Published online Jan 28, 2015. doi: 10.3748/wjg.v21.i4.1268
Peer-review started: May 4, 2014
First decision: June 27, 2014
Revised: July 22, 2014
Accepted: September 18, 2014
Article in press: September 19, 2014
Published online: January 28, 2015
Processing time: 272 Days and 0.9 Hours
AIM: To determine whether the endoscopic findings of depressed-type early gastric cancers (EGCs) could precisely predict the histological type.
METHODS: Ninety depressed-type EGCs in 72 patients were macroscopically and histologically identified. We evaluated the microvascular (MV) and mucosal surface (MS) patterns of depressed-type EGCs using magnifying endoscopy (ME) with narrow-band imaging (NBI) (NBI-ME) and ME enhanced by 1.5% acetic acid, respectively. First, depressed-type EGCs were classified according to MV pattern by NBI-ME. Subsequently, EGCs unclassified by MV pattern were classified according to MS pattern by enhanced ME (EME) images obtained from the same angle.
RESULTS: We classified the depressed-type EGCs into the following 2 MV patterns using NBI-ME: a fine-network pattern that indicated differentiated adenocarcinoma (25/25, 100%) and a corkscrew pattern that likely indicated undifferentiated adenocarcinoma (18/23, 78.3%). However, 42 of the 90 (46.7%) lesions could not be classified into MV patterns by NBI-ME. These unclassified lesions were then evaluated for MS patterns using EME, which classified 33 (81.0%) lesions as MS patterns, diagnosed as differentiated adenocarcinoma. As a result, 76 of the 90 (84.4%) lesions were matched with histological diagnoses using a combination of NBI-ME and EME.
CONCLUSION: A combination of NBI-ME and EME was useful in predicting the histological type of depressed-type EGC.
Core tip: Prediction of the histological diagnosis of early gastric cancer (EGC) using endoscopy is important for determining the appropriate therapeutic approach. In the present study, we combined magnifying endoscopy (ME) with narrow-band imaging (NBI) and enhanced ME (EME) to determine the associations between microvascular (MV) and mucosal surface (MS) patterns of depressed-type EGCs and the histological type. Indeed, 82 of the 90 lesions (91.1%) were classified according to MV or MS pattern, and 76 of the 90 lesions (84.4%) were diagnosed according to histological type. Therefore, our study suggested that the NBI-EME combination was useful for diagnosing the histological type in depressed-type EGC.
- Citation: Matsuo K, Takedatsu H, Mukasa M, Sumie H, Yoshida H, Watanabe Y, Akiba J, Nakahara K, Tsuruta O, Torimura T. Diagnosis of early gastric cancer using narrow band imaging and acetic acid. World J Gastroenterol 2015; 21(4): 1268-1274
- URL: https://www.wjgnet.com/1007-9327/full/v21/i4/1268.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i4.1268
Narrow-band imaging (NBI) is a video endoscopic imaging technique that enhances the display of microstructures and capillaries in the superficial mucosal layer, using narrow band filters that change the spectral feature of the observation light[1]. Microvascular (MV) patterns detected using standard magnifying endoscopy (ME) have been reported to be useful for the diagnosis of early gastric cancer (EGC)[2]. Furthermore, Nakayoshi et al[3] reported associations between images obtained by ME combined with NBI (NBI-ME) and histological findings. Their report concluded that the histological type of gastric cancer could be predicted using NBI-ME, which yielded clear images of the fine mucosal structure and the microvasculature of the gastric mucosa; however, endoscopic pathology by NBI-ME is not sufficient to replace conventional histology. Additional methods and techniques are necessary for predicting the histological type of EGC using endoscopy.
Enhanced-magnification endoscopy (EME) is a technique that combines ME with 1.5% acetic acid instillation. This technique was initially used to observe the specialized columnar epithelium of Barrett’s esophagus[4], and it was later adopted for the assessment of gastric neoplasms[5,6]. When the epithelial surfaces are sprayed with acetic acid, they transiently whiten because of a reversible alteration in the tertiary structure of cellular proteins[5]. EME allows for the visualization of the actual villi and cryptal areas, which appear similar when observed with a stereoscopic microscope. Several studies have demonstrated that EME was useful in the diagnosis of EGC[7] and the detection of gastric cancer margins[8-10]. In contrast, only a few studies have reported on the associations between EME findings and histological diagnosis of EGC[11,12]. Lee et al[12] reported that the accuracy of EME in diagnosing undifferentiated adenocarcinoma seemed unsatisfactory when compared with its accuracy in diagnosing differentiated adenocarcinoma.
Therefore, in the present study, we combined NBI-ME and EME (NBI-EME combination) to determine whether the MV and mucosal surface (MS) patterns of depressed-type EGCs could precisely predict the histological type.
The study group included 72 consecutive patients diagnosed with depressed-type EGC by four expert endoscopists at Kurume University Hospital between September 2007 and October 2011. Eighteen patients had two EGC lesions, and a total of 90 lesions were evaluated. Some of the clinical characteristics of the patients with EGC are summarized in Table 1. In all of the patients, the diagnosis of gastric cancer was based on the examination of biopsy specimens and was later confirmed by histopathology. The hospital ethics committee approved the study protocol, and all of the participating patients provided prior written informed consent.
| Characteristics | |
| No. of patients | 72 |
| Sex (M/F) | 47/25 |
| Tumors | 90 |
| Mean age | 64 (28-89) |
| Size (major axis) | 16.5 mm (5-52 mm) |
| Histological diagnosis | |
| Differentiated | 67 |
| Undifferentiated | 23 |
| Depth | |
| Mucosal | 84 |
| Submucosal | 6 |
All of the procedures were performed using a GIF-H260Z magnifying endoscope and a CV260SL/CLV260SL endoscopic system (Olympus Medical Systems Co., Tokyo, Japan). The GIF-H260Z instrument not only maintains the capabilities of a standard videoendoscope, but it also affords a continuous range of image magnification adjustment. A black hood (MB-46, Olympus Medical Systems Co., Tokyo, Japan) was attached to the tip of the endoscope to maintain the focal distance during the procedure. The same endoscopy system settings (image enhancement mode-B8; and color enhancement mode-1) were maintained for all of the methods. For EME, 20-30 mL of 1.5% acetic acid were sprinkled onto the lesion using a syringe at low pressure, through the endoscope accessory channel. When the gastric mucosa whitened transiently, enhancing the contrast of the surface patterns, EME images were obtained from the same angle used to obtain the NBI-ME images. The shape and regularity on EME images were classified according to the form of the mucosal surface, and the width of crypt was classified by comparison with normal crypt size. All of the observations were made on optimal foci and at the highest achievable magnification ratios. Four endoscopists performed the endoscopic procedures, using a digital filing system to record the images. For each lesion, endoscopic NBI-ME and EME images were evaluated for MV and MS patterns, respectively, by four expert endoscopists.
All of the EGC patients underwent endoscopic submucosal dissection (ESD) without any complications. The resected EGC specimens were then extended on boards with pins for fixation in 20% formalin. Each lesion, together with the surrounding mucosa, was cut into 2- to 5-mm-wide serial-step sections. The histologic criteria for diagnosing EGC were based on the Japanese classification of gastric carcinomas[13].
The NBI-ME/EME and histological findings were evaluated by Fisher’s exact test and Pearson’s χ2 test. All of the statistical tests were two sided with a significance level of 0.05. Statistical analysis was performed using JMP software (JMP, version 10.0; SAS Institute Inc., Cary, NC, United States).
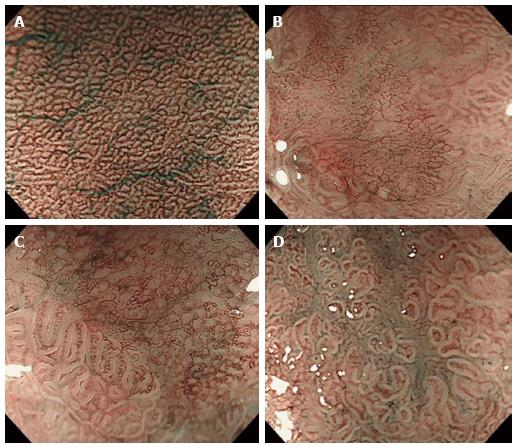
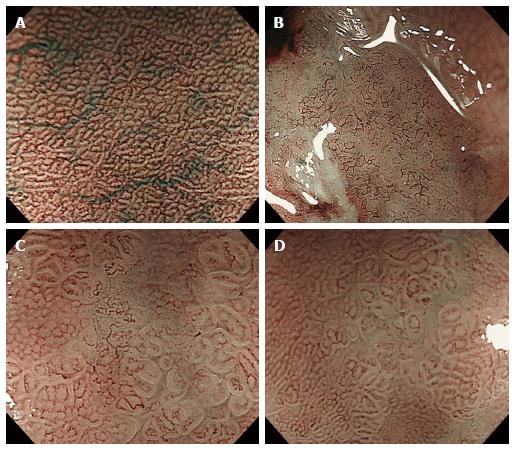
All of the depressed-type EGCs were macroscopically and histopathologically identified. The clinical characteristics of the patients enrolled in this study are summarized in Table 1. A total of 90 EGC lesions in 72 patients were analyzed. Histopathologically, 67 lesions (74.4%) were diagnosed as differentiated adenocarcinomas, and 23 lesions (25.6%) were undifferentiated adenocarcinomas. The depth of tumor invasion was mucosal in 93.3% (84/90) and submucosal in 6.7% (6/90). Previously reported[14] MV patterns include irregular, regular, and absent patterns (Figure 1). Table 2 shows that these regularity patterns were not associated with the differentiation of adenocarcinoma (P = 0.4174). Therefore, we used another previously reported[3] MV pattern classification for depressed-type EGCs in the present study: a fine-network pattern, a corkscrew pattern, and an unclassified pattern (Figure 2). Table 2 shows that the fine-network pattern indicated differentiated adenocarcinoma (25/25, 100%) and that the corkscrew pattern was likely to indicate undifferentiated adenocarcinoma (18/23, 78.3%). It was significantly useful for diagnosing the histological type (P < 0.001). However, 42 of the 90 (46.7%) lesions were not classifiable under any MV pattern by NBI-ME, and an unclassified pattern is incapable of predicting the histological type of depressed-type EGCs. Therefore, we considered it necessary to evaluate the unclassified MV patterns on NBI-ME images by additional methods.
| Differentiated | Undifferentiated | Total | P value | |
| #1 | ||||
| Irregular | 50 | 17 | 67 | 0.4174 |
| Regular | 8 | 1 | 9 | |
| Absent | 9 | 5 | 14 | |
| #2 | ||||
| Fine network | 25 | 0 | 25 | < 0.001 |
| Corkscrew | 5 | 18 | 23 | |
| Unclassified | 37 | 5 | 42 |
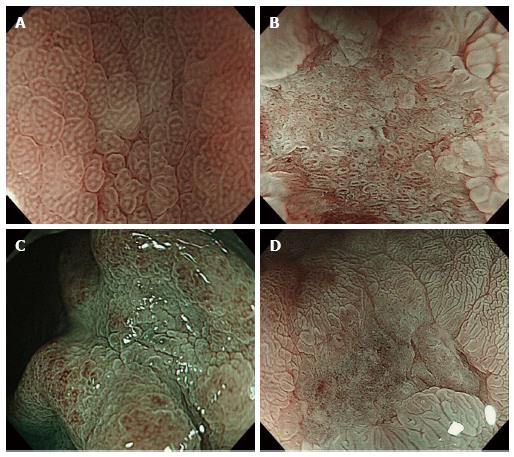
To evaluate the different characteristics of unclassified MV patterns (42 cases), we investigated the associations between MS patterns evaluated by ME and EME and the differentiation of adenocarcinoma according to the following three categories: shape (Figure 3); width of crypt; and regularity. Table 3 shows that sessile barnacle (4/4, 100%) and villous type (21/22, 95.5%) shapes, narrow (16/16, 100%) and wide (9/10, 90%) crypts, and irregular (19/19, 100%) and regular (6/7, 85.7%) patterns indicated differentiated adenocarcinoma. However, there were no associations between MS patterns evaluated by ME and histological type because several lesions remained unclassified (16/42, 38.1%) by ME. Next, we used EME to evaluate MS patterns because EME is useful for visualizing the mucosal surface structure. Table 4 shows that sessile barnacle (10/10, 100%) and villous type (23/24, 95.8%) shapes, narrow (29/29, 100%) and wide (4/5, 80%) crypts, and irregular (31/31, 100%) and regular (2/3, 66.7%) patterns indicated differentiated adenocarcinoma of EGC. Thirty-four of the 42 (78.6%) lesions unclassified by NBI-ME were classified for MS patterns by EME. Therefore, 82 of the 90 (91.1%) lesions were classified into either MV or MS patterns, and 76 of the 90 (84.4%) lesions were matched with histological diagnoses. In 59 lesions, including 25 lesions classified into fine-network patterns by NBI-ME (Table 2) and 34 lesions classified into MS patterns by EME (Table 4), 58 lesions (98.3%) were adequately diagnosed as differentiated adenocarcinoma. With regard to the diagnosis of undifferentiated adenocarcinoma, there were no associations with MS patterns diagnosed by EME, although the corkscrew pattern using NBI-ME was an exception (18/23, 78.3%). With regard to the diagnosis of undifferentiated adenocarcinoma, only 4 of the 8 lesions (50%) unclassified by NBI-EME combination demonstrated undifferentiated adenocarcinoma.
| Differentiated | Undifferentiated | Total | P value | |
| Shape | ||||
| Sessile barnacle | 4 | 0 | 4 | 0.1169 |
| Villous | 21 | 1 | 22 | |
| Unclassified | 12 | 4 | 16 | |
| Width of Crypt | ||||
| Narrow | 16 | 0 | 16 | 0.0901 |
| Wide | 9 | 1 | 10 | |
| Unclassified | 12 | 4 | 16 | |
| Regularity | ||||
| Irregular | 19 | 0 | 19 | 0.0735 |
| Regular | 6 | 1 | 7 | |
| Unclassified | 12 | 4 | 16 | |
| Differentiated | Undifferentiated | Total | P value | |
| Shape | ||||
| Sessile barnacle | 10 | 0 | 10 | 0.0010 |
| Villous | 23 | 1 | 24 | |
| Unclassified | 4 | 4 | 8 | |
| Width of Crypt | ||||
| Narrow | 29 | 0 | 29 | 0.0005 |
| Wide | 4 | 1 | 5 | |
| Unclassified | 4 | 4 | 8 | |
| Regularity | ||||
| Irregular | 31 | 0 | 31 | 0.0003 |
| Regular | 2 | 1 | 3 | |
| Unclassified | 4 | 4 | 8 | |
Recently, ESD treatment has been increasingly used to treat a subset of patients with EGC in Japan[15,16]. Accurate preoperative diagnosis, which includes the determination of the depth, spread of invasion, and histological findings, is critical for safe endoscopic therapy and for ensuring complete resection. Several endoscopic modalities have been developed to determine endoscopic pathology, resulting in a more accurate endoscopic diagnosis than with histological diagnosis. Early detection and accurate diagnosis of depressed-type gastric cancers have been effective in decreasing mortality because this morphological type is most predominant among all gastric cancers[17,18]. Moreover, the detection of EGCs measuring ≤ 20 mm diameter is ideal because they are curable with minimally invasive treatment, such as endoscopic mucosal resection and ESD[19]. However, the conventional white-light imaging endoscopic approach alone is inadequate for determining an accurate diagnosis. NBI-ME is more reliable for characterizing gastric cancers and for evaluating the area of EGCs[14,20]. MV patterns, detected using standard ME and NBI-ME, are reportedly capable of predicting the histological type of gastric cancer[2,3]. Several studies have demonstrated a fine-network pattern and a corkscrew pattern specific to differentiated and undifferentiated adenocarcinoma, respectively, using NBI-ME[3,21]. The present study also demonstrated a fine-network pattern indicating differentiated adenocarcinoma (25/25, 100%) and a corkscrew pattern indicating undifferentiated adenocarcinoma (18/23, 78.3%). However, Nakayoshi et al[3] reported that 39 of the 165 (23.6%) lesions were not classifiable according to MV patterns. Because 46.7% of lesions remained unclassified in the present study, it was necessary to evaluate the MV patterns unclassifiable by NBI-ME using additional methods.
Several studies have reported associations between MS patterns and histological type[5,22]. Standard ME demonstrated that the depressed-type EGC had a finer-pit pattern, characterized by the destruction or disappearance of the mucosal microstructure[2]. Otsuka et al[22] classified MS patterns of gastric cancers as evaluated by ME into the following three patterns: small and regular patterns of sulci and ridges; an irregular pattern of sulci and ridges; and a lack of visible structure. With regard to depressed-type EGCs, the latter two patterns were more frequently associated with undifferentiated adenocarcinoma (14/18), while the former pattern was more frequently associated with differentiated adenocarcinoma (22/45). However, ME was not sufficient for predicting the histological type, because 23 of 45 lesions were not adequately diagnosed by histological type. Furthermore, Tanaka et al[5] reported that the mucosal surface of gastric cancer, as evaluated by EME, could be characterized into five surface structure patterns: type I (small round pits of uniform size and shape), type II (slit-like pits), type III (a fine villous or gyrus pattern), type IV (irregular arrangements and size of pattern types I, II, and III), and type V (destructive pattern of type I, II, and III). Thirty lesions of depressed-type EGCs were characterized by two clearly recognizable surface patterns: type IV (70%, 21/30) and type V (30%, 9/30). 8 (88.9%) of the 9 lesions with type V patterns demonstrated undifferentiated adenocarcinoma. Although absent patterns of depressed-type EGCs were associated with undifferentiated adenocarcinoma, the diagnosis of differentiated adenocarcinoma remained unclear. In our study, 82 of the 90 (91.1%) lesions were classified into either MV or MS patterns. The decrease in unclassified lesions with the NBI-EME combination resulted in improvement in the diagnosis of differentiated adenocarcinoma. In fact, 76 of the 90 (84.4%) lesions were matched with histological diagnoses. The results of the present study suggested that the prediction of the histological type of depressed-type EGCs was more precise with the NBI-EME combination than with NBI-ME alone or EME alone.
Previous studies have reported the usefulness of NBI-ME and EME in predicting the histological type of EGC, although the diagnostic accuracy was low because many lesions remained unclassified in those studies. In the present study, most of the lesions were classified into MV and MS patterns using NBI-ME combined with EME, which showed increased diagnostic accuracy compared with that of NBI-ME alone or EME alone. Therefore, combination methods, e.g., NBI-ME followed by EME, are more useful in identifying the histological type of depressed-type EGCs. Increased accuracy of the histological diagnosis of depressed-type EGCs using endoscopy is necessary for determining an appropriate therapeutic approach during the early phase of the disease.
The prediction of the histological diagnosis of early gastric cancer (EGC) using endoscopy is important for determining the appropriate therapeutic approach. However, an endoscopic technique for the histological diagnosis of EGC has not been completely established. In the present study, we combined magnifying endoscopy (ME) with narrow-band imaging (NBI) (NBI-ME) and enhanced ME (EME) to determine whether the microvascular (MV) and mucosal surface (MS) patterns of depressed-type EGCs could precisely predict the histological type.
Previous studies reported associations between images obtained by NBI-ME and histological findings. This report concluded that the histological type of gastric cancer could be predicted using NBI-ME; however, endoscopic pathology by NBI-ME was insufficient to replace conventional histology. Furthermore, only a few studies have reported on the associations between EME findings and histological diagnosis of EGC. Research attention to this area could help to establish methods and techniques for predicting the histological type of EGC using endoscopy.
Previous studies have reported the usefulness of NBI-ME and EME in predicting the histological type of EGC, although the diagnostic accuracy was low because many lesions remained unclassified in those studies. In the present study, most of the lesions were classified into MV and MS patterns using NBI-ME combined with EME, which showed increased diagnostic accuracy compared to that of NBI-ME alone or EME alone. Therefore, combination methods, e.g., NBI-ME followed by EME, were more useful in identifying the histological type of depressed-type EGC.
The study results suggested that the increased accuracy of histological diagnosis of depressed-type EGCs using the combination of NBI-ME and EME was necessary to determine the appropriate therapeutic approach during the early phases of gastric cancer.
NBI is a video endoscopic imaging technique that enhances the display of the microstructures and capillaries in the superficial mucosal layer using narrow band filters that change the spectral features of the observation light. EME is a technique that combines magnification endoscopy with 1.5% acetic acid instillation. EME allows for the visualization of the actual villi and cryptal areas, which appear similar when observed with a stereoscopic microscope.
Matsuo et al presented an interesting paper concerning the combination of NBI-ME and EME in the diagnosis of early gastric cancer. In fact, as we can see in previous reports, either of the methods could be applied to predict the histological type of lesion; however, both of them lacked sufficient accuracy when the samples were finally assessed by histological methods. In the current study, the authors attempted to increase the accuracy further by combining both of the above methods, and the results indicated that 84.4% of lesions were finally matched with histological diagnoses.
P- Reviewer: Albuquerque A, Fan XM, Gresta LT, Kato M, Wei PK S- Editor: Qi Y L- Editor: A E- Editor: Zhang DN
| 1. | Dinis-Ribeiro M, da Costa-Pereira A, Lopes C, Lara-Santos L, Guilherme M, Moreira-Dias L, Lomba-Viana H, Ribeiro A, Santos C, Soares J. Magnification chromoendoscopy for the diagnosis of gastric intestinal metaplasia and dysplasia. Gastrointest Endosc. 2003;57:498-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 110] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 2. | Tajiri H, Doi T, Endo H, Nishina T, Terao T, Hyodo I, Matsuda K, Yagi K. Routine endoscopy using a magnifying endoscope for gastric cancer diagnosis. Endoscopy. 2002;34:772-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 84] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 3. | Nakayoshi T, Tajiri H, Matsuda K, Kaise M, Ikegami M, Sasaki H. Magnifying endoscopy combined with narrow band imaging system for early gastric cancer: correlation of vascular pattern with histopathology (including video). Endoscopy. 2004;36:1080-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 335] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 4. | Guelrud M, Herrera I. Acetic acid improves identification of remnant islands of Barrett’s epithelium after endoscopic therapy. Gastrointest Endosc. 1998;47:512-515. [PubMed] |
| 5. | Tanaka K, Toyoda H, Kadowaki S, Kosaka R, Shiraishi T, Imoto I, Shiku H, Adachi Y. Features of early gastric cancer and gastric adenoma by enhanced-magnification endoscopy. J Gastroenterol. 2006;41:332-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Yagi K, Aruga Y, Nakamura A, Sekine A, Umezu H. The study of dynamic chemical magnifying endoscopy in gastric neoplasia. Gastrointest Endosc. 2005;62:963-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Tao G, Xing-Hua L, Ai-Ming Y, Wei-Xun Z, Fang Y, Xi W, Li-Yin W, Chong-Mei L, Gui-Jun F, Hui-Jun S. Enhanced magnifying endoscopy for differential diagnosis of superficial gastric lesions identified with white-light endoscopy. Gastric Cancer. 2014;17:122-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Iizuka T, Kikuchi D, Hoteya S, Yahagi N. The acetic acid + indigocarmine method in the delineation of gastric cancer. J Gastroenterol Hepatol. 2008;23:1358-1361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Sakai Y, Eto R, Kasanuki J, Kondo F, Kato K, Arai M, Suzuki T, Kobayashi M, Matsumura T, Bekku D. Chromoendoscopy with indigo carmine dye added to acetic acid in the diagnosis of gastric neoplasia: a prospective comparative study. Gastrointest Endosc. 2008;68:635-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 10. | Yamada S, Doyama H, Yao K, Uedo N, Ezoe Y, Oda I, Kaneko K, Kawahara Y, Yokoi C, Sugiura Y. An efficient diagnostic strategy for small, depressed early gastric cancer with magnifying narrow-band imaging: a post-hoc analysis of a prospective randomized controlled trial. Gastrointest Endosc. 2014;79:55-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 11. | Eleftheriadis N, Inoue H, Ikeda H, Onimaru M, Yoshida A, Maselli R, Santi G, Kudo SE. Acetic acid spray enhances accuracy of narrow-band imaging magnifying endoscopy for endoscopic tissue characterization of early gastric cancer. Gastrointest Endosc. 2014;79:712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Lee BE, Kim GH, Park do Y, Kim DH, Jeon TY, Park SB, You HS, Ryu DY, Kim DU, Song GA. Acetic acid-indigo carmine chromoendoscopy for delineating early gastric cancers: its usefulness according to histological type. BMC Gastroenterol. 2010;10:97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 13. | Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2390] [Cited by in RCA: 2873] [Article Influence: 205.2] [Reference Citation Analysis (0)] |
| 14. | Yao K, Anagnostopoulos GK, Ragunath K. Magnifying endoscopy for diagnosing and delineating early gastric cancer. Endoscopy. 2009;41:462-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 337] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 15. | Kim JJ, Lee JH, Jung HY, Lee GH, Cho JY, Ryu CB, Chun HJ, Park JJ, Lee WS, Kim HS. EMR for early gastric cancer in Korea: a multicenter retrospective study. Gastrointest Endosc. 2007;66:693-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 97] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 16. | Min BH, Lee JH, Kim JJ, Shim SG, Chang DK, Kim YH, Rhee PL, Kim KM, Park CK, Rhee JC. Clinical outcomes of endoscopic submucosal dissection (ESD) for treating early gastric cancer: comparison with endoscopic mucosal resection after circumferential precutting (EMR-P). Dig Liver Dis. 2009;41:201-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 17. | Hirasawa T, Gotoda T, Miyata S, Kato Y, Shimoda T, Taniguchi H, Fujisaki J, Sano T, Yamaguchi T. Incidence of lymph node metastasis and the feasibility of endoscopic resection for undifferentiated-type early gastric cancer. Gastric Cancer. 2009;12:148-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 368] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 19. | Ono H, Kondo H, Gotoda T, Shirao K, Yamaguchi H, Saito D, Hosokawa K, Shimoda T, Yoshida S. Endoscopic mucosal resection for treatment of early gastric cancer. Gut. 2001;48:225-229. [PubMed] |
| 20. | Nonaka K, Namoto M, Kitada H, Shimizu M, Ochiai Y, Togawa O, Nakao M, Nishimura M, Ishikawa K, Arai S. Usefulness of the DL in ME with NBI for determining the expanded area of early-stage differentiated gastric carcinoma. World J Gastrointest Endosc. 2012;4:362-367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Yokoyama A, Inoue H, Minami H, Wada Y, Sato Y, Satodate H, Hamatani S, Kudo SE. Novel narrow-band imaging magnifying endoscopic classification for early gastric cancer. Dig Liver Dis. 2010;42:704-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 22. | Otsuka Y, Niwa Y, Ohmiya N, Ando N, Ohashi A, Hirooka Y, Goto H. Usefulness of magnifying endoscopy in the diagnosis of early gastric cancer. Endoscopy. 2004;36:165-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 72] [Article Influence: 3.4] [Reference Citation Analysis (0)] |