INTRODUCTION
Inflammatory bowel disease (IBD) is a chronic relapsing-remitting immune disorder of unknown etiology that afflicts millions of individuals throughout the world with debilitating symptoms, which impair performance and quality of life[1]. IBD is precipitated by a complex interaction of environmental, genetic, and immunoregulatory factors. Higher rates of IBD are seen in northern, industrialized countries[2]. Recurrent inflammation with ulceration and tissue restitution confers an increased risk of colorectal cancer in both ulcerative colitis (UC) and Crohn’s disease (CD)[3]. Although colorectal cancer occurs in a minority of IBD patients (1%), it carries a high mortality and accounts for 20% of IBD-related mortality[4].
Homocysteine is a sulfur-containing amino acid derived from the metabolism of methionine via methyl group metabolism[5]. There is little doubt that hyperhomocysteinemia plays a role in the development of cardiovascular disease. This is not only supported by human population studies identifying it as an independent risk factor, but strong evidence resides in animal models, as well[5]. More recently, a relationship between hyperhomocysteinemia and increased risk of different cancers has been indicated[6-11]. In the present article, we review the association between hyperhomocysteinemia and increased risk of colorectal cancer in IBD and the possible mechanisms.
INFLAMMATORY BOWEL DISEASE
IBD, including UC and CD, is characterized by chronic inflammation of the gastrointestinal tract in genetically susceptible individuals that are exposed to environmental risk factors[12]. CD may affect all parts of the gastrointestinal tract, from mouth to anus, but most commonly involves the distal part of the small intestine or ileum, and colon. UC results in colonic inflammation that can affect the rectum only (proctitis) or can cause continuous disease from the rectum proximally, to involve part of or the entire colon. Clinical symptoms include diarrhea, abdominal pain, gastrointestinal bleeding, and weight loss[13]. IBD has become one of the most common chronic inflammatory conditions worldwide[14]. The incidence and prevalence of IBD are increasing with time and in different regions around the world, indicating its emergence as a global disease[12]. In Canada, there are approximately 280000 patients with medically diagnosed IBD, which accounts for 0.8% of the population[15]. Although IBD has long been considered a disease that affects predominantly Western populations, recent data have shown significantly higher rates in Asians and time trend studies have shown an increase in its incidence across Asia[16]. IBD is mostly prevalent in young adults and currently is not curable, with patients usually requiring lifelong medication and may undergo devastating surgeries[17].
COLORECTAL CANCER IN INFLAMMATORY BOWEL DISEASE
The development of colorectal cancer is a serious long-term complication of chronic inflammation[13]. Colorectal cancer still accounts for 10%-15% of deaths in patients with IBD. Herrinton et al[18] demonstrated a 60% greater relative risk of colorectal cancer among individuals with CD and UC compared with an age- and gender-matched cohort of patients without IBD. IBD-associated colorectal cancer affects patients at a younger age than sporadic colorectal cancer. The prognosis for sporadic colorectal cancer and IBD associated colorectal cancer is similar, with a 5-year survival of approximately 50%. The increased risk of colorectal cancer in association with IBD is thought to be due to genetic and acquired factors[19]. The relationship between inflammation and cancer has been well established in the gastrointestinal system. The role of toll-like receptors and tumour necrosis factor-α (TNF-α) in the activation of nuclear factor κB, which induces transcription of genes involved in tumorigenesis, including COX-2 have been indicated in colitis-associated cancer. Defective signaling via p53 may be an early event in the progression of colitis-induced dysplasia to cancer. Without p53-induced apoptosis, aberrant cells are not eliminated and cancer may develop[20].
RISK FACTORS OF COLORECTAL CANCER DEVELOPMENT IN INFLAMMATORY BOWEL DISEASE
The extent and duration of colonic disease, the co-existence of primary sclerosing cholangitis, and a family history of sporadic colorectal cancer have been confirmed as risk factors of colorectal cancer in IBD patients. The risk of UC-associated colorectal cancer starts to increase after 7 years of extensive colonic disease[21]. In a meta-analysis of 41 studies the cumulative incidence of IBD associated colorectal cancer in patients with UC was 2% at 10 years, 8% at 20 years, and 18% after 30 years of disease[22]. The extent of mucosal inflammation has also been correlated with the risk of developing colorectal cancer. While patients with extensive disease (pancolitis and left-sided colitis) have an increased risk of developing colorectal cancer, patients with only proctitis or proctosigmoiditis do not[21]. There is conflicting evidence as to whether younger age at diagnosis of IBD is an independent risk factor for colorectal cancer in IBD. This evidence is not easy to evaluate, as children tend to have more extensive and severe colitis than those diagnosed as adults, and younger people have the potential for longer colitis duration, which is itself a risk factor[19]. IBD patients with a first-degree relative with colorectal cancer have twice the risk of developing colorectal cancer than those who do not. Moreover, if a first-degree relative suffered from colorectal cancer before the age of 50 years, the risk of developing colorectal cancer in IBD patients increases nine-fold[21]. Some genetic polymorphisms have been proposed to be associated with the risk of colorectal cancer in UC patients[23]. So far, there has not been any specific biomarker useful to identify the high-risk patients for progression to colorectal cancer in IBD patients[21].
HOMOCYSTEINE METABOLISM AND PATHOGENESIS OF HYPERHOMOCYSTEINEMIA
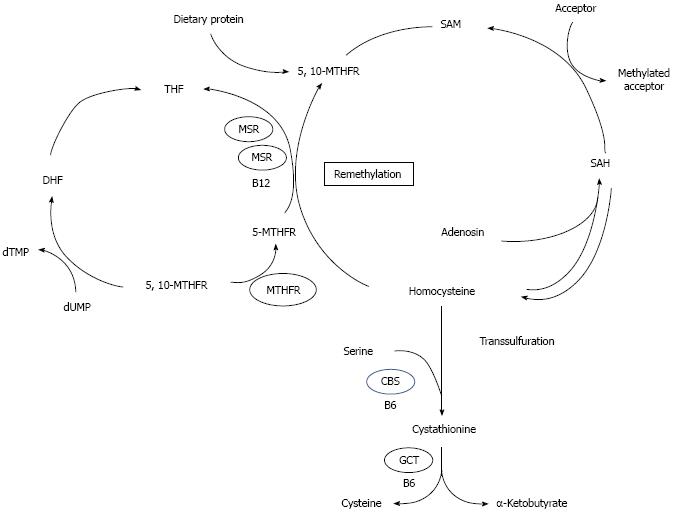
Homocysteine is a non-protein-forming, sulfur amino acid whose metabolism is at the intersection of two metabolic pathways[24]: remethylation and transsulfuration (Figure 1). In remethylation, homocysteine acquires a methyl group from N-5-methyl-tetrahydrofolate (MTHF) or from betaine to form methionine. The reaction with MTHF occurs in all tissues and is vitamin B12-dependent, while the reaction with betaine is confined mainly to the liver and is vitamin B12-independent. ATP then activates a considerable proportion of methionine to form S-adenosylmethionine (SAM). SAM serves primarily as a universal methyl donor to a variety of acceptors including guanidinoacetate, nucleic acids, neurotransmitters, phospholipids, and hormones. S-adenosylhomocysteine (SAH), the by-product of these methylation reactions, is subsequently hydrolyzed, thus regenerating homocysteine, which then becomes available to start a new cycle of methyl-group transfer. In the transsulfuration pathway, homocysteine condenses with serine to form cystathionine in an irreversible reaction catalyzed by the pyridoxal-5’-phosphate (PLP)-containing enzyme, cystathionine β-synthase (CBS). Cystathionine is hydrolyzed by a second PLP-containing enzyme, gamma-cystathionase, to form cysteine and alpha-ketobutyrate. Excess cysteine is oxidized to taurine and inorganic sulfates or excreted in the urine. Thus, in addition to the synthesis of cysteine, this transsulfuration pathway effectively catabolizes excess homocysteine which is not required for methyltransfer, and delivers sulfate for the synthesis of heparin, heparan sulfate, dermatan sulfate, and chondroitin sulfate. It is important to note that since homocysteine is not a normal dietary constituent, the only source of homocysteine is methionine[25].
Figure 1 Metabolism of homocysteine[7].
dUMP: Desoxyuridine monophosphate; dTMP: Desoxytimidine monophosphste; THF: Tetrahydrofolate; DHF: Dihydrofolate; 5-MTHF: 5-methyltetrahydrofolate; 5,10-MTHF: 5,10-methyltetrahydrofolate; 5,10 MTHFR: 5,10- methyltetrahydrofolate reductase; MS: Metionin synthase; MSR: Metionin synthase reductase; B12: Vitamin B12; SAM: S-adenosylmethionine; SAH: S-adenosylhomocysteine; CBS: Cystathionine β-synthase; GCT: γ-cystathionase; B6: Vitamin B6.
Two enzymes and three vitamins play a key role in the regulation of circulating homocysteine levels. Of the enzymes, cystathionine-β-synthase controls the breakdown of homocysteine to cystathionine in the transsulfuration pathway, while methylene tetrahydrofolate reductase (MTHFR) is involved in the remethylation pathway, in which homocysteine is converted back to methionine. Folic acid, vitamin B6 and vitamin B12 are essential cofactors in homocysteine metabolism and a lack of them due to a deficient diet or disease can produce elevated plasma homocysteine[26]. In addition, a genetic defect in one of the enzymes of homocysteine metabolism can lead to metabolic disruption and potentially to hyperhomocysteinemia[24]. Of the gene defects, the most common is the C-to-T substitution at nucleotide 677 in the coding region of the gene for MTHFR, the so-called thermolabile variant. There is an elevated homocysteine concentration and a decreased plasma folate concentration in the homozygous mutant genotype of C677TMTHFR gene[26].
Depending on its severity, hyperhomocysteinemia is classified into several categories: (1) severe hyperhomocysteinemia which is characterized by high homocysteine levels at all times, caused by deficiencies in CBS, MTHFR, or enzymes of vitamin B12 metabolism; (2) mild hyperhomocysteinemia during fasting which is characterized by moderately high homocysteine levels under fasting conditions and reflects impaired homocysteine methylation (folate, vitamin B12, or moderate enzyme defects (e.g., thermolabile MTHFR); and (3) mild hyperhomocysteinemia during post-methionine load that is defined as abnormal increase in homocysteine after methionine load which reflects impaired homocysteine transsulfuration (heterozygous CBS defects, vitamin B6 deficiency)[25].
It should be noted that in addition to the above mentioned key enzymes and vitamins, a variety of other factors affect the regulation and function of these enzymes, including diet, age, physiological state, and hormonal imbalance. Moreover, and in addition to the MTHFR C677T polymorphisms, the majority of these enzymes exhibit polymorphic forms that certainly have the potential to influence homocysteine balance for specific individuals, as has been discussed[27].
ROLE OF HYPERHOMOCYSTEINEMIA IN CANCER DEVELOPMENT
Many malignant cells are methionine dependent, resulting from the inability of malignant cells to convert homocysteine to methionine[28]. Elevated total homocysteine could be an early marker of carcinogenesis and a sensitive marker for detecting recurrence. The change of serum levels of homocysteine paralleled that of different tumor markers in cases of ovarian, breast, pancreatic and colon cancer suggesting that serum total homocysteine level, like tumor markers, reflected the tumor cell activity or the rapid proliferation rate of tumor cells. In addition, hyperhomocysteinemia caused by the proliferation of tumor cells was also demonstrated from the study of cell tissue cultures[28].
Several biochemical changes indicate that elevated homocysteine in blood creates a risk for cancer, and it is likely that hyperhomocysteinemia is a risk factor for carcinogenesis. Hyperhomocysteinemia is frequently associated with folate deficiency. In fact, homocysteine has become a sensitive marker for the deficiency of folate and the majority of the cancer risk derived from hyperhomocysteinemia is likely to be related to folate status. Polymorphism of MTHFR may reduce the production of its product, 5-MTHF, and increase the risk for cancer. 5-MTHF is the major form of folate in serum that provides the methyl group for DNA methylation. Reduction of 5-MTHF results in global genomic hypomethylation[28], which is an early and consistent event in carcinogenesis. Global hypomethylation of the coding and noncoding regions and demethylation of repetitive DNA sequences may contribute to the development of cancer through the following mechanisms: chromosomal instability, increased mutations, reactivation of intragenomic parasitic sequences that could be transcribed and moved to other sites, where they could disrupt normal cellular genes mitotic recombination leading to loss of heterozygosity and promotion of rearrangements, aneuploidy, loss of imprinting, and up-regulation of proto-oncogeneses[29]. Hyperhomocysteinemia has been shown to be a potential oxidative stress indicator via its impact on folate status. The overproduction of oxygen free radicals generated from the oxidation of homocysteine causes of endothelial injury and DNA damage. As reduced free homocysteine contains a free sulfhydryl group, free radicals including hydrogen peroxide can be generated upon oxidation of homocysteine, forming a disulfide linkage with free sulfhydryl group of albumin, cysteine or homocysteine. Actually, the plasma level of reduced free homocysteine affects and enhances oxidative stress. The endogenous attack on DNA by hydrogen peroxide and oxygen free radicals may cause gene mutations such as P53 and ras gene, and eventually lead to carcinogenesis[28,30,31]. However, a recent case-control study by Chiang et al[32] indicated that that increased homocysteine concentration is strongly associated with the risk of colorectal cancer independently of oxidative stress indicators and antioxidant capacities.
Another mechanism by which homocysteine might predispose to cancer is the activation of proinflammatory genes due to region-specific hypomethylation. Results of in vitro and in vivo experiments have suggested that homocysteine might provoke intestinal mucosal injury by modulating TNF-α-mediated cytotoxicity. Indeed, plasma homocysteine has been regarded as a determinant of TNF-α in pathological conditions characterized by low-grade inflammation and targeting the TNF pathway can significantly reduce homocysteine, suggesting a role for this cytokine in homocysteine metabolism[33]. Finding out the biological mechanisms in which hyperhomocysteinemia plays its carcinogenic effects requires further investigations including well-designed experimental studies.
HOMOCYSTEINE STATUS IN INFLAMMATORY BOWEL DISEASE
In 1996, Lambert et al[34]. were the first who reported elevated homocysteine levels in patients suffering from CD in comparison with healthy controls Since then, several studies reported the higher prevalence of hyperhomocysteinemia in IBD patients in comparison with healthy subjects. Recently, Oussalah et al[35] performed a meta-analysis of 28 studies that had evaluated plasma homocysteine level and/or hyperhomocysteinemia risk in IBD patients. They found that the mean plasma homocysteine level was significantly higher in IBD patients when compared with controls and the mean plasma homocysteine level did not differ between UC and CD. In addition, they reported that the risk of hyperhomocysteinemia was significantly higher in IBD patients when compared with controls (OR = 4.65; 95%CI: 3.04-7.09). Hyperhomocysteinemia in IBD patients has been mainly attributed to low folate[36-38], vitamin B12[36-38], and vitamin B6 status[39]. A meta-analysis on genetic variants of homocysteine metabolism pathway in IBD did not find a relationship between MTHFR C677T polymorphism and IBD risk[40]. It should be noted that the impact of MTHFR C677T polymorphism on IBD risk according to plasma folate concentration was not assessed in this study. However, in another meta-analysis, Oussalah et al[35] found that MTHFR 677TT genotype was associated with higher IBD risk in patients with low plasma folate status. As mentioned before, this genotype is accompanied by elevated homocysteine concentration[26]. Furthermore, the hyperhomocysteinemia in IBD patients is suggested to be associated with advanced age, male sex, vitamin B12 deficiency or lower vitamin B12 serum levels, multivitamin therapy, and disease severity[41].
HYPERHOMOCYSTEINEMIA IN THE PATHOGENESIS OF COLORECTAL CANCER
It has been shown that homocysteine enhances growth of colon cancer cells in culture and the growth-promoting effect of homocysteine is reversed by folate[42]. In 1999, Kato et al[43] published the first epidemiological study showing the relationship between biological markers for folate status and colorectal cancer risk among women. Since 1999, different studies that have investigated the potential role of homocysteine status in the pathogenesis of colorectal cancer reported controversial results.
In a case-control study, total homocysteine levels were significantly higher in cancer patients (18 cases of breast cancer and 29 cases of colorectal cancer) compared to controls[44]. Univariate analysis demonstrated that total homocysteine levels significantly correlated with both interleukin-6 and TNF-α both in breast and colorectal cancer patients. In addition, TNF-α was independently associated with total homocysteine in patients with breast or colorectal cancer suggesting that cancer-related inflammation may be associated with elevated total homocysteine levels. The authors concluded that homocysteine-induced damage related to cell adhesion molecules, cytokines and chemokines might therefore contribute to the biology of cancer[44]. Battistelli et al[26] reported that non-metastatic colorectal cancer patients, who were eligible for curative surgery, had statistically higher levels of homocysteine than healthy individuals did. They also found that the increase of plasma homocysteine observed in the C/C and C/T genotype of C677TMTHFR gene carriers in the cancer group might be related to the methionine-dependent proliferation rate of colorectal cancer cells and might act as a permissive factor for thrombosis in the context of cancer thrombophilia. The homocysteine increase observed in T/T genotype carriers in both groups, on the other hand, was probably dependent on the enzymatic deficit associated with the homocysteine conversion to methionine and/or the depletion of folate. However, it should be mentioned that conflicting data exists on the relationship between different C677TMTHFR polymorphisms and risk of colorectal cancer development. For instance, while The TT genotype of MTHFR was found to associated with an increased risk of CRC in older populations, possibly due to age related disturbances in folate metabolism[45], the C677T was reported to have a protective effect on colorectal cancer development in a population with low allelic variability and an optimal intake of folic acid[46]. A recent meta-analysis of 70 published studies concluded that the MTHFR 677TT allele was associated with a decreased risk of colorectal cancer in comparison to CT + CC polymorphisms (OR = 0.86; 95%CI: 0.76-0.96)[47].
The mean plasma homocysteine level in 226 cases of colorectal cancer and 437 matched referents from the population-based Northern Sweden Health and Disease Study did not differ significantly and plasma homocysteine concentrations were not significantly associated with colorectal cancer risk[48]. Although high homocysteine concentration was reported to be inversely correlated with colorectal tumorigenesis in patients suffering from end-stage renal disease[49], the association between increasing plasma total homocysteine levels and colorectal cancer was reported in three other case-control studies[50-52]. Kim et al[53] performed an observational study on 30 persons with colorectal polyps and found that the mean concentration of serum homocysteine was 22% higher in patients with adenomatous polyps than in those with hyperplastic polyps. It should be noted that hyperplastic polyps are generally regarded as not having malignant potential. A recent study among 422 Korean patients with colorectal adenoma and 617 controls indicated a higher plasma homocysteine concentration to be significantly correlated with increased risk of adenoma among women[54]. In a nested case-control study within the Norwegian JANUS cohort, total homocysteine was associated with increased risk of colorectal cancer[55]. Odds ratio (OR) for the upper vs lower tertile was 1.32 (95%CI: 1.04-1.68; P-value for trend = 0.02). In addition, no interaction between MTHFR polymorphisms and total homocysteine was detected. However, in a case-control study nested within the Multiethnic Cohort study in United States, investigators analyzed prospectively collected blood samples from 224 incident colorectal cancer cases and 411 matched controls and reported no association between plasma homocysteine levels and risk of colorectal cancer[56]. Similarly, in another nested case control study from the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study cohort in Finland, serum homocysteine was unrelated to risk of colon or rectal cancer[57]. In a recent nested case-control study, Miller et al[58] demonstrated that high plasma homocysteine was associated with increased risk of colorectal cancer among a large sample (n = 988/group) of United States postmenopausal women. In this study, multivariate-adjusted OR (95%CI) for colorectal cancer was 1.46 (1.05, 2.04) for the highest quartile of homocysteine compared with the lowest quartile. In another recent case-control study in Taiwan, high serum homocysteine level was significantly associated with increased odds of colorectal before and after adjustment for different potential confounders including oxidative stress indicators and antioxidant capacities[32].
One of the most promising molecular markers of outcome that have been investigated in colorectal cancer is microsatellite instability (MSI). Approximately 15% of colorectal cancers are characterized by MSI, reflecting inactivation of the mismatch repair genes. The remaining 85% of colorectal cancers develop from the chromosomal-instability (microsatellite-stable) pathway. In comparison to patients with microsatellite stable tumors, those with tumors having a high degree of MSI (MSI-H) have a significantly better prognosis[59]. A strong association between sporadic MSI-H and plasma homocysteine has been indicated in Danish patients with colorectal cancer[57]. In addition, the authors indicated that systemic folate did not reflect the level of folate in tumor tissue and systemic homocysteine but not systemic folate found to be a biomarker for MSI-H[60]. Hyperhomocysteinemia has also been suggested as the missing link between type 2 diabetes mellitus and colorectal cancer risk[61].
The relationship between homocysteine status and colorectal cancer has been investigated in clinical trials, as well. Martínez et al[62] assessed the relation of plasma folate and homocysteine and colorectal adenoma recurrence separately in two studies. The first involved an intervention of a cereal supplement that contained folic acid, wheat bran fiber (WBF), and the second was conducted primarily during postfortification of the food supply using ursodeoxycholic acid (UDCA). It is worthy to note that UDCA may prevent colonic neoplastic transformation by countering the tumor-promoting effects of secondary bile acids, such as deoxycholic acid (DCA). UDCA exerts cytoprotective effects and has been shown to antagonize DCA-induced cell death of transformed colonocytes[63]. In these trials, among non-multivitamin users, individuals in the highest vs the lowest quartile of homocysteine had higher odds of adenoma recurrence, in both the WBF (OR = 2.25) and UDCA (OR = 1.93) populations. Using the data from WBF trial, Martínez et al[64] found that relative to subjects in the highest quartile of plasma homocysteine, those in the lowest quartile had an OR of adenoma recurrence of 0.69 (P-value for trend = 0.02) after adjustment for confounding factors. They reported a significant dose response between plasma homocysteine and adenoma recurrence. Using the data from 627 participants from the control arm of Polyp Prevention Trial, a large 4-year multicenter randomized, controlled trial in United States the authors found that high homocysteine concentrations were positively associated with two times increased likelihood of any and multiple adenoma recurrence[65]. Also, there was a suggestive positive association between high homocysteine concentrations and high-risk adenoma recurrence[65]. In the analysis of subjects, participating in a randomized clinical trial of folate and/or aspirin for the prevention of colorectal adenomas there was no association between baseline plasma total homocysteine and adenoma recurrence risk in either the placebo or the folic acid supplementation groups[66]. The lack of association between plasma total homocysteine and recurrence risk was similar for all adenoma end-points. In this study, baseline plasma total homocysteine was associated with the number of adenomas at the baseline examination, but this association was attenuated and no longer statistically significant after controlling for potential confounders, including plasma total folate and other B vitamins. About half the subjects in the study were recruited after voluntary folate fortification of the United States food supply began in 1996, and the first 3-year observation period overlapped a time of gradually increasing folic acid availability in United States and Canadian diets, with consequently decreasing total homocysteine levels. The authors discussed that it was possible that their negative results were due to the progressively lower plasma total homocysteine, which might have fallen to levels below a threshold for an association with adenoma risk. They concluded that their data would suggest one of two possibilities: there is no independent association between plasma total homocysteine and adenoma recurrence risk or that any association between plasma total homocysteine and adenoma recurrence may be limited to plasma total homocysteine levels higher than their study population who were largely folic acid-fortified[66].
ROLE OF HYPERHOMOCYSTEINEMIA IN THE DEVELOPMENT OF COLORECTAL CANCER IN INFLAMMATORY BOWEL DISEASE
Although the role of folate deficiency in the increased risk of colorectal cancer in IBD patients has been indicated in different studies[67-69], to date only one study investigated the relationship between homocysteine status and colorectal cancer in IBD patients. Phelip et al[29] performed a cross-sectional study to analyze the factors (especially hyperhomocysteinemia and folate deficiency) associated with the development of dysplasia-associated lesions or masses, or colorectal carcinoma in 114 IBD patients. In univariate analysis, the risk of oncogenesis in the IBD patients was significantly associated with low level of folate, and hyperhomocysteinemia. In multivariate analysis, neither hyperhomocysteinemia nor folate deficiency were associated with increased risk of colorectal cancer. However, when hyperhomocysteinemia was associated with folate deficiency, there was a significant increased risk of carcinogenesis (OR = 16.9, 95%CI: 2.3-126.7). Although, patients with hyperhomocysteinemia without folate deficiency had 2.5 times as many carcinogenic lesions as patients with normal homocysteinemia, the association was not statistically significant (P = 0.08). They concluded that hyperhomocysteinemia was significantly associated with oncogenesis when there was concomitant folate deficiency and in the subgroup of patients with low folate and no hyperhomocysteinemia, no increased risk of oncogenesis or preoncogenesis was shown.
CONCLUSION
Overall, studies investigating the relationship between hyperhomocysteinemia and risk of colorectal cancer have shown a tendency toward increased risk of colorectal cancer in association with elevated homocysteine levels. Although, most studies have also demonstrated that the effect of hyperhomocysteinemia on carcinogenesis is associated with low folate status and other vitamin B deficiencies mainly due to the underlying metabolic pathways that cause hyperhomocysteinemia, there is some evidence from well-designed studies showing independent effects of hyperhomocysteinemia on colorectal cancer development. In addition, there is some evidence suggesting that hyperhomocysteinemia may be a risk factor for cancer development in IBD. There should be further well designed prospective studies to investigate if hyperhomocysteinemia is associated with increased colorectal cancer risk in IBD patients. Currently, the primary strategy for managing colorectal risk in IBD is to conduct high quality colonoscopy screening at regular intervals in at risk individuals[70]. With the finding that hyperhomocysteinemia is associated with increased risk of colorectal cancer, it is highly suggested to include IBD patients with elevated levels of homocysteine as an “at risk” group of patients to perform regular colonoscopic screening, and in addition, to provide hyperhomocysteinemia lowering therapy using B vitamins (e.g., folic acid, B6 and B12).
ACKNOWLEDGMENTS
We wish to thank Dr. Paula Robson for her constructive input during darfting this manuscript.
P- Reviewer: Matsuda A, Niu ZS, Ozen H, Tomizawa M S- Editor: Qi Y L- Editor: A E- Editor: Zhang DN