Published online Oct 21, 2015. doi: 10.3748/wjg.v21.i39.11199
Peer-review started: March 31, 2015
First decision: June 2, 2015
Revised: June 29, 2015
Accepted: August 30, 2015
Article in press: August 30, 2015
Published online: October 21, 2015
Processing time: 202 Days and 18.5 Hours
Primary splenic angiosarcoma (PSA) is the most unusual type of malignancy with early multifocal metastasis through hematogenous spread. PSA is generally believed to originate from splenic sinusoidal vascular endothelium with a high rate of metastasis and to have a poor prognosis. Its etiology and pathogenetic mechanisms have not yet been clearly described. Thus far, only approximately 200 cases have been reported. PSA has variable symptomatology with the potential to present with life-threatening complications. The diagnosis of PSA is challenging; and often late. PSA should be considered in the differential diagnosis of patients with splenomegaly and anemia of unknown etiology. Surgical treatment with splenectomy is considered the only curative intervention for potential long-term disease-free survival. Early diagnosis and treatment are very important. It is important that clinical doctors improve the understanding of PSA. Herein, we report one rare case of PSA with hepatic metastases, along with a review of the current literature.
Core tip: Primary splenic angiosarcoma (PSA) is an aggressive malignancy with poor prognosis. It has variable symptomatology with the potential to present with life-threatening complications. Its etiology has not yet been established, and its clinical presentation may confuse even experienced physicians. Early diagnosis and treatment are very important. It is important that clinical doctors improve the understanding of PSA. Herein, we report one rare case of PSA with hepatic metastases, along with a review of the current literature.
- Citation: Chen F, Jin HF, Fan YH, Cai LJ, Zhang ZY, Lv B. Case report of primary splenic angiosarcoma with hepatic metastases. World J Gastroenterol 2015; 21(39): 11199-11204
- URL: https://www.wjgnet.com/1007-9327/full/v21/i39/11199.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i39.11199
PSA is generally believed to originate from splenic sinusoidal vascular endothelium and has a high rate of metastasis and poor prognosis. Thus far, only approximately 200 cases have been reported. Primary splenic angiosarcoma (PSA) has variable symptomatology with the potential to present with life-threatening complications. PSA should be considered in the differential diagnosis of patients with splenomegaly and anemia of unknown etiology. Definitive diagnosis of PSA is challenging and often late. Surgical treatment with splenectomy is considered the only curative intervention for potential long-term disease-free survival. We report herein a rare case of PSA with hepatic metastases and a review of the current literature.
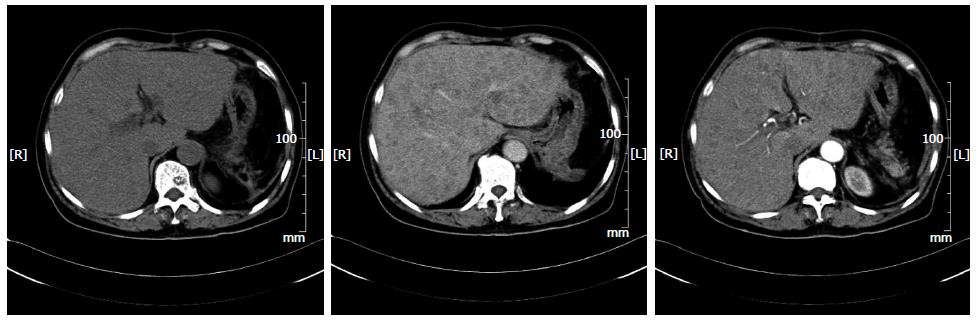
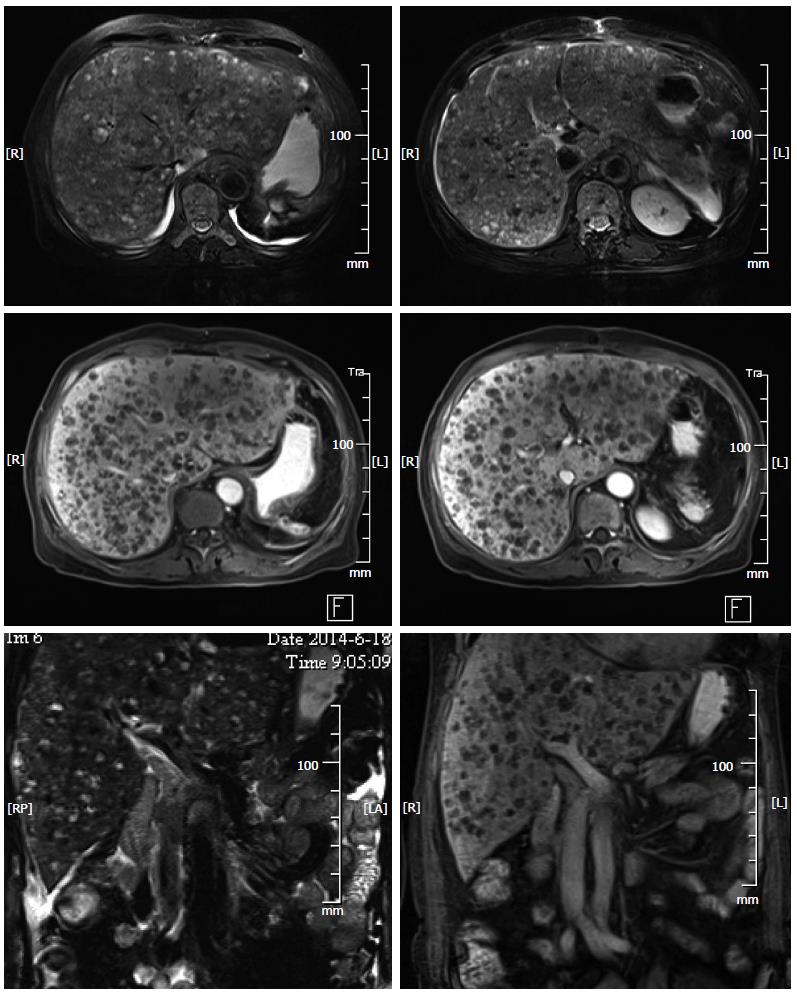
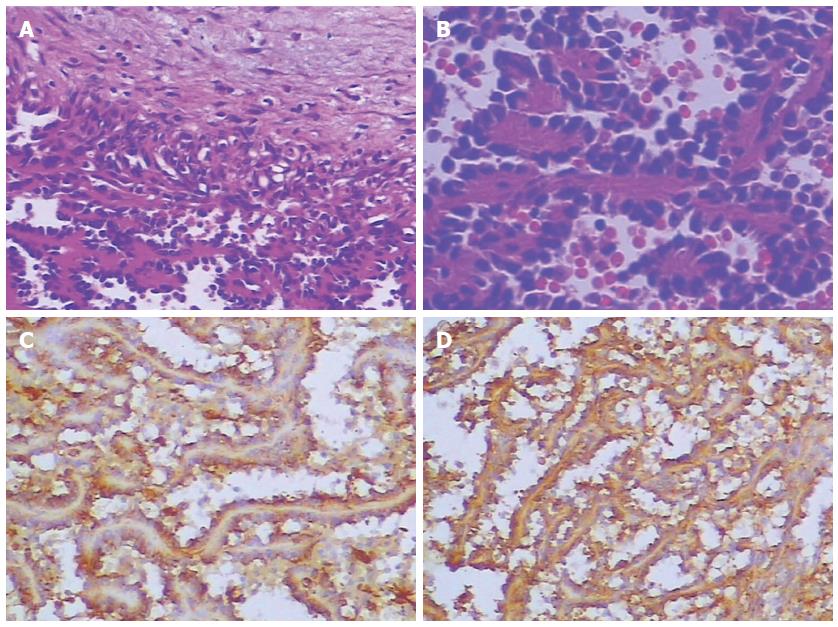
A 72-year-old woman presented to our emergency services with right upper quadrant abdominal pain and fatigue for one week. The pain was distending within a sustainable degree lasting for one week. The patient did not present with radiating pain or weight loss. Physical examination was notable for mild jaundice and hepatomegaly (5 cm below the costophrenic margin). A 30-cm-long oblique line of old scars was visible on the left upper quadrant. Blood work at admission revealed a leukocyte count of 4.3 × 109/L, with 69.7% neutrophils, 19% lymphocytes, 7.9% monocytes, 1.2% eosinophils and 2.2% basophils, hemoglobin of 70 g/L and a platelet count of 14 × 109/L. Evidence of significant coagulopathy, with an D-dimer level of 50.87 mg/L and deranged liver function (ALP 593 U/L, ALT 109 U/L, AST 163 U/L, total bilirubin 96.7 μmol/L, direct bilirubin 67.6 μmol, indirect bilirubin 29.1 U/L, total protein 51.40 g/L, and albumin 27.8 g/L) was noted. Other blood examination results, including ANA, ANCA and tumor markers (CA-199, AFP and CEA), were normal. Imaging studies at admission including abdominal computed tomography (CT) (Figure 1) and magnetic resonance imaging (MRI) (Figure 2) demonstrated obvious hepatomegaly with multiple liver nodules and loss of the spleen. The radiological differential diagnosis included hematological system diseases, such as lymphoma and metastatic carcinoma. A subsequent bone marrow biopsy indicated poor platelet production by megakaryocytes. From her medical history, splenectomy and resection of a left liver tumor after trauma 2 mo previously in another hospital were significant to the diagnosis. Preoperative abdominal CT revealed a massive splenomegaly with hemorrhage, whereas the liver did not exhibit any abnormality. Postoperative pathological findings indicated splenic littoral cell angioma and hepatic cavernous hemangioma. We believed that the multiple nodules of the liver were related to the previous surgery. To confirm the diagnosis, we conducted a multidisciplinary case discussion and rechecked the postoperative pathological section. Histology showed spindled vascular proliferation and area of necrosis (Figure 3). A primary splenic angiosarcoma and hepatic cavernous hemangioma were detected. Immunohistochemical staining of the spleen showed that the lesion was positive for CD31, CD34, F8, and Vim, partially positive for CD68 and CD8, and negative for P53, SMA and CK. The Ki67 index was 20% higher than normal. Postoperative thrombosis, which expended platelets, could explain the thrombocytopenia. Due to the patient’s thrombocytopenia, the risk of performing a liver biopsy was extremely high. Therefore a liver biopsy examination was not performed. Based on the immunohistochemical staining, rapid development of disease, clinical and radiological findings, a primary splenic angiosarcoma with hepatic metastases was finally diagnosed. The patient passed away within four weeks after admission.
PSA is an aggressive malignancy with a rare incidence of 0.14-0.23 cases per million. The half year survival rate is only 20%[1-3]. The mean age at presentation ranges from 50 to 79 years, with a slight male preponderance but no genetic predisposition[1,4]. This aggressive malignant neoplasm is commonly observed in adults, but can also be observed in pediatric groups[5,6]. The disease was first reported in 1879, with only 200 cases currently reported in the literature, largely as isolated case reports[7,8].
The pathogenesis of this tumor remains unclear. For every type of angiosarcoma, thorium dioxide, vinyl chloride, arsenic and chemotherapy for lymphoma or radiation therapy for other malignancies[7,9] have been considered as causative factors. However, no clear relationship between these factors and splenic angiosarcoma has been established. Benign splenic tumors, such as hemangiomas or hemangioendotheliomas, may act as a precursor to splenic angiosarcoma[10,11]. However, there is no evidence that these factors were involved in this patient.
Clinical presentation is nonspecific and may vary from asymptomatic diseases revealed by investigations for unrelated reasons to splenic rupture and lethal hemorrhage[12-14]. Over 75% of patients presented with left upper abdominal pain in one series[15], making it one of the most common presenting symptoms. Other possible complaints include fatigue, anorexia, and weight loss. High temperature, as an associated finding, has been observed in nearly 10% of PSA patients. On physical examination, in addition to splenomegaly as the most consistent sign[7,16], hepatomegaly and a palpable left upper quadrant mass can often be revealed. Blood anomalies, such as anemia and thrombocytopenia, are the most common laboratory abnormalities[17], as in our case, but schistocytes and echinocytes are also common[18]. Spontaneous splenic rupture is observed in 13%-32% of patients presenting with acute abdominal pain[4]. In our case, the patient underwent a splenectomy for traumatic rupture and presented hepatic metastatic cancer as the first manifestation. We considered postoperative thrombosis as the cause of her thrombocytopenia.
Traumatic rupture of angiosarcoma in the spleen is associated with the worst prognosis, with an immediate risk of death from hypovolemic shock and disseminated intravascular coagulopathy. Furthermore, it increases the risk of peritoneal dissemination and hematogenous spread. Reported rates of metastasis range from 69%-100%[4,7,19]. Further, the postoperative metastasis rate remains high. Similar to other forms of angiosarcomas, splenic angiosarcoma commonly has early multifocal metastasis through hematogenous spread[20-22]. Metastasis has been reported by hematogenous routing to the liver, lung, lymph nodes, bone and ovaries[23,24]. In our case, the previous surgery may have accelerated the metastasis, which led to the deterioration.
Imaging modalities are useful for establishing a splenomegaly diagnosis, but are not the determinants of a diagnosis. Specifically, ultrasound, CT and MRI all display supportive evidence of splenomegaly; the most common findings on ultrasound are solitary or multiple, solid and cystic mass lesions with heterogeneous echotexture. On CT imaging, splenic enlargement in the presence of a heterogeneous mass is observed in 60% of cases[25,26]. Contrast CT scanning may reveal non-enhanced areas due to necrosis or enhancement with a blush suggesting active bleeding. There may also be punctuate or widespread calcification. CT imaging is valuable for both diagnosis and acute assessment of complications. Additionally, angiosarcomas may exhibit peripheral or heterogeneous enhancement similar to that of hepatic cavernous hemangiomas. On MRI, both T1-weighted and T2-weighted images show ill-defined nodular lesions with increased or decreased signal intensity, which is related to necrosis or the presence of hemorrhage or fibrosis within the tumor, respectively[25].
The histologic appearance and immunohistochemical analysis of splenic angiosarcoma may be the gold standard for diagnosing the tumor. This tumor had typical features of angiosarcoma, including vasoformative architecture, highly pleomorphic tumor cells with irregular, hyperchromatic and prominent nucleoli, and some mitotic figures. The tumor exhibited “biphasic” immunoreactivity for vascular and histiocytic markers[5]. Immunohistochemically, pathologists always search for at least two vascular proliferation markers (CD31, CD34, and factor VIII) and at least one histiocytic differentiation marker (lysozyme and/or CD68)[7]. Mark et al[27] found that histological appearance or grade was not related to clinical outcome because well-differentiated tumors can behave aggressively. Naka et al[28] conducted a multivariate analysis of 55 angiosarcoma cases and found that tumor size, mode of treatment, and mitotic count were independent prognostic factors. Splenectomy is the preferred treatment for localized disease. Montemayor et al[23] found that patients had a longer survival time if splenectomy was performed prior to rupture compared with after rupture (14.4 mo vs 4.4 mo). There is no specific chemotherapeutic regimen for treating splenic angiosarcoma. Recently, Hara et al[29] reported the use of autologous peripheral blood stem cell transplantation combined with high-dose chemotherapy in splenic angiosarcoma. We suggest that older people may attach great importance to the annual medical examination. The longest survival case was a 7-year-old boy reported by Jun-Te Hsu. The boy retained disease-free at 14.8 years after surgery[30].
In conclusion, primary angiosarcoma of the spleen is an aggressive disease that often presents with metastatic disease. Surgery is the only potentially long-term therapeutic option. Early diagnosis and treatment are very important for prognosis. It is important that clinical doctors improve the understanding of PSA.
A 72-year-old woman with a history of splenectomy and resection of hepatic cavernous hemangioma after trauma two months prior to presenting at our emergency services with right upper quadrant abdominal pain and fatigue for one week.
Mild jaundice and obvious hepatomegaly.
Hematological system diseases and metastatic carcinoma.
WBC 4.3 × 109/L; HGB 70 g/L; PLT 14 × 109/L; D-dimer 50.87 mg/L; ALP 593 U/L; ALT 109 U/L; AST 163 U/L; total bilirubin 96.7μmol/l; direct bilirubin 67.6μmol; indirect bilirubin 29.1 U/L; total protein 51.40 g/L; albumin 27.8 g/L; ANA, ANCA and tumor markers (CA-199, AFP and CEA) were within normal limits.
Abdominal computed tomography and magnetic resonance imaging demonstrated obvious hepatomegaly with multiple liver nodules and spleen loss.
Immunohistochemical staining of the spleen indicated that the lesion was positive for CD31, CD34, F8, and Vim, partially positive for CD68 and CD8, and negative for P53, SMA and CK. The Ki67 index was 20% higher than normal.
The patient was treated with the best supportive treatment.
Primary splenic angiosarcoma is an aggressive malignancy with poor prognosis and we must improve the understanding of this rare disease.
Immunohistochemical staining is based on antigen-antibody reactions to detect whether there is a target antigen in cells or tissue.
This case report presents a case of PSA to improve understanding.
This is a case report on primary angiosarcoma of the spleen with hepatic metastases.
P- Reviewer: Boscá L, Owczarek D S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Wang CH
| 1. | Manouras A, Giannopoulos P, Toufektzian L, Markogiannakis H, Lagoudianakis EE, Papadima A, Papanikolaou D, Filis K, Kekis P. Splenic rupture as the presenting manifestation of primary splenic angiosarcoma in a teenage woman: a case report. J Med Case Rep. 2008;2:133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Naka N, Ohsawa M, Tomita Y, Kanno H, Uchida A, Aozasa K. Angiosarcoma in Japan. A review of 99 cases. Cancer. 1995;75:989-996. [PubMed] |
| 3. | Maddox JC, Evans HL. Angiosarcoma of skin and soft tissue: a study of forty-four cases. Cancer. 1981;48:1907-1921. [PubMed] |
| 4. | Falk S, Krishnan J, Meis JM. Primary angiosarcoma of the spleen. A clinicopathologic study of 40 cases. Am J Surg Pathol. 1993;17:959-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 142] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 5. | Hsu JT, Chen HM, Lin CY, Yeh CN, Hwang TL, Jan YY, Chen MF. Primary angiosarcoma of the spleen. J Surg Oncol. 2005;92:312-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | den Hoed ID, Granzen B, Granzen B, Aronson DC, Pauwels P, de Kraker J, van Heurn LW. Metastasized angiosarcoma of the spleen in a 2-year-old girl. Pediatr Hematol Oncol. 2005;22:387-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Neuhauser TS, Derringer GA, Thompson LD, Fanburg-Smith JC, Miettinen M, Saaristo A, Abbondanzo SL. Splenic angiosarcoma: a clinicopathologic and immunophenotypic study of 28 cases. Mod Pathol. 2000;13:978-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 128] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 8. | Despoina M, Dionysios D, Georgios A, Konstantinos S, Efstratios K, Adamantia ZS. Primary angiosarcoma of the spleen: an oncological enigma. Case Rep Oncol Med. 2014;2014:193036. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Zwi LJ, Evans DJ, Wechsler AL, Catovsky D. Splenic angiosarcoma following chemotherapy for follicular lymphoma. Hum Pathol. 1986;17:528-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | He P, Yan XD, Wang JR, Guo RC, Zhang HB. Splenic littoral cell hemangioendothelioma: report of a case with hepatic metastases and review of the literature. J Clin Ultrasound. 2014;42:308-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Kamocki Z, Steward A, Zaręba KP, Kukliński A, Kędra B. Primary splenic angiosarcoma - the same diagnosis yielding two different clinical pictures. Case report. Contemp Oncol (Pozn). 2013;17:218-221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Badiani R, Schaller G, Jain K, Swamy R, Gupta S. Angiosarcoma of the spleen presenting as spontaneous splenic rupture: A rare case report and review of the literature. Int J Surg Case Rep. 2013;4:765-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Duan YF, Jiang Y, Wu CX, Zhu F. Spontaneous rupture of primary splenic angiosarcoma: a case report and literature review. World J Surg Oncol. 2013;11:53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Yoshida K, Endo T, Kamata K, Aisawa H, Konuma Y, Ogasawara H, Yoshihara A, Kusumi T, Fukuda S. [A case of angiosarcoma of the spleen with intraperitoneal bleeding]. Nihon Shokakibyo Gakkai Zasshi. 2014;111:549-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Hai SA, Genato R, Gressel I, Khan P. Primary splenic angiosarcoma: case report and literature review. J Natl Med Assoc. 2000;92:143-146. [PubMed] |
| 16. | Sordillo EM, Sordillo PP, Hajdu SI. Splenic angiosarcoma. Am J Surg Pathol. 1995;19:119-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Aqil B, Green LK, Lai S. Primary splenic angiosarcoma associated with anemia, leukocytosis and thrombocytopenia. Ann Clin Lab Sci. 2014;44:217-221. [PubMed] |
| 18. | Rosenblatt P, Koka R, Chen Q, Papadimitriou JC, Sausville EA, Emadi A. Schistocytes, echinocytes, iron deficiency anemia, and thrombocytopenia - hematologic manifestations of splenic angiosarcoma. Arch Iran Med. 2013;16:602-605. [PubMed] |
| 19. | Falk S, Stutte HJ, Frizzera G. Littoral cell angioma. A novel splenic vascular lesion demonstrating histiocytic differentiation. Am J Surg Pathol. 1991;15:1023-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 160] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 20. | Abraham JA, Hornicek FJ, Kaufman AM, Harmon DC, Springfield DS, Raskin KA, Mankin HJ, Kirsch DG, Rosenberg AE, Nielsen GP. Treatment and outcome of 82 patients with angiosarcoma. Ann Surg Oncol. 2007;14:1953-1967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 209] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 21. | Rupolo M, Berretta M, Buonadonna A, Stefanovski P, Bearz A, Bertola G, Canzonieri V, Morassut S, Frustaci S. Metastatic angiosarcoma of the spleen. A case report and treatment approach. Tumori. 2001;87:439-443. [PubMed] |
| 22. | Hsu JT, Lin CY, Wu TJ, Chen HM, Hwang TL, Jan YY. Splenic angiosarcoma metastasis to small bowel presented with gastrointestinal bleeding. World J Gastroenterol. 2005;11:6560-6562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Montemayor P, Caggiano V. Primary hemangiosarcoma of the spleen associated with leukocytosis and abnormal spleen scan. Int Surg. 1980;65:369-373. [PubMed] |
| 24. | Valbuena JR, Levenback C, Mansfield P, Liu J. Angiosarcoma of the spleen clinically presenting as metastatic ovarian cancer. A case report and review of the literature. Ann Diagn Pathol. 2005;9:289-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Thompson WM, Levy AD, Aguilera NS, Gorospe L, Abbott RM. Angiosarcoma of the spleen: imaging characteristics in 12 patients. Radiology. 2005;235:106-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 73] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 26. | Vrachliotis TG, Bennett WF, Vaswani KK, Niemann TH, Bova JG. Primary angiosarcoma of the spleen--CT, MR, and sonographic characteristics: report of two cases. Abdom Imaging. 2000;25:283-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Mark RJ, Poen JC, Tran LM, Fu YS, Juillard GF. Angiosarcoma. A report of 67 patients and a review of the literature. Cancer. 1996;77:2400-2406. [PubMed] |
| 28. | Naka N, Ohsawa M, Tomita Y, Kanno H, Uchida A, Myoui A, Aozasa K. Prognostic factors in angiosarcoma: a multivariate analysis of 55 cases. J Surg Oncol. 1996;61:170-176. [PubMed] |
| 29. | Hara T, Tsurumi H, Kasahara S, Ogawa K, Takada J, Imai K, Takai K, Kitagawa J, Kiyama S, Imai N. Long-term survival of a patient with splenic angiosarcoma after resection, high-dose chemotherapy, and autologous peripheral blood stem cell transplantation. Intern Med. 2010;49:2253-2257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Hsu JT, Ueng SH, Hwang TL, Chen HM, Jan YY, Chen MF. Primary angiosarcoma of the spleen in a child with long-term survival. Pediatr Surg Int. 2007;23:807-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |