Published online Jan 28, 2015. doi: 10.3748/wjg.v21.i4.1099
Peer-review started: May 27, 2014
First decision: June 27, 2014
Revised: July 11, 2014
Accepted: September 5, 2014
Article in press: September 5, 2014
Published online: January 28, 2015
Processing time: 245 Days and 16.3 Hours
Fatty liver disease (FLD) is a growing public health problem worldwide. There is an urgent requirement for alternative and natural medicine to treat this disease. As phytochemicals, isoflavones have attracted considerable attention for the prevention of FLD. Numerous studies have revealed that isoflavones protect against FLD through various pathways which modulate fatty acid β-oxidation, lipid synthesis, and oxidative stress. Recently, the aldose reductase (AR)/polyol pathway has been reported to be involved in the development of FLD by modulating hepatic fructose production, peroxisome proliferator-activated receptor (PPAR)α activity, cytochrome P450 (CYP)2E1 expression, and gut bacterial endotoxin-induced cytokine release. It has been reported that some isoflavones are potent AR inhibitors. Here, we review the anti-FLD actions of isoflavones and the proposed mechanism whereby isoflavones protect against FLD, with regard to the AR/polyol pathway. We propose that isoflavones block the AR/polyol pathway and in turn reduce fructose production and subsequent fat accumulation in the liver in diabetic or high-glucose-diet mice. In addition, in rodents with alcoholic liver disease or nonalcoholic fatty liver disease/nonalcoholic steatohepatitis, inhibition of AR by isoflavones may improve PPARα-mediated fatty acid oxidation, reduce hepatic steatosis, and attenuate CYP2E1-mediated oxidative stress or AR/gut bacterial endotoxin-mediated cytokine overproduction, to alleviate progression of FLD.
Core tip: The aldose reductase (AR)/polyol pathway has recently been reported to be involved in the development of fatty liver disease (FLD) via various pathways. Some isoflavones have been reported to be potent AR inhibitors. Here, we review the anti-FLD actions of isoflavones and the proposed mechanism whereby isoflavones protect against FLD, with regard to the AR/polyol pathway. We propose that isoflavones block the AR/polyol pathway to suppress fructose production in the liver, improve peroxisome-proliferator-activated-receptor-α-mediated fatty acid oxidation, ameliorate cytochrome-P450-2E1-mediated oxidative stress, and attenuate AR/gut bacterial endotoxin-mediated cytokine overproduction, which in turn alleviates the progression of FLD.
- Citation: Qiu LX, Chen T. Novel insights into the mechanisms whereby isoflavones protect against fatty liver disease. World J Gastroenterol 2015; 21(4): 1099-1107
- URL: https://www.wjgnet.com/1007-9327/full/v21/i4/1099.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i4.1099
Fatty liver disease (FLD) is a condition where neutral fat accumulates in liver cells, and may be accompanied by progressive inflammation of the liver. In light of the contribution of alcohol, fatty liver may be termed alcoholic liver disease (ALD) or non-alcoholic fatty liver disease (NAFLD), and the more severe forms of NAFLD as non-alcoholic steatohepatitis (NASH). It is difficult to distinguish ALD from NAFLD histologically. The histological spectrum of ALD includes steatosis, hepatitis and fibrosis, and NAFLD can mimic the entire spectrum of hepatic changes in ALD.
FLD is a growing public health problem worldwide. The prevalence of NAFLD is approximately 30% in developed countries and nearly 10% in developing nations[1]. FLD is increasingly recognized as an important cause of end-stage liver disease[2]. Current treatments for FLD focus on the factors that may cause the disease. In general, these treatments include weight loss, cholesterol management, blood glucose control, or treatment of alcoholism. Although several pharmacological agents for the prevention of FLD have been investigated, they have been found to be effective, but have side effects[3]. Thus, there is an urgent requirement for alternative and natural medicine to treat this disease. Isoflavones are phytochemicals and have been reported to prevent FLD in numerous studies through the regulation of peroxisome proliferator-activated receptors (PPARs), carbohydrate responsive element binding protein and Wnt signaling, to regulate fatty acid β-oxidation, lipid synthesis and oxidative stress[4]. Recently, the aldose reductase (AR)/polyol pathway has been reported to be involved in the development of FLD[5-7]. Of note, isoflavones such as genistein, daidzein and puerarin have been recognized as AR inhibitors[8,9]. However, only a few studies have investigated the effect of isoflavones on FLD by inhibition of AR. Thus, this article reviews the biological effects of isoflavones on FLD, and the mechanisms whereby isoflavones protect against ALD and NAFLD/NASH, with regard to the AR/polyol pathway.
The causes of FLD are alcoholism, toxins, inherited metabolic disorders, and certain drugs. Almost all heavy drinkers develop fatty liver. NAFLD has been consistently associated with insulin resistance and the metabolic syndrome (obesity, diabetes mellitus, hypertension, and dyslipidemia)[10]. Although many investigations have been carried out to elucidate the mechanisms of ALD development, the pathogenesis of ALD is still not fully understood. It is generally accepted that increased release of proinflammatory cytokines, induced oxidative stress, and elevated gut bacterial endotoxins play important roles in the development of ALD[11,12]. In contrast, the underlying cause of NAFLD/NASH is still not clear. However, there are several factors, which may be involved including insulin resistance[13,14], toxic inflammatory cytokines[15], oxidative stress inside liver cells[14,16], gut microbiota[17], endoplasmic reticulum stress[18], and genetics[19]. Day et al[20] proposed the hypothesis of “two hits” to clarify the mechanisms underlying the progression from steatosis to steatohepatitis. The first hit is insulin resistance, which causes hepatic steatosis and excess fatty acids. The second hit is oxidative stress and associated lipid peroxidation and cytokines within the liver, which may initiate progression from steatosis to steatohepatitis and ultimately to cirrhosis. Recently, Basaranoglu et al[21] suggested that possible candidates for the second hit included increased oxidative stress, lipid peroxidation and release of toxic products, decreased antioxidants, adipocytokines, transforming growth factor-β, Fas ligand, mitochondrial dysfunction, fatty acid oxidation by cytochrome P450s, peroxisomes, excess iron, small intestinal bacterial overgrowth, and the generation of gut-derived toxins such as lipopolysaccharide and ethanol. In addition to the two-hit hypothesis, a “multiple parallel hits” hypothesis was recently proposed by Tilg et al[18] to clarify the mechanisms underlying the development of liver inflammation. Many parallel hits derived from the gut and/or the adipose tissue may promote liver inflammation, such as endoplasmic reticulum stress, adipocytokines, and innate immunity.
Isoflavones are phytochemicals found in various legumes including soybean, kudzu, red clover, fava beans, alfalfa, chickpeas and peanuts. Numerous reports indicate that the consumption of isoflavones has many health benefits, including protection against menopausal symptoms, osteoporosis, cardiovascular disease, atherosclerosis, hyperlipidemia, and cancer[22,23].
Recent studies have demonstrated that isoflavones can protect against ALD or NAFLD (Table 1). The most studied isoflavones are soy isoflavones, including genistein and daidzein. Among the soy isoflavones, genistein is the most beneficial and protects against both ALD and NAFLD/NASH in rodents[24-33]. In addition to soy isoflavones, kudzu isoflavones and their main bioactive component, puerarin, have received considerable attention due to their beneficial effect on ALD and NAFLD/NASH[34-39]. Red clover isoflavones have also attracted attention. We reported that red clover isoflavones can improve hepatic steatosis in db/db obese diabetic mice[40] and methionine-choline-deficient (MCD) diet-induced NASH mice[41]. However, we did not find that red clover isoflavones alleviated liver inflammation in MCD-diet-induced NASH mice. Surprisingly, formononetin, one of the major isoflavones in red clover, is reported to induce hepatic steatosis and decrease markers of inflammation and liver injury in mice fed a cholesterol-enriched diet[42]. There are few data on the effect of biochanin A, the other major isoflavone in red clover, on FLD. It is known that biochanin A can protect against CCl4-induced liver fibrosis[43]. Therefore, the effect of biochanan A and formononetin on FLD cannot be concluded from the present studies and requires further investigation.
| Experimental model | Treatment | Effects | Ref. |
| Mice fed high-fat diet | Genistein | Alleviates NAFLD by stimulating hepatic fatty acid β-oxidation and increasing antioxidative enzyme | Lee et al[24] |
| Rats fed high-fat diet | Genistein | Prevents emergence of NASH by attenuating oxidative stress | Yalniz et al[25] |
| Rats fed MCD diet | Soy isoflavone | Prevents liver damage by decreasing lipid peroxidation in NASH model | Ustundag et al[26] |
| Rats fed high-fructose diet | Genistein | Reduces NAFLD via activation of antioxidant profiles and decreases IL-6 and TNF-α | Mohamed Salih et al[27] |
| Mice fed high-fat diet | Genistein | Reduces NAFLD by regulating adipocyte fatty acid β-oxidation and adipogenesis | Kim et al[28] |
| Rats fed high-fat diet | Genistein | Slows down NASH progression by inhibiting IκB-α phosphorylation, nuclear translocation of NF-κB p65 subunit, and activation of JNK | Ji et al[29] |
| Rats provided with ethanol | Genistein | Ameliorates alcoholic liver injury and liver fibrosis by reducing lipid peroxidation, recruiting the anti-oxidative defense system, inhibiting CYP2El activity, and promoting extracellular matrix degradation | Huang et al[30] |
| ApoE-/- mice fed high-fat diet | Genistein | Alleviates metabolic abnormalities including hypercholesterolemia and NASH in ApoE-/- mice | Kwon et al[31] |
| Mice fed high-fat diet | Daidzein | Prevents NAFLD through the direct regulation of hepatic de novo lipogenesis and insulin signaling, and the indirect control of adiposity and adipocytokines | Kim et al[32] |
| Rats fed high-fat diet | Daidzein | Reduces weight gain and fat content in liver by affecting PPARα/γ and stearoyl coenzyme A desaturase 1 | Crespillo et al[33] |
| Rats fed high-fat diet | Puerarin | Reduces NAFLD via hepatic leptin signaling activation (leptin receptor/JAK2/STAT3) | Zheng et al[34] |
| Rats provided with ethanol | Puerarin | Prevents acute alcoholic liver injury by inhibiting oxidative stress | Zhao et al[35] |
| Mice provided with ethanol | Tectoridin | Protects against ethanol-induced liver steatosis by modulating disturbance of PPARα pathway and ameliorating mitochondrial function | Xiong et al[36] |
| Hepatocytes treated with ethanol | Puerarin | Restores viability of cells and reduces lipid accumulation in ethanol-treated hepatocytes by activating autophagy via AMPK/mTOR-mediated signaling | Noh et al[37] |
| Mice fed high-fat diet | Puerariae flower extract (isoflavone-rich) | Exerts anti-fatty liver effects by suppressing lipogenesis in the liver | Kamiya et al[38] |
| Rats provided with the Liber-DeCarli liquid diet | Puerarin | Alleviates chronic alcoholic liver injury by inhibiting endotoxin gut leakage, Kupffer cell activation, and endotoxin receptors expression | Peng et al[39] |
| db/db diabetic mice | Red clover extract (isoflavone-rich) | Reduces liver TG and cholesterol levels by activating hepatic PPARα and inhibiting hepatic fatty acid synthase | Qiu et al[40] |
| Mice fed cholesterol-enriched diet | 2-heptyl-formononetin, formononetin | Induces hepatic steatosis, but decreases markers of inflammation and liver injury | Andersen et al[42] |
| Mice fed MCD diet | Red clover extract (isoflavone-rich) | Improves hepatic steatosis, but does not alleviate liver inflammation | Qiu et al[41] |
The polyol pathway is a glucose metabolic shunt that is defined by two enzymatic reactions catalyzed respectively by AR (EC1.1.1.21) and sorbitol dehydrogenase (SDH, EC1.1.1.14). AR catalyzes the rate-limiting reduction of glucose to sorbitol with the aid of co-factor NADPH and then SDH converts sorbitol to fructose using NAD+[44].
It is well documented that the AR/polyol pathway is involved in the development of diabetes complications[45,46]. Elevated AR can lead to serious diseases affecting the heart and blood vessels, eyes, kidneys and nerves. AR is induced in diseased liver, other than in the above mentioned tissues that are vulnerable to complications of diabetes. AR was detected in the livers of two human subjects with ALD, but was undetected in healthy humans[47]. Moreover, AR is induced in human livers obtained from patients undergoing liver transplantation for fulminant (acute) liver failure or end-stage liver disease from cirrhosis due to various chronic liver diseases, including ALD, chronic hepatitis B and C, primary biliary cirrhosis, autoimmune hepatitis, and hepatocellular carcinoma[48]. These studies indicate that AR may play an important role in the development of liver injuries. Recently, investigations were conducted to elucidate the role of AR in the development of FLD. Lanaspa et al[5] demonstrated that genetic ablation of the AR gene resolved high-glucose-diet-induced hepatic steatosis in mice. We previously demonstrated that inhibition of AR ameliorated hepatic steatosis in db/db diabetic mice[6], and lentivirus-mediated knockdown of the AR gene alleviated MCD-diet-induced NASH in db/db mice[7]. These studies confirm the involvement of AR in the development of FLD.
Numerous reports show that isoflavones have significant AR inhibitory activity. Park et al[9] reported that genistein, daidzein and puerarin inhibited AR in rat lens with IC50 values of 4.5, 7.9 and 44.7 μmol/L, respectively, whereas formononetin exhibited weak AR inhibitor activity (IC50 > 100 μmol/L). Hsieh et al[49] reported that genistein inhibited AR in rat lens with an IC50 of 16.9 μmol/L, while Choi et al[50] reported that genistein inhibited AR in pig lens with an IC50 of 20 μmol/L. Moreover, tectoridin has also exhibited potent activity, with an IC50 value of 1.08 μmol/L[51]. Furthermore, biochanin A shows better binding interactions with AR than epalrestat, a synthetic AR-specific inhibitor, which indicates that biochanin A possesses significant AR inhibitory activity[52].
Recently, Lanaspa et al[5] found that mice deficient in AR were protected against fatty liver after exposure to 10% glucose for 14 wk. They demonstrated that the metabolic conversion of glucose to endogenous fructose by the AR/polyol pathway in the liver is a key step in the development of glucose-induced fatty liver in mice. In addition, we demonstrated that inhibition of AR ameliorates hepatic steatosis in db/db diabetic mice[6]. Therefore, we postulate that isoflavones can block the AR/polyol pathway and subsequently reduce fructose production in the liver and alleviate fatty liver in humans and animals with high glucose diet or in diabetic conditions. Indeed, genistein, daidzein and red clover isoflavones improve hepatic steatosis and dyslipidemia in diabetic mice, although their mechanisms of action are reported to be through different pathways[40,53].
It is well established that PPARα, a nuclear receptor, is a central regulator for hepatic lipid catabolism[54]. It regulates the enzymes involved in fatty acid oxidation, for example, acyl-CoA oxidase (ACO), carnitine palmitoyl transferase (CPT)-1, and liver fatty acid binding protein. The ablation of PPARα gene causes the development of FLD[55,56]. Administration of PPARα agonists improves MCD-diet-induced NASH[57,58] and ethanol-induced liver injury[59]. Genistein, daidzein, biochanin A and formononetin are well known PPARα agonists[60,61]. Genistein and daidzein alleviate NAFLD in animals fed a high-fat diet by stimulating the hepatocyte and adipocyte PPARα pathway and fatty acid β-oxidation[24,28,33]. Red clover isoflavones also reduce liver triglycerides and cholesterol levels in db/db mice by activating hepatic PPARα[40]. These studies indicate that some isoflavones may act as PPARα agonists to prevent FLD.
Overexpression of AR in hepatocytes stimulates extracellular signal-regulated kinase (ERK)1/2 activation, sequentially phosphorylates hepatic PPARα at the OH group of serine 12 and 21, and reduces mRNA expression of ACO and CPT-1, two target genes transcriptionally regulated by PPARα[62]. This study indicates that AR overexpression in hepatocytes inhibits lipid degradation by suppressing PPARα activity. In diabetic db/db mice with hepatic steatosis, elevated hepatic AR also stimulates ERK1/2 activation and phosphorylates PPARα and suppresses its activity. The AR inhibitor, zopolrestat, attenuates the phosphorylation of PPARα and the suppression of PPARα activity, which improves hepatic steatosis in db/db mice[6]. These studies indicate that AR inhibitors may improve hepatic steatosis by modulating the phosphorylation of PPARα and its transcriptional activity. Indeed, genistein can reduce the level of phosphorylated PPARα and increase the mRNA expression of ACO in high-glucose-treated HepG2 cells (unreported data). Mezei et al[63] demonstrated that soy isoflavones modulate lipid metabolism in part via a PPARα-dependent mechanism in mice fed a high-fat diet. CPT-1 mRNA is consistently found to be induced by soy isoflavones in obese Zucker rats[64] and ACO mRNA is induced by soy isoflavones in Agouti (A(vy)/a) mice fed an AIN-93G diet to alleviate hepatic steatosis[65]. These studies suggest that isoflavones may improve FLD, at least in part, via the regulation of AR/PPARα mediated fatty acid oxidation.
Oxidative stress within the liver may act as the second hit and initiate the progression from steatosis alone to steatohepatitis and ultimately to cirrhosis[16,20]. Isoflavones as antioxidants have been well documented[66,67]. Genistein is reported to activate antioxidative enzymes and attenuate oxidative stress in animals fed a high-fat diet, thus alleviating NAFLD and preventing the emergence of NASH[24,25]. Puerarin is also reported to prevent acute alcoholic liver injury by inhibiting oxidative stress[35].
There is accumulating evidence that cytochrome P450 (CYP)2E1 plays an important role in the pathogenesis of liver tissue injury[68]. Upregulation of CYP2E1 may initiate lipid peroxidation by the production of reactive oxygen species (ROS) and promote liver inflammation[69]. Previous studies have shown that CYP2E1 activity correlates with ethanol-induced liver injury, and alcohol-induced hepatotoxicity is reduced when CYP2E1 is inhibited by inhibitors or by ablation of the CYP2E1 gene[70-73]. In addition to ALD, elevated CYP2E1 protein expression and activity are also found in both humans and animals with NAFLD/NASH and promote the progression of NAFLD/NASH[74-76].
We found that overexpression of AR in hepatocytes results in induction of CYP2E1 mRNA and protein, and simultaneously, ROS production is also induced by AR overexpression. Lentivirus-mediated knockdown of the AR gene attenuates MCD-diet-induced CYP2E1 expression, reduces the levels of lipid peroxidation, suppresses expression of proinflammatory cytokines, and alleviates NASH in db/db and C57BL/6 mice[7]. Our observation indicates that CYP2E1 expression is induced by elevated AR in fatty liver and generates ROS production, resulting in oxidative stress. AR inhibitors may alleviate steatohepatitis by attenuating CYP2E1 induction.
Several studies have revealed that isoflavones can reduce expression of CYP2E1 in healthy or diseased animals with liver injury. Soybean extract (rich in isoflavones) significantly decreases hepatic CYP2E1 expression in healthy rats[77] or in rats with high-fat-diet-induced NASH[78]. In addition, genistein significantly inhibits CYP2E1 activity and protects against alcohol-induced chronic liver injury in rats[30]. Moreover, in mice, pretreatment with puerarin prior to the administration of CCl4 significantly suppresses the expression of CYP2E1 protein, and prevents hepatic malondialdehyde formation[79]. These studies suggest that the protective effects of isoflavones against hepatotoxicity possibly involve mechanisms related to its ability to block CYP2E1 activity. We propose that isoflavones inhibit AR activity and, at least in part, cause the subsequent suppression of CYP2E1 activity to alleviate oxidative stress and improve liver inflammation in humans and animals with ALD or NASH, although there is no direct evidence that isoflavones suppress CYP2E1 activity by blocking the AR/polyol pathway.
In addition to oxidative stress, the gut bacterial endotoxin, toxic lipopolysaccharide (LPS), plays an important role in the development of alcoholic liver injury[12] or NAFLD/NASH[80]. Bacterial endotoxin reaches the liver through the portal circulation to activate hepatic Kupffer cells (special macrophages located in the liver) and stimulate their production of NO and cytokines, which subsequently cause damage to hepatocytes. Inhibition of the AR prevents nuclear factor (NF)-κB-dependent activation of tumor necrosis factor (TNF)-α, interleukin (IL)-12, IL-6, and macrophage chemoattractant protein-1 in livers of mice injected with LPS[81]. Moreover, pharmacological inhibition or siRNA ablation of AR prevents the biosynthesis of inflammatory cytokines and chemokines in LPS-activated RAW264.7 murine macrophages[81,82]. These studies indicate that inhibition of AR can prevent LPS-induced production of cytokines and chemokines in mice.
Pretreatment of RAW264.7 macrophages with genistein, luteolin, luteolin-7-glucoside and quercetin inhibits LPS-stimulated TNF-α and IL-6 release, whereas eriodictyol and hesperetin only inhibit TNF-α release[83]. Of these, luteolin and quercetin are the most potent inhibitors of cytokine production, with an IC50 < 1 and 5 μmol/L for TNF-α release, respectively. The cytokine-production-inhibiting potential of these flavonids is in accordance with their AR inhibitory activity (IC50: luteolin 0.5-0.6 μmol/L, quercetin 3.3-7.73 μmol/L, and genistein 4.5-16.9 μmol/L against rat lens AR), suggesting that these compounds inhibit LPS-stimulated cytokine production, at least in part, through inhibition of AR activity.
Genistein is reported to have a beneficial effect on LPS-induced injury in rodent liver, RAW264.7 murine macrophages, and murine Kupffer cells. Zhao et al[84] reported that genistein suppresses hepatic production of LPS-induced TNF-α, IL-1β and IL-6 in rats. In vitro preincubation of liver slices from naïve rats with genistein suppresses LPS-induced TNF-α production in a dose-dependent manner. Both in vivo and in vitro administration of genistein suppresses LPS-induced liver proinflammatory cytokine overproduction. Lin et al[85] reported that genistein treatment significantly protects against LPS/D-galactosamine-induced liver injury in mice, and alleviates proinflammatory cytokines, including TNF-α and NO/inducible NO synthase, by inhibiting NF-κB activity.
The preventive effect of genistein on LPS-stimulated cytokine production has been found to be through inhibition of tyrosine kinase activity[86]. Genistein is a well-known inhibitor of tyrosine kinase, whereas daidzein does not inhibit tyrosine kinase activity. Genistein attenuates the liver injury caused by LPS in rats, whereas daidzein does not, which indicates the involvement of tyrosine kinase in LPS-induced liver injury. However, the AR inhibitory potential of daidzein is also weaker than that of genistein. Inhibiting AR activity is not an exclusive mechanism by which isoflavones protect against endotoxin-induced liver injury.
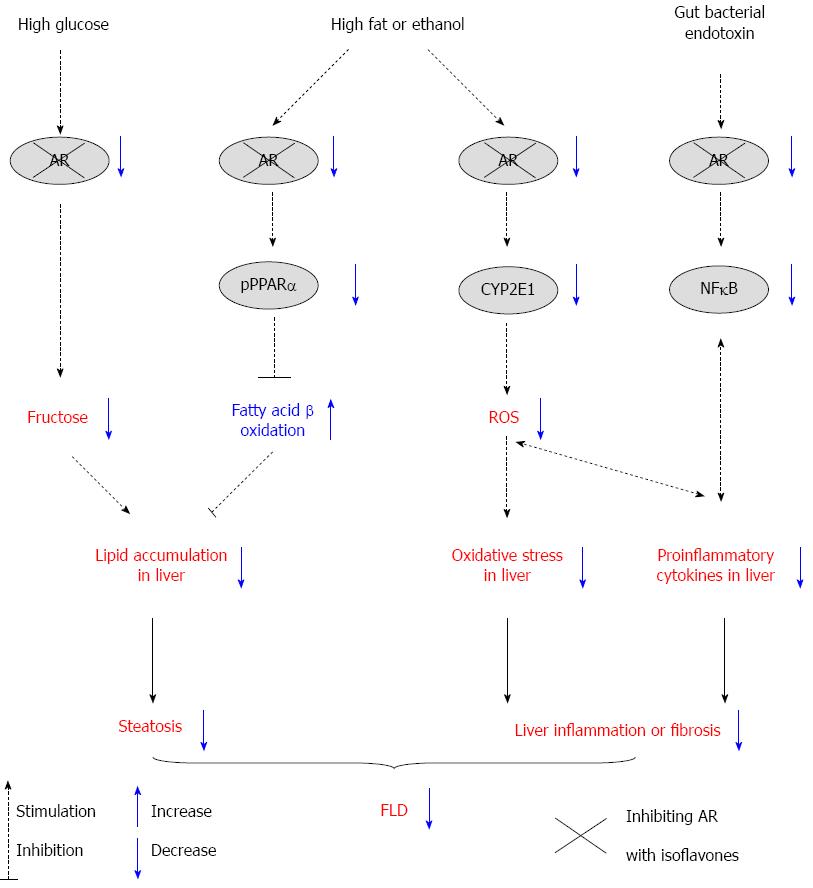
Collectively, isoflavones have been found to alleviate ALD and NAFLD/NASH in rodents, and these effects are partially achieved by the following mechanisms: (1) blocking the AR/polyol pathway to reduce fructose production in the liver under high-glucose conditions; (2) suppressing hepatic AR activity, which in turn improves PPARα-mediated fatty acid oxidation; (3) inhibiting AR activity and subsequently ameliorating CYP2E1-mediated oxidative stress; and (4) attenuating AR/gut bacterial endotoxin-mediated cytokine overproduction. The proposed mechanisms of action of isoflavones regarding the AR/polyol pathway are depicted in Figure 1.
Therefore, isoflavones may be useful in preventing ALD and NAFLD/NASH. Clarifying the mechanisms of action of isoflavones regarding the AR/polyol pathway will help to develop efficient anti-FLD medications. However, the literature reviewed in this paper was limited to animal models. Human data on the anti-FLD effect of isoflavones are scarce. Further clinical trials are necessary to affirm the beneficial effect of isoflavones on FLD in humans.
P- Reviewer: Balaban YH, Daltro C, Kim HS, Loguercio C, Rocha R S- Editor: Ma YJ L- Editor: Webster JR E- Editor: Zhang DN
| 1. | Smith BW, Adams LA. Non-alcoholic fatty liver disease. Crit Rev Clin Lab Sci. 2011;48:97-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 231] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 2. | Cao HX, Fan JG. Editorial: Fatty liver disease: a growing public health problem worldwide. J Dig Dis. 2011;12:1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Dowman JK, Armstrong MJ, Tomlinson JW, Newsome PN. Current therapeutic strategies in non-alcoholic fatty liver disease. Diabetes Obes Metab. 2011;13:692-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 4. | Kim MH, Kang KS. Isoflavones as a smart curer for non-alcoholic fatty liver disease and pathological adiposity via ChREBP and Wnt signaling. Prev Med. 2012;54 Suppl:S57-S63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 5. | Lanaspa MA, Ishimoto T, Li N, Cicerchi C, Orlicky DJ, Ruzycki P, Rivard C, Inaba S, Roncal-Jimenez CA, Bales ES. Endogenous fructose production and metabolism in the liver contributes to the development of metabolic syndrome. Nat Commun. 2013;4:2434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 188] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 6. | Qiu L, Lin J, Xu F, Gao Y, Zhang C, Liu Y, Luo Y, Yang JY. Inhibition of aldose reductase activates hepatic peroxisome proliferator-activated receptor-α and ameliorates hepatosteatosis in diabetic db/db mice. Exp Diabetes Res. 2012;2012:789730. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Qiu L, Lin J, Ying M, Chen W, Yang J, Deng T, Chen J, Shi D, Yang JY. Aldose reductase is involved in the development of murine diet-induced nonalcoholic steatohepatitis. PLoS One. 2013;8:e73591. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Veeresham C, Rama Rao A, Asres K. Aldose reductase inhibitors of plant origin. Phytother Res. 2014;28:317-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 9. | Park CH, Lim SS, Lee DU. Structure-activity relationships of components from the roots of pueraria thunbergiana having aldose reductase inhibitory and antioxidative activity. Bull Korean Chem Soc. 2007;28:493-495. |
| 10. | Day CP. Non-alcoholic fatty liver disease: a massive problem. Clin Med. 2011;11:176-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 11. | Gramenzi A, Caputo F, Biselli M, Kuria F, Loggi E, Andreone P, Bernardi M. Review article: alcoholic liver disease--pathophysiological aspects and risk factors. Aliment Pharmacol Ther. 2006;24:1151-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 125] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 12. | Rao R. Endotoxemia and gut barrier dysfunction in alcoholic liver disease. Hepatology. 2009;50:638-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 374] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 13. | Larter CZ, Farrell GC. Insulin resistance, adiponectin, cytokines in NASH: Which is the best target to treat? J Hepatol. 2006;44:253-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 112] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 14. | Voican CS, Perlemuter G. Insulin resistance and oxidative stress: two therapeutic targets in non-alcoholic steatohepatitis. J Hepatol. 2011;54:388-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Carter-Kent C, Zein NN, Feldstein AE. Cytokines in the pathogenesis of fatty liver and disease progression to steatohepatitis: implications for treatment. Am J Gastroenterol. 2008;103:1036-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 153] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 16. | Koek GH, Liedorp PR, Bast A. The role of oxidative stress in non-alcoholic steatohepatitis. Clin Chim Acta. 2011;412:1297-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 244] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 17. | Goel A, Gupta M, Aggarwal R. Gut microbiota and liver disease. J Gastroenterol Hepatol. 2014;29:1139-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 18. | Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52:1836-1846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1543] [Cited by in RCA: 1807] [Article Influence: 120.5] [Reference Citation Analysis (0)] |
| 19. | Li YY. Genetic and epigenetic variants influencing the development of nonalcoholic fatty liver disease. World J Gastroenterol. 2012;18:6546-6551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 62] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 20. | Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114:842-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2953] [Cited by in RCA: 3120] [Article Influence: 115.6] [Reference Citation Analysis (36)] |
| 21. | Basaranoglu M, Basaranoglu G, Sentürk H. From fatty liver to fibrosis: a tale of “second hit”. World J Gastroenterol. 2013;19:1158-1165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 114] [Cited by in RCA: 134] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 22. | Barnes S. Evolution of the health benefits of soy isoflavones. Proc Soc Exp Biol Med. 1998;217:386-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 153] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 23. | Setchell KD, Cassidy A. Dietary isoflavones: biological effects and relevance to human health. J Nutr. 1999;129:758S-767S. [PubMed] |
| 24. | Lee YM, Choi JS, Kim MH, Jung MH, Lee YS, Song J. Effects of dietary genistein on hepatic lipid metabolism and mitochondrial function in mice fed high-fat diets. Nutrition. 2006;22:956-964. [PubMed] |
| 25. | Yalniz M, Bahcecioglu IH, Kuzu N, Poyrazoglu OK, Bulmus O, Celebi S, Ustundag B, Ozercan IH, Sahin K. Preventive role of genistein in an experimental non-alcoholic steatohepatitis model. J Gastroenterol Hepatol. 2007;22:2009-2014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 26. | Ustundag B, Bahcecioglu IH, Sahin K, Duzgun S, Koca S, Gulcu F, Ozercan IH. Protective effect of soy isoflavones and activity levels of plasma paraoxonase and arylesterase in the experimental nonalcoholic steatohepatitis model. Dig Dis Sci. 2007;52:2006-2014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Mohamed Salih S, Nallasamy P, Muniyandi P, Periyasami V, Carani Venkatraman A. Genistein improves liver function and attenuates non-alcoholic fatty liver disease in a rat model of insulin resistance. J Diabetes. 2009;1:278-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 74] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 28. | Kim MH, Kang KS, Lee YS. The inhibitory effect of genistein on hepatic steatosis is linked to visceral adipocyte metabolism in mice with diet-induced non-alcoholic fatty liver disease. Br J Nutr. 2010;104:1333-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 29. | Ji G, Yang Q, Hao J, Guo L, Chen X, Hu J, Leng L, Jiang Z. Anti-inflammatory effect of genistein on non-alcoholic steatohepatitis rats induced by high fat diet and its potential mechanisms. Int Immunopharmacol. 2011;11:762-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 30. | Huang Q, Huang R, Zhang S, Lin J, Wei L, He M, Zhuo L, Lin X. Protective effect of genistein isolated from Hydrocotyle sibthorpioides on hepatic injury and fibrosis induced by chronic alcohol in rats. Toxicol Lett. 2013;217:102-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 31. | Kwon YH, Jeon S, Park YJ. Inhibitory effect of genistein on nonalcoholic fatty liver disease development in ApoE–/– mice fed a high-fat diet. FASEB J. 2013;27:862. |
| 32. | Kim MH, Park JS, Jung JW, Byun KW, Kang KS, Lee YS. Daidzein supplementation prevents non-alcoholic fatty liver disease through alternation of hepatic gene expression profiles and adipocyte metabolism. Int J Obes (Lond). 2011;35:1019-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 33. | Crespillo A, Alonso M, Vida M, Pavón FJ, Serrano A, Rivera P, Romero-Zerbo Y, Fernández-Llebrez P, Martínez A, Pérez-Valero V. Reduction of body weight, liver steatosis and expression of stearoyl-CoA desaturase 1 by the isoflavone daidzein in diet-induced obesity. Br J Pharmacol. 2011;164:1899-1915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 34. | Zheng P, Ji G, Ma Z, Liu T, Xin L, Wu H, Liang X, Liu J. Therapeutic effect of puerarin on non-alcoholic rat fatty liver by improving leptin signal transduction through JAK2/STAT3 pathways. Am J Chin Med. 2009;37:69-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 35. | Zhao M, Du YQ, Yuan L, Wang NN. Protective effect of puerarin on acute alcoholic liver injury. Am J Chin Med. 2010;38:241-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 36. | Xiong Y, Yang Y, Yang J, Chai H, Li Y, Yang J, Jia Z, Wang Z. Tectoridin, an isoflavone glycoside from the flower of Pueraria lobata, prevents acute ethanol-induced liver steatosis in mice. Toxicology. 2010;276:64-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 37. | Noh BK, Lee JK, Jun HJ, Lee JH, Jia Y, Hoang MH, Kim JW, Park KH, Lee SJ. Restoration of autophagy by puerarin in ethanol-treated hepatocytes via the activation of AMP-activated protein kinase. Biochem Biophys Res Commun. 2011;414:361-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 38. | Kamiya T, Sameshima-Kamiya M, Nagamine R, Tsubata M, Ikeguchi M, Takagaki K, Shimada T, Aburada M. The crude extract from puerariae flower exerts antiobesity and antifatty liver effects in high-fat diet-induced obese mice. Evid Based Complement Alternat Med. 2012;2012:272710. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 39. | Peng JH, Cui T, Huang F, Chen L, Zhao Y, Xu L, Xu LL, Feng Q, Hu YY. Puerarin ameliorates experimental alcoholic liver injury by inhibition of endotoxin gut leakage, Kupffer cell activation, and endotoxin receptors expression. J Pharmacol Exp Ther. 2013;344:646-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 40. | Qiu L, Chen T, Zhong F, Hong Y, Chen L, Ye H. Red clover extract exerts antidiabetic and hypolipidemic effects in db/db mice. Exp Ther Med. 2012;4:699-704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 41. | Chen T, Zhong FJ, Hong YM, Su WJ, Zhuang LL, Qiu LX. Effect of Trifolium pratense extract on methionine-choline-deficient diet-induced steatohepatitis in C57BL/6 mice. Chin J Nat Med. 2014;12:194-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 42. | Andersen C, Schjoldager JG, Tortzen CG, Vegge A, Hufeldt MR, Skaanild MT, Vogensen FK, Kristiansen K, Hansen AK, Nielsen J. 2-heptyl-formononetin increases cholesterol and induces hepatic steatosis in mice. Biomed Res Int. 2013;2013:926942. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 43. | Breikaa RM, Algandaby MM, El-Demerdash E, Abdel-Naim AB. Multimechanistic antifibrotic effect of biochanin a in rats: implications of proinflammatory and profibrogenic mediators. PLoS One. 2013;8:e69276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 44. | Hers HG. [Aldose reductase]. Biochim Biophys Acta. 1960;37:120-126. [PubMed] |
| 45. | Oates PJ, Mylari BL. Aldose reductase inhibitors: therapeutic implications for diabetic complications. Expert Opin Investig Drugs. 1999;8:2095-2119. [PubMed] |
| 46. | Yabe-Nishimura C. Aldose reductase in glucose toxicity: a potential target for the prevention of diabetic complications. Pharmacol Rev. 1998;50:21-33. [PubMed] |
| 47. | O’connor T, Ireland LS, Harrison DJ, Hayes JD. Major differences exist in the function and tissue-specific expression of human aflatoxin B1 aldehyde reductase and the principal human aldo-keto reductase AKR1 family members. Biochem J. 1999;343 Pt 2:487-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 48. | Brown KE, Broadhurst KA, Mathahs MM, Kladney RD, Fimmel CJ, Srivastava SK, Brunt EM. Immunodetection of aldose reductase in normal and diseased human liver. Histol Histopathol. 2005;20:429-436. [PubMed] |
| 49. | Hsieh PC, Huang GJ, Ho YL, Lin YH, Huang SS, Chiang YC, Tseng MC, Chang YS. Activities of antioxidants, α-Glucosidase inhibitors and aldose reductase inhibitors of the aqueous extracts of four Flemingia species in Taiwan. Bot Stud. 2010;51:293-302. |
| 50. | Choi SW, Yang JS, Jung EA, Choi HJ, Lee HS, Shin CS, Kim DS, Hur NY, Baik MY. Isolation and structural determination of aldose reductase inhibitor from Korean fermented soybean paste. Food Sci Biotechnol. 2005;14:344-349. |
| 51. | Jung SH, Lee YS, Lee S, Lim SS, Kim YS, Shin KH. Isoflavonoids from the rhizomes of Belamcanda chinensis and their effects on aldose reductase and sorbitol accumulation in streptozotocin induced diabetic rat tissues. Arch Pharm Res. 2002;25:306-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 52. | Madeswaran A, Umamaheswari M, Asokkumar K, Sivashanmugam T, Subhadradevi V, Jagannath P. In silico docking studies of aldose reductase inhibitory activity of commercially available flavonoids. Bangladesh J Pharmacol. 2012;7:266-271. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 53. | Ae Park S, Choi MS, Cho SY, Seo JS, Jung UJ, Kim MJ, Sung MK, Park YB, Lee MK. Genistein and daidzein modulate hepatic glucose and lipid regulating enzyme activities in C57BL/KsJ-db/db mice. Life Sci. 2006;79:1207-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 131] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 54. | Kota BP, Huang TH, Roufogalis BD. An overview on biological mechanisms of PPARs. Pharmacol Res. 2005;51:85-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 464] [Cited by in RCA: 483] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 55. | Abdelmegeed MA, Yoo SH, Henderson LE, Gonzalez FJ, Woodcroft KJ, Song BJ. PPARalpha expression protects male mice from high fat-induced nonalcoholic fatty liver. J Nutr. 2011;141:603-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 215] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 56. | Djouadi F, Weinheimer CJ, Saffitz JE, Pitchford C, Bastin J, Gonzalez FJ, Kelly DP. A gender-related defect in lipid metabolism and glucose homeostasis in peroxisome proliferator- activated receptor alpha- deficient mice. J Clin Invest. 1998;102:1083-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 321] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 57. | Ip E, Farrell G, Hall P, Robertson G, Leclercq I. Administration of the potent PPARalpha agonist, Wy-14,643, reverses nutritional fibrosis and steatohepatitis in mice. Hepatology. 2004;39:1286-1296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 308] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 58. | Ip E, Farrell GC, Robertson G, Hall P, Kirsch R, Leclercq I. Central role of PPARalpha-dependent hepatic lipid turnover in dietary steatohepatitis in mice. Hepatology. 2003;38:123-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 380] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 59. | Kong L, Ren W, Li W, Zhao S, Mi H, Wang R, Zhang Y, Wu W, Nan Y, Yu J. Activation of peroxisome proliferator activated receptor alpha ameliorates ethanol induced steatohepatitis in mice. Lipids Health Dis. 2011;10:246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 60. | Mueller M, Hobiger S, Jungbauer A. Red clover extract: a source for substances that activate peroxisome proliferator-activated receptor alpha and ameliorate the cytokine secretion profile of lipopolysaccharide-stimulated macrophages. Menopause. 2010;17:379-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 61. | Qiu L, Lin B, Lin Z, Lin Y, Lin M, Yang X. Biochanin A ameliorates the cytokine secretion profile of lipopolysaccharide-stimulated macrophages by a PPARγ-dependent pathway. Mol Med Rep. 2012;5:217-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 62. | Qiu L, Wu X, Chau JF, Szeto IY, Tam WY, Guo Z, Chung SK, Oates PJ, Chung SS, Yang JY. Aldose reductase regulates hepatic peroxisome proliferator-activated receptor alpha phosphorylation and activity to impact lipid homeostasis. J Biol Chem. 2008;283:17175-17183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 63. | Mezei O, Li Y, Mullen E, Ross-Viola JS, Shay NF. Dietary isoflavone supplementation modulates lipid metabolism via PPARalpha-dependent and -independent mechanisms. Physiol Genomics. 2006;26:8-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 64. | Iqbal MJ, Yaegashi S, Ahsan R, Lightfoot DA, Banz WJ. Differentially abundant mRNAs in rat liver in response to diets containing soy protein isolate. Physiol Genomics. 2002;11:219-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 65. | Badger TM, Ronis MJ, Wolff G, Stanley S, Ferguson M, Shankar K, Simpson P, Jo CH. Soy protein isolate reduces hepatosteatosis in yellow Avy/a mice without altering coat color phenotype. Exp Biol Med (Maywood). 2008;233:1242-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 66. | Rimbach G, De Pascual-Teresa S, Ewins BA, Matsugo S, Uchida Y, Minihane AM, Turner R, VafeiAdou K, Weinberg PD. Antioxidant and free radical scavenging activity of isoflavone metabolites. Xenobiotica. 2003;33:913-925. [PubMed] |
| 67. | Ruiz-Larrea MB, Mohan AR, Paganga G, Miller NJ, Bolwell GP, Rice-Evans CA. Antioxidant activity of phytoestrogenic isoflavones. Free Radic Res. 1997;26:63-70. [PubMed] |
| 68. | Leung TM, Nieto N. CYP2E1 and oxidant stress in alcoholic and non-alcoholic fatty liver disease. J Hepatol. 2013;58:395-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 384] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 69. | Leclercq IA, Farrell GC, Field J, Bell DR, Gonzalez FJ, Robertson GR. CYP2E1 and CYP4A as microsomal catalysts of lipid peroxides in murine nonalcoholic steatohepatitis. J Clin Invest. 2000;105:1067-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 552] [Cited by in RCA: 590] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 70. | Lu Y, Cederbaum AI. CYP2E1 and oxidative liver injury by alcohol. Free Radic Biol Med. 2008;44:723-738. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 611] [Cited by in RCA: 587] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 71. | Nanji AA, Zhao S, Sadrzadeh SM, Dannenberg AJ, Tahan SR, Waxman DJ. Markedly enhanced cytochrome P450 2E1 induction and lipid peroxidation is associated with severe liver injury in fish oil-ethanol-fed rats. Alcohol Clin Exp Res. 1994;18:1280-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 191] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 72. | Robin MA, Sauvage I, Grandperret T, Descatoire V, Pessayre D, Fromenty B. Ethanol increases mitochondrial cytochrome P450 2E1 in mouse liver and rat hepatocytes. FEBS Lett. 2005;579:6895-6902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 75] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 73. | Bardag-Gorce F, Yuan QX, Li J, French BA, Fang C, Ingelman-Sundberg M, French SW. The effect of ethanol-induced cytochrome p4502E1 on the inhibition of proteasome activity by alcohol. Biochem Biophys Res Commun. 2000;279:23-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 82] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 74. | Chtioui H, Semela D, Ledermann M, Zimmermann A, Dufour JF. Expression and activity of the cytochrome P450 2E1 in patients with nonalcoholic steatosis and steatohepatitis. Liver Int. 2007;27:764-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 75. | Orellana M, Rodrigo R, Varela N, Araya J, Poniachik J, Csendes A, Smok G, Videla LA. Relationship between in vivo chlorzoxazone hydroxylation, hepatic cytochrome P450 2E1 content and liver injury in obese non-alcoholic fatty liver disease patients. Hepatol Res. 2006;34:57-63. [PubMed] |
| 76. | Kathirvel E, Morgan K, French SW, Morgan TR. Overexpression of liver-specific cytochrome P4502E1 impairs hepatic insulin signaling in a transgenic mouse model of nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2009;21:973-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 77. | Mrozikiewicz PM, Bogacz A, Czerny B, Karasiewicz M, Kujawski R, Mikolajczak PL, Seremak-Mrozikiewicz A, Grzeskowiak E, Bobkiewicz-Kozlowska T. The influence of a standardized soybean extract (Glycine max) on the expression level of cytochrome P450 genes in vivo. Ginekol Pol. 2010;81:516-520. [PubMed] |
| 78. | Yang HY, Tzeng YH, Chai CY, Hsieh AT, Chen JR, Chang LS, Yang SS. Soy protein retards the progression of non-alcoholic steatohepatitis via improvement of insulin resistance and steatosis. Nutrition. 2011;27:943-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 79. | Hwang YP, Choi CY, Chung YC, Jeon SS, Jeong HG. Protective effects of puerarin on carbon tetrachloride-induced hepatotoxicity. Arch Pharm Res. 2007;30:1309-1317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 80. | Fukunishi S, Sujishi T, Takeshita A, Ohama H, Tsuchimoto Y, Asai A, Tsuda Y, Higuchi K. Lipopolysaccharides accelerate hepatic steatosis in the development of nonalcoholic fatty liver disease in Zucker rats. J Clin Biochem Nutr. 2014;54:39-44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 81. | Ramana KV, Fadl AA, Tammali R, Reddy AB, Chopra AK, Srivastava SK. Aldose reductase mediates the lipopolysaccharide-induced release of inflammatory mediators in RAW264.7 murine macrophages. J Biol Chem. 2006;281:33019-33029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 311] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 82. | Shoeb M, Yadav UC, Srivastava SK, Ramana KV. Inhibition of aldose reductase prevents endotoxin-induced inflammation by regulating the arachidonic acid pathway in murine macrophages. Free Radic Biol Med. 2011;51:1686-1696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 83. | Xagorari A, Papapetropoulos A, Mauromatis A, Economou M, Fotsis T, Roussos C. Luteolin inhibits an endotoxin-stimulated phosphorylation cascade and proinflammatory cytokine production in macrophages. J Pharmacol Exp Ther. 2001;296:181-187. [PubMed] |
| 84. | Zhao JH, Arao Y, Sun SJ, Kikuchi A, Kayama F. Oral administration of soy-derived genistin suppresses lipopolysaccharide-induced acute liver inflammation but does not induce thymic atrophy in the rat. Life Sci. 2006;78:812-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 85. | Lin X, Zhang S, Huang R, Wei L, Liang C, Chen Y, Lv S, Liang S, Wu X, Huang Q. Protective effect of genistein on lipopolysaccharide/D-galactosamine- induced hepatic failure in mice. Biol Pharm Bull. 2014;37:625-632. [PubMed] |
| 86. | Ruetten H, Thiemermann C. Effects of tyrphostins and genistein on the circulatory failure and organ dysfunction caused by endotoxin in the rat: a possible role for protein tyrosine kinase. Br J Pharmacol. 1997;122:59-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 83] [Article Influence: 3.0] [Reference Citation Analysis (0)] |