’s disease. Cochrane Database Syst Rev. 2007;CD000542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 145] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 6. | Watanabe O, Ando T, Ishiguro K, Takahashi H, Ishikawa D, Miyake N, Kato T, Hibi S, Mimura S, Nakamura M. Enteral nutrition decreases hospitalization rate in patients with Crohn’s disease. J Gastroenterol Hepatol. 2010;25 Suppl 1:S134-S137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Matsui T, Sakurai T, Yao T. Nutritional therapy for Crohn’s disease in Japan. J Gastroenterol. 2005;40 Suppl 16:25-31. [PubMed] |
| 8. | Gerasimidis K, Talwar D, Duncan A, Moyes P, Buchanan E, Hassan K, O’Reilly D, McGrogan P, Edwards CA. Impact of exclusive enteral nutrition on body composition and circulating micronutrients in plasma and erythrocytes of children with active Crohn’s disease. Inflamm Bowel Dis. 2012;18:1672-1681. [PubMed] |
| 9. | Takagi S, Utsunomiya K, Kuriyama S, Yokoyama H, Takahashi S, Iwabuchi M, Takahashi H, Takahashi S, Kinouchi Y, Hiwatashi N. Effectiveness of an ‘half elemental diet’ as maintenance therapy for Crohn’s disease: A randomized-controlled trial. Aliment Pharmacol Ther. 2006;24:1333-1340. [PubMed] |
| 10. | Yamamoto T, Nakahigashi M, Umegae S, Kitagawa T, Matsumoto K. Impact of long-term enteral nutrition on clinical and endoscopic recurrence after resection for Crohn’s disease: A prospective, non-randomized, parallel, controlled study. Aliment Pharmacol Ther. 2007;25:67-72. [PubMed] |
| 11. | Burnham JM, Shults J, Semeao E, Foster BJ, Zemel BS, Stallings VA, Leonard MB. Body-composition alterations consistent with cachexia in children and young adults with Crohn disease. Am J Clin Nutr. 2005;82:413-420. [PubMed] |
| 12. | Rocha R, Santana GO, Almeida N, Lyra AC. Analysis of fat and muscle mass in patients with inflammatory bowel disease during remission and active phase. Br J Nutr. 2009;101:676-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 13. | Wiskin AE, Wootton SA, Hunt TM, Cornelius VR, Afzal NA, Jackson AA, Beattie RM. Body composition in childhood inflammatory bowel disease. Clin Nutr. 2011;30:112-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Geerling BJ, Badart-Smook A, Stockbrügger RW, Brummer RJ. Comprehensive nutritional status in patients with long-standing Crohn disease currently in remission. Am J Clin Nutr. 1998;67:919-926. [PubMed] |
| 15. | Hill RJ, Cleghorn GJ, Withers GD, Lewindon PJ, Ee LC, Connor F, Davies PS. Resting energy expenditure in children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2007;45:342-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Wiskin AE, Wootton SA, Culliford DJ, Afzal NA, Jackson AA, Beattie RM. Impact of disease activity on resting energy expenditure in children with inflammatory bowel disease. Clin Nutr. 2009;28:652-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Zoli G, Katelaris PH, Garrow J, Gasbarrini G, Farthing MJ. Increased energy expenditure in growing adolescents with Crohn’s disease. Dig Dis Sci. 1996;41:1754-1759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Kushner RF, Schoeller DA. Resting and total energy expenditure in patients with inflammatory bowel disease. Am J Clin Nutr. 1991;53:161-165. [PubMed] |
| 19. | Al-Jaouni R, Hébuterne X, Pouget I, Rampal P. Energy metabolism and substrate oxidation in patients with Crohn’s disease. Nutrition. 2000;16:173-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 54] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Schneeweiss B, Lochs H, Zauner C, Fischer M, Wyatt J, Maier-Dobersberger T, Schneider B. Energy and substrate metabolism in patients with active Crohn’s disease. J Nutr. 1999;129:844-848. [PubMed] |
| 21. | Diamanti A, Basso MS, Gambarara M, Papadatou B, Bracci F, Noto C, Castro M. Positive impact of blocking tumor necrosis factor alpha on the nutritional status in pediatric Crohn’s disease patients. Int J Colorectal Dis. 2009;24:19-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Steiner SJ, Pfefferkorn MD, Fitzgerald JF, Denne SC. Protein and energy metabolism response to the initial dose of infliximab in children with Crohn’s disease. Inflamm Bowel Dis. 2007;13:737-744. [PubMed] |
| 23. | Varille V, Cézard JP, de Lagausie P, Bellaiche M, Tounian P, Besnard M, Faure C, Aigrain Y, Girardet JP, Navarro J. Resting energy expenditure before and after surgical resection of gut lesions in pediatric Crohn’s disease. J Pediatr Gastroenterol Nutr. 1996;23:13-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Hsu A, Heshka S, Janumala I, Song MY, Horlick M, Krasnow N, Gallagher D. Larger mass of high-metabolic-rate organs does not explain higher resting energy expenditure in children. Am J Clin Nutr. 2003;77:1506-1511. [PubMed] |
| 25. | Hart JW, Bremner AR, Wootton SA, Beattie RM. Measured versus predicted energy expenditure in children with inactive Crohn’s disease. Clin Nutr. 2005;24:1047-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Sasaki M, Johtatsu T, Kurihara M, Iwakawa H, Tanaka T, Tsujikawa T, Fujiyama Y, Andoh A. Energy metabolism in Japanese patients with Crohn’s disease. J Clin Biochem Nutr. 2010;46:68-72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |