Published online Oct 21, 2015. doi: 10.3748/wjg.v21.i39.11185
Peer-review started: February 26, 2015
First decision: March 30, 2015
Revised: April 6, 2015
Accepted: August 25, 2015
Article in press: August 25, 2015
Published online: October 21, 2015
Processing time: 238 Days and 14.1 Hours
AIM: To investigate the efficacy (survival) and safety of treatments for recurrent hepatocellular carcinoma (HCC) in liver transplantation (LT) patients.
METHODS: Literature search was performed on available online databases without a time limit until January 2015. Clinical studies describing survival after HCC recurrence in LT patients were retrieved for a full-text evaluation. A total of 61 studies were selected: 13 case reports, 41 retrospective case series, and 7 retrospective comparative studies.
RESULTS: Based on all included studies, the mean HCC recurrence rate was 16% of all LTs for HCC. A total of 1021 LT patients experienced HCC recurrence. The median time from LT to HCC recurrence was 13 mo (range 2-132 mo). The majority of patients (67%) presented with HCC extra-hepatic recurrences, involving lung, bone, adrenal gland, peritoneal lymph nodes, and rarely the brain. Overall survival after HCC recurrence was 12.97 mo. Surgical resection of localized HCC recurrence and Sorafenib for controlling systemic spread of HCC recurrence were associated with the higher survival rates (42 and 18 mo, respectively). However, Sorafenib, especially when combined with mTOR, was frequently associated with severe side effects that required dose reduction or discontinuation
CONCLUSION: Management of recurrent HCC in LT patients is challenging and associated with poor prognosis independently of the type of treatment.
Core tip: The present systematic review analyzes the current trends in the management of hepatocellular carcinoma recurrence after liver transplantation (LT). A great variety of treatment options, ranging from surgical resection to systemic therapies (e.g., Sorafenib), are tailored to the different clinical scenarios and aimed to increase patient survival. However, tumor recurrence after LT is still associated with poor prognosis. By summarizing the available literature, the present article provides to clinicians and surgeons the body of knowledge for a better decision-making process and supports researchers in future clinical trials.
- Citation: de’Angelis N, Landi F, Carra MC, Azoulay D. Managements of recurrent hepatocellular carcinoma after liver transplantation: A systematic review. World J Gastroenterol 2015; 21(39): 11185-11198
- URL: https://www.wjgnet.com/1007-9327/full/v21/i39/11185.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i39.11185
Hepatocellular carcinoma (HCC) is the fifth most common cancer and third leading cause of cancer-related deaths worldwide[1]. In Europe, the age-adjusted incidence rate of HCC is currently 6.2 per 100000 people[2]; however, owing to widespread viral hepatitis infections and nonalcoholic fatty liver disease, HCC incidence and related mortality are expected to increase in western countries over the next 10 years[2-5].
Liver transplantation (LT) is the best treatment option in selected patients for early HCC[6]. When the Milan criteria[7] are fulfilled, the long-term survival following LT for HCC is similar to that following transplantation in patients without HCC[7-9]. However, despite a restrictive patient selection policy, the post-transplant HCC recurrence is reported in up to 25% of cases and drastically affects patient survival[10-13].
Predictors of post-transplant HCC recurrence and prognostic factors have been extensively studied. They have been applied in the selection of candidates for LT and for the construction of models integrating the classic volume-related features (tumor number and maximal size) with histological differentiation, vascular invasion, and, more recently, markers of tumor biological behavior, such as alpha-fetoprotein (AFP) serum level[14-17]. On the contrary, a few well-conducted studies have addressed the management of HCC recurrence in LT patients by describing and comparing clinical outcomes and survival. Currently, the evidence level of recommendations is weak, and there is still a considerable debate about how to treat HCC recurrence in LT patients[6]. Current treatment options include a wide range of therapies, such as surgical resection, transarterial chemoembolization, immunosuppression, and multi-target tyrosine kinases inhibitor (Sorafenib)[18].
The objective of the present systematic review is to summarize and analyze the current available literature in order to evaluate the efficacy and safety of various treatments for HCC recurrence in LT patients. Owing to the lack of international consensus, a systematic approach was chosen to provide an exhaustive report of the clinical experience and current strategies to treat recurrent HCC in LT patients and to support the development of guidelines that will help clinicians in the challenging decision making process.
The methodological approach included the development of selection criteria, definition of search strategies, assessment of study quality, and abstraction of relevant data. The PRISMA statements checklist for reporting a systematic review was followed[19].
The study selection criteria were defined before initiating data collection for proper identification of studies eligible for the analysis. All studies in which the primary objective was to evaluate the efficacy, safety, and/or survival of treatments for HCC recurrence in LT patients were retrieved and analyzed if they met the following selection criteria.
Types of study: All types of prospective and retrospective clinical studies, including case reports, were eligible for inclusion without trial duration limitation. Review articles, commentaries, and conference abstracts were not considered.
Types of participants: Adult LT patients with HCC recurrence were eligible. LT from either deceased or living donor were eligible. Patients with hepato-cholangiocarcinoma and fibrolamellar hepatocellular carcinoma were excluded.
Types of interventions: All types of surgical and non-surgical therapies reported in the literature to treat HCC recurrence in LT patients were eligible.
Types of outcome measures: The primary outcome was survival after treatment of recurrence. The secondary outcomes included safety, tolerability, efficacy, and all other possible clinical parameters evaluated in each study.
A literature search was performed on the following online databases: MEDLINE (through PubMed), EMBASE, Scopus, Cochrane Oral Health Group Specialized Register, and ProQuest Dissertations and Thesis Database. To increase the probability of identifying all relevant articles, a specific research equation was formulated for each database, using the following keywords and/or MeSH terms: hepatocellular carcinoma, recurrence, recurrent hepatocellular carcinoma, liver transplantation, liver transplant, treatment, therapy, management, and Sorafenib (i.e., Nexavar®). In addition, reference lists from eligible studies and relevant review articles (not included in the systematic review) were crosschecked to identify additional studies. A grey literature search was also performed by using the OpenGrey database. No time limitation was applied. Studies written in English, French, Spanish or Italian and meeting the selection criteria were reviewed.
The titles and abstracts of the retrieved studies were independently and blindly screened for relevance by two reviewers (NdeA and MCC). To enhance sensitivity, records were removed only if both reviewers excluded the record at the title screening level. All disagreements were resolved by discussion with a third reviewer (FL). Subsequently, both reviewers performed a full-text analysis of the selected articles. The two reviewers independently assessed the risk of bias using appropriate tools according to the study design. Briefly, the Cochrane criteria described in the Cochrane Handbook for Systematic Reviews of Interventions[20] were applied for RCT, and the Newcastle-Ottawa Scale (NOS)[21] was used for the non-randomized studies. Additionally, the Grading of Recommendations Assessment Development and Evaluation (GRADE) system was used to grade the “body of evidence” merging from this review[22].
Data from the studies included in the systematic review were processed for qualitative and possibly quantitative analyses. Outcome measures (mean values, standard deviation, and ranges) were extracted for each treatment approach. Average survival was calculated as the weighted mean (and standard deviation) of median survivals reported in the included studies. Data from case reports were not pooled into the measurement of the outcomes of interest due to the low level of evidence of this study design.
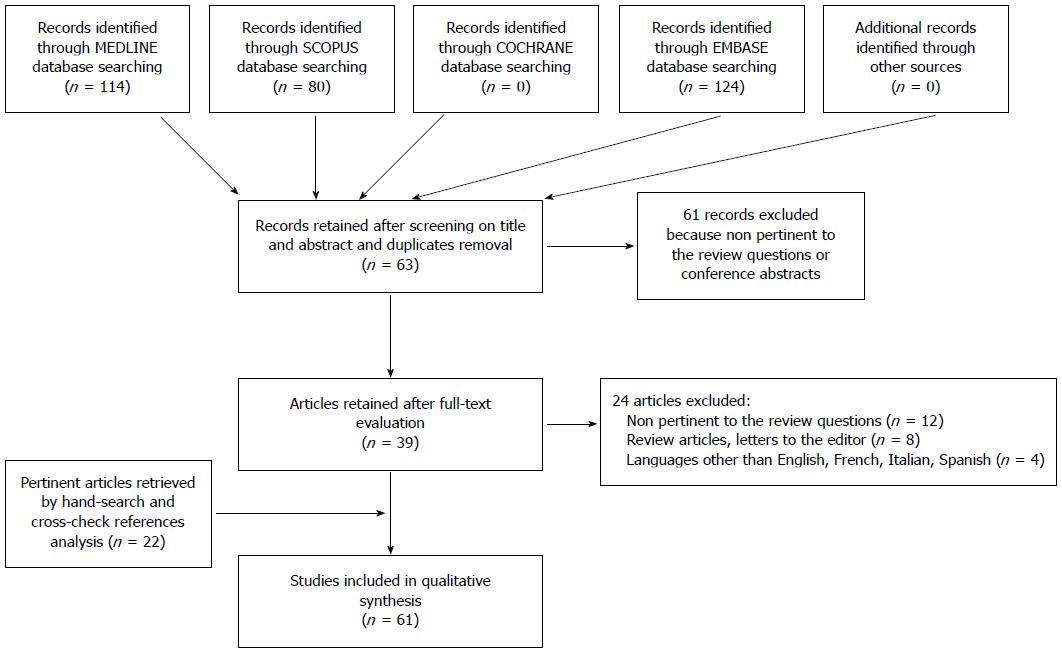
All database searches were performed without time limit until January 2015. Overall, the combined search identified 124 articles (after removing duplicates); of these, 61 were rejected based upon title and abstract evaluation. Out of the remaining 63 articles (which underwent a full-text evaluation), 24 were excluded because they were not pertinent to the review questions, had non-relevant study design, or had language limitations. By reference crosscheck analysis and manual search, we identified 22 additional publications that were included in the total count. Finally, 61 articles were found eligible for the systematic review and were evaluated for both qualitative and quantitative analyses. The flow chart of the study identification and inclusion/exclusion process is shown in Figure 1.
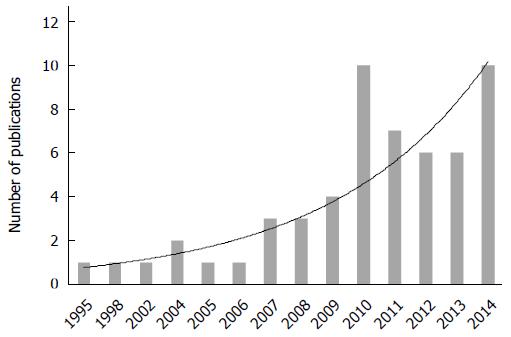
The selected studies included 13 case reports[23-35], 41 retrospective case series[36-76], and 7 retrospective case-control/comparative studies[77-83]. Studies were conducted in 12 different countries including those in Europe (n = 28), North America (n = 13), Asia, and the Pacific (n = 20). Six (9.8%) studies out of 61 were multicentric. The study time frame ranged from 1987 to 2013. Since the first report published in 1995, a progressive increase in the number of publications was noted (Figure 2).
Based on the included studies, the mean rate of HCC recurrence was an average 16% of all patients receiving LT for HCC. The median time from LT to HCC recurrence was 13 mo (range 2-132 mo). The mean age at HCC recurrence diagnosis was 53.8 years, and 84.3% of affected patients being males. Nearly 51% of LT recipients were classified as beyond the Milan Criteria (upon examination of the explanted liver), and 44.5% of LTs were performed from living donors (LDLT).
Overall, 1021 LT patients presenting with HCC recurrence were treated by different modalities. The majority (67%) were extra-hepatic HCC recurrences involving lung, bone, adrenal gland, peritoneal lymph nodes, and, more rarely, the brain. Only 33% of the described cases were limited to hepatic HCC recurrence at diagnosis. All included studies are summarized in Supplementary file.
HCC recurrence was managed with surgical resections, radiofrequency ablation (RFA), microwave ablation, percutaneous ethanol injection (PEI), selective internal radiotherapy treatment (SIRT), stereotactic body radiation therapy, brachytherapy, transarterial chemoembolization (TACE), re-transplantation, immunosuppression (e.g., mTOR), systemic chemotherapy, administration of a multi-kinase inhibitor agent (Sorafenib), and best supportive care (BSC). Most of the studies applied multimodality therapies, which usually involved surgery and systemic treatments.
The overall median survival after HCC recurrence was 12.97 mo (mean range: 0.1-112.5 mo) (Supplementary file). In order to estimate the survival per treatment, the included studies were grouped based on the type of management applied and were considered into the estimation only if they reported the median survival for the specific treatment modality[34,38,39,45,47,50,54,58,63-65,73,74,76-80,84] (Table 1). Among the loco-regional treatments, surgical resection of HCC recurrence was associated with the highest survival (42 mo), whereas in the case of systemic spread of HCC recurrence, a longer survival was related to the use of Sorafenib combined with immunosuppression (mTOR) (18.3 mo). In all related studies, BSC was associated with the lowest survival (3.3 mo) (Table 1).
| Type of treatment for HCC recurrence in LT patients | No. of patients | Median survival1 (mo) (weighted mean ± SD) | Ref. |
| Loco-regional treatments for resectable local recurrence of HCC | |||
| Surgery | 27 | 42 ± 24.45 | Bates et al[38] |
| Kornberg et al[50] | |||
| Pfiffer et al[54] | |||
| Kim et al[47] | |||
| Chen et al[77] | |||
| Sommacale et al[74] | |||
| TACE | 40 | 11.2 ± 8.81 | Tan et al[80] |
| Kim et al[47] | |||
| Pfiffer et al[54] | |||
| Carr[39] | |||
| Chen et al[77] | |||
| Yamagami et al[64] | |||
| Systemic treatments for unresectable, advanced, multifocal recurrence of HCC | |||
| Sorafenib | 76 | 12.1 ± 9.95 | Tan et al[80] |
| Yoon et al[65] | |||
| Pfiffer et al[54] | |||
| Staufer et al[76] | |||
| Sposito et al[79] | |||
| Pfeiffenberger et al[78] | |||
| Alsina et al[73] | |||
| Sorafenib + mTOR | 68 | 18.2 ± 6.53 | Waidmann et al[34] |
| Gomez-Martin et al[45] | |||
| Weimann et al[63] | |||
| Staufer et al[76] | |||
| Sotiropoulos et al[58] | |||
| Systemic chemotherapy | 35 | 5.79 ± 2.7 | Lee et al[84] |
| Kim et al[47] | |||
| Best supportive care | 54 | 3.3 ± 2.12 | Kim et al[47] |
| Pfiffer et al[54] | |||
| Yoon et al[82] | |||
| Sposito et al[79] |
Surgical resection was performed for either localized intra-hepatic or extra-hepatic HCC recurrence. Resection of isolated HCC recurrence appeared to be safe and effective, with no reported post-operative mortality. The post-operative period was also reported as uneventful in the majority of the study[24,40,71,75,83]; one study, however, reported a very high morbidity rate after liver resection for post-LT HCC recurrence[74] (Table 2).
| Ref. | No. of patients | Surgical treatments | Post-operative morbidity | Post-operative mortality |
| (No. of patients) | ||||
| Regalia et al[75] | 7 | Liver resection (2), pulmonary lobectomy (2), omentectomy (1), bone resection (1), skin resection (1) | Uneventful | 0 |
| Castroagudìn et al[24] | 1 | Bilateral adrenalectomies in a successive manner | Uneventful | 0 |
| Catalano et al[40] | 2 | Liver resection (2) | Uneventful | 0 |
| Roayaie et al[55] | 15 | Liver resection (5), pulmonary resection (7), adrenalectomy (2), chest wall resection (1) | NR | 0 |
| Bates et al[38] | 5 | Pulmonary lobectomy (4), pulmonary lobectomy + rib resection after pre-transplant tumor biopsy (1) | NR | 0 |
| Kwon et al[71] | 7 | Pulmonary resection (7) | Uneventful | 0 |
| Marangoni et al[52] | 4 | Liver resection (4) | NS | 0 |
| Han et al[69] | 12 | Pulmonary resection (12) | NS | 0 |
| Kornberg et al[50] | 7 | Liver resection (2), pulmonary resection (2), cerebral tumor extirpation (1), adrenalectomy (1), chest wall resection after pre-transplant tumor biopsy (1) | NR | 0 |
| Valdivieso et al[61] | 8 | Liver resection (2), adrenalectomy (2), abdominal lymph node resection (2), pulmonary resection (2) | NR | 0 |
| Kitano et al[70] | 3 | Pulmonary resection (3) | 0 | |
| Pfiffer et al[54] | 7 | Liver resection (1), extra-hepatic resection (6) | NR | NR |
| Kim et al[47] | 3 | Left adrenalectomy (1), splenectomy (1), lymph node resection | NR | 0 |
| Chen et al[77] | 2 | Pulmonary resection (1), adrenalectomy (1) | NR | 0 |
| Hwang et al[83] | 23 | Pulmonary resection (23) | Uneventful | 0 |
| Sommacale et al[74] | 3 | Liver resection (3) | 100% (renal failure, respiratory sepsis, sub phrenic abscess) | 0 |
Among the loco-regional therapies for HCC recurrence, TACE was the most frequently reported. Overall, TACE was well tolerated and not associated with major adverse events. Anecdotal reports described SIRT, RFA, and CT guided brachytherapy as effective, well-tolerated and safe loco-regional treatment approaches (Table 3).
| Ref. | No. of patients | Treatment | No. of treatments per patient | Efficacy | Side effects | Tolerability | Safety |
| (No.of patients) | (No. of patients) | ||||||
| Rivera et al[31] | 1 | SIRT (Y-90) | 1 | Efficacy demonstrated by tumor necrosis on imaging and decreased AFP level | Intermittent nausea Mild right upper quadrant abdominal pain | Well tolerated | No adverse consequence |
| Ho et al[27] | 1 | RFA | 1 | No evidence of local progression and normalization of AFP levels | None | Well tolerated | No adverse consequence |
| Ko et al[49] | 28 | TACE | 2.5 | Complete response (3), partial response (11), minimal response (5), stable disease (3), progressive disease (6) | In 17.9% of patients: Nausea, vomiting, diarrhea Hypertension, tachycardia Mild right upper quadrant abdominal pain | Well tolerated | No adverse consequence |
| Tan et al[80] | 10 | TACE | NR | According to RECIST criteria, partial response (1), stable disease (3), and progressive disease (6) | NR | Well tolerated | No adverse consequence |
| Carr[39] | 6 | TACE | 8.2 | Complete response (1), partial response (2), stable disease (1), progression (2) | Bilirubin toxicity (Grade 2) (1) Granulocyte toxicity (Grade 3) (3) | Well tolerated | No adverse consequence |
| Chen et al[77] | 4 | TACE | 2.8 | According to mRECIST criteria, complete or partial response in all patients | None | Well tolerated | No adverse consequence |
| Cheng et al[41] | 11 | TACE | NR | NR | NR | Well tolerated | No adverse consequence |
| Zhang et al[67] | 10 | CT 125I guided brachytherapy | 3.9 | Complete local control of HCC recurrence 72% of patients at 2 yr | Minor displacement of radioactive seeds (2) | Well tolerated | Hemothorax (1) |
| Mild increase of white blood cell counts (3) | Hemosputum (3) | ||||||
| Fever (4) |
Efficacy and safety of Sorafenib: Since 2009, several studies investigated the safety and effectiveness of Sorafenib alone or in combination with modified immunosuppressors, namely Everolimus or Sirolimus (mTOR). As summarized in Table 4, the most common dose of Sorafenib was 400 mg/bid. However, this dosage was rarely tolerated, and dose reduction or discontinuation was reported in the majority of the studies. Overall, 197 patients were treated with Sorafenib: 42.1% of these required dose reduction, whereas 9.6% discontinued the therapy due to intolerable side effects. The most common side effects observed in almost all studies included gastrointestinal symptoms, hand foot skin reactions, hypertension, and fatigue. Six out of 23 studies[26,34,45,53,66,76] reported severe adverse events following the administration of Sorafenib combined with mTOR; among these, 4 cases reported death (Table 4).
| Ref. | n | Treatments | Type of immunosuppression | Efficacy(No. of patients) | Most frequent side effects1 | Tolerability | Safety(No. of patients) |
| Yeganeh et al[35] | 1 | Sorafenib (400 m bid) | Tacrolimus + mycophenolate mofetil | Complete resolution of his lung lesion | Diarrhea Hand-foot skin reactions | Dose reduction needed (200 mg bid) | No major adverse consequence |
| Bhoori et al[23] | 1 | Sorafenib (400 mg bid) | Everolimus | 50% response according to RECIST criteria modifications after introduction of Everolimus | Hand-foot skin reactions (Grade 1) | Well tolerated | No major adverse consequence |
| Herden et al[26] | 1 | Sorafenib (200 mg bid) | Cyclosporine A | NR | Nausea Fever up to 39 °C Jaundice | Discontinuation after 5 d of treatment | Centrolobular hepatocellular necrosis and lymphoplasmacellular and granulocytic infiltration of portal tracts with significant eosinophilia (hyper allergic drug reaction) |
| Kim et al[48] | 9 | Sorafenib (200 to 400 mg bid) | Tacrolimus ± Sirolimus | Complete radiographic response (1), stable disease (4), progression (3) | Hand-foot skin reactions Fatigue and anorexia Diarrhea Mucositis | Discontinuation in 5 patients | No major adverse consequence nor deterioration of liver graft function |
| Tan et al[80] | 10 | Sorafenib (400 mg bid) | Tacrolimus | Stable disease (7), progressive disease (3) | Rash Hypertension Agrypnia Hand-foot skin reaction Diarrhea | Discontinuation in 1 patient | No major adverse consequence |
| Valdivieso et al[61] | 5 | Sorafenib (400 mg bid) | Everolimus | NR | NR | NR | NR |
| Wang et al[37] | 1 | Sorafenib (400 mg bid) | Tacrolimus/Sirolimus | Partial response (after introduction of Sirolimus) | Hand-foot skin reactions | Dose reduction needed (200 mg bid) | No major adverse consequence |
| Yoon et al[65] | 13 | Sorafenib (400 mg bid) | Calcineurin inhibitors ± mycophenolate mofetil | Stable disease (6) | Chest wall pain Hand-foot skin reactions Neutropenia, thrombocytopenia, anemia Rash Diarrhea | Dose reduction (200 mg bid) in 4 patients | No major adverse consequence |
| Kim et al[28] | 1 | Sorafenib (200 to 400 mg bid) | Sirolimus | Complete radiologic response | Hand-foot skin reactions Fatigue Mucositis | Dose reduction needed (200 mg bid) | No major adverse consequence |
| Takahara et al[33] | 2 | Sorafenib (200 to 400 mg bid) | Cyclosporine/Tacrolimus | Complete response (1) | Hypertension Diarrhea, anorexia | Dose reduction (200 mg bid) for 1 patient | No major adverse consequence |
| Waidmann et al[34] | 3 | Sorafenib (400 mg bid) | Everolimus/Sirolimus | Partial response after introduction of mTOR (1), progression (1) | Hand-foot skin reactions (Grade 3) Fatigue (Grade 3) | Discontinuation for 1 patient, dose reduction (200 mg bid) for 1 patient | Death for sepsis and multi-organ failure (1) after 3 wk of Sorafenib treatment |
| Gomez-Martin et al[45] | 31 | Sorafenib (400 mg bid) | Everolimus | Complete response (1), partial response (1), and stable disease (13) | Mild graft dysfunction Hand-foot skin reactions Asthenia Hypertension Diarrhea Thrombocytopenia | Dose reduction (200-300 mg bid) in 8 patients | Central nervous system hemorrhaging (1), severe biventricular heart failure (1), and upper digestive hemorrhaging (2) leading to death (1) |
| Sotiropoulos et al[58] | 14 | Sorafenib (400 mg bid) | mTOR | Progression (4) | NR | Discontinuation for 4 patients, dose reduction (100 to 200 mg bid) for 2 patients | NR |
| Staufer et al[76] | 13 | Sorafenib (400 mg bid) | mTOR/Cyclosporine A | Partial response (1), stable disease (4), progression (7) | Anemia, leukopenia Hand-foot skin reactions Increase of liver function tests Fatigue | Poor tolerability. Dose reduction (200 mg bid) in 10 patients | Centrolobular hepatocellular necrosis and lymphoplasmacellular infiltration of portal tracts (2) with eosinophilia (2) |
| Vitale et al[62] | 10 | Sorafenib (400 mg bid) | mTOR/Tacrolimus | According to mRECIST criteria, partial response (2), stable disease (6), progression (2) | Diarrhea Diarrhea Hand-foot skin reactions Fatigue | Dose reduction in 7 patients | No major adverse consequence |
| Weinmann et al[63] | 11 | Sorafenib (400 mg bid) | Sirolimus | Stable disease (4), progression (7) | Diarrhea Fatigue Nausea Hand-foot skin reactions Hair loss Weight loss | Discontinuation and dose reduction (200 mg bid) for 7 patients | No major adverse consequence |
| Pfeiffenberger et al[78] | 8 | Sorafenib (400 mg bid) | Tacrolimus/Tacrolimus + mycophenolate mofetil/Cyclosporin A | Progression (1) | Hand-foot skin reaction Diarrhea | Dose reduction for 6 patients | No major adverse consequence |
| Sposito et al[79] | 15 | Sorafenib (400 mg bid) | mTOR/Cyclosporine A/Tacrolimus | According to RECIST, stable disease (11), partial response (4) | Hand-foot skin reactions Diarrhea Fatigue | Dose reduction (200 mg bid) for 8 patients | No major adverse consequence |
| Waghray et al[81] | 17 | Sorafenib (400 mg bid) | Sirolimus | Complete response (2), partial response (1), stable disease (2), progression (5) | Diarrhea, nausea Fatigue Increase of liver function tests Hand-foot skin reactions | Dose reduction (100 to 200 mg bid) for 14 patients | No major adverse consequence |
| Zavaglia et al[66] | 11 | Sorafenib (400 mg bid) | Everolimus/Cyclosporine A | Partial response (2), stable disease (1), progression (6) | Fatigue, anorexia Hypophosphatemia Diarrhea, nausea, vomiting Hand-foot skin reactions | Discontinuation in 4 patients, dose reduction (200 mg bid) for 7 patients | Massive gastrointestinal bleeding leading to death after 4 mo of Sorafenib + mTOR |
| Alsina et al[73] | 9 | Sorafenib (400 mg bid) | Tacrolimus/Cyclosporine A ± mycophenolate mofetil | NR | Hand-foot skin reaction Rash Seizure Skin flushing | Dose reduction for 2 patients | No major adverse consequence |
| De Simone et al[43] | 7 | Sorafenib (400 mg bid) | Everolimus | According to mRECIST, progression (5) | Hand-foot skin reactions Hypertension Diarrhea Anorexia, asthenia Hoarseness Alopecia | Dose reduction for 3 patients, temporary discontinuation for 2 patients | No major adverse consequence |
| Perricone et al[53] | 4 | Sorafenib (200 mg bid) | Everolimus | Stable disease (1), progression (3) | Diarrhea (Grade 3) | Discontinuation for 1 patient | Severe diarrhea with progressive worsening of clinical condition, leading to coma then death (1) 4 mo after Sorafenib + mTOR |
Based on the RECIST classification (Response Evaluation Criteria in Solid Tumors) or its modified version (mRECIST)[85] used in some studies, Sorafenib appeared to have only a partial effect; indeed, stable disease was observed in the majority of the cases (46.5%), whereas a considerable amount of patients (37%) showed progression of the disease. Only in a minority of cases (5.5%), a complete response to Sorafenib was found.
Study quality assessment: Two reviewers (NdeA and MCC) scored the methodological qualities of the included studies according to the criteria described above. The majority of the studies were case reports or case series without any comparison between different treatments. No RCT was found, therefore the Cochrane criteria were not applied. Only 12 retrospective studies (19.6%) performed statistical comparisons between groups of treatments for HCC recurrence in LT patients. The quality and risk of bias in these studies were assessed according to the Newcastle-Ottawa Criteria and are summarized in Supplementary file. Overall, 3 studies were classified at a low risk of bias, and 9 studies were at high risk of bias.
In addition, the GRADE system was used to enable consistent judgment of the quality of the available evidence included in this systematic review. The quality of the evidence was rated according to the following aspects: study design, study quality, consistency, and directness of results. Fourteen studies[42,47,49,55-57,59,68,72,75,79-81,83] (22.9%) were judged as being of low quality, and the remaining 47 studies[23-41,43-46,50,52-54,58,60-67,69-71,73,74,76-78,82,84,86] had a very low quality of evidence. Of note, the majority of studies were retrospective, which, by definition, are susceptible of major selection bias as well as misclassification or information bias due to the unknown accuracy of record keeping.
Despite the stringent selection of LT candidates and measures to control the recipient[16,56,87] and, more recently, donor-related[88] risk factors, post-transplant HCC recurrence is a reality that occurs in average 16% of patients and drastically affects their survival. Treatments for HCC recurrence have been receiving increasing attention in the literature, as shown by the rising number of publications in the recent years. However, there are no randomized clinical trial or large sample prospective studies available on the topic. The present systematic review might provide a better understanding of the clinical experience and thus support the development of treatment strategies tailored for different clinical settings.
As supported by several studies[50,57,59,60,72], the time between LT and HCC recurrence is a key predictor of survival, with worse prognosis associated with early HCC recurrence (within 24 mo). From a physiopathological perspective, early HCC recurrence could occur due to non-detectable extra-hepatic metastases that were present before LT, as well as a consequence of circulating HCC cell clones engrafting and growing in a target organ in the post-transplant period[89]. Conversely, late HCC recurrence appeared to be the consequence of late engrafting of HCC cells that remained latent and less numerous for a longer time post LT[60,89]. In LT patients, immunosuppression may also play a role in the recurrence of HCC[90,91]; however, it remains controversial whether the administration of mTOR as a first line of management after LT can control both the inherent risk of graft rejection and the potential risk of tumor recurrence[6,92,93] or impact on the delay and extension of HCC recurrence. Based on all included studies, the median HCC recurrence time was 13 mo; thus, the majority of HCC recurrence appeared early after LT. It was not possible from the available data to make distinctions and comparisons between early and late HCC recurrence. Although described in some studies, the survival was usually reported for all patients together. Notwithstanding, early HCC recurrence can be considered a negative prognostic factor for survival[42,47,50,60].
The majority of patients presented as extra-hepatic HCC recurrence at diagnosis, most commonly located at the lung, bone, adrenal gland, and abdominal lymph nodes. Hepatic recurrence, especially late recurrence, might be indicative of de novo HCC development from recurrent hepatitis and cirrhosis in the liver graft. However, in the absence of molecular profiling analyses to distinguish recipient origin from donor origin, it is impossible to determinate the nature of HCC recurrence[94]. As it is rarely described, the actual incidence of de novo HCC post-LT remains unclear.
Another prognostic factor that emerged from the analysis of the literature is the pattern of HCC recurrence, either as localized, isolated nodule(s) or multifocal metastases. This is shown to impact on patient survival as well as on the choice of treatment strategies. For both isolated hepatic and extra-hepatic metastases, surgical resection appeared to be the best treatment option offering longer survival chance[50,52,59,61,72,75,83]. However, a major selection bias has to be highlighted: LT patients with HCC recurrence undergoing surgery are usually the ones with the best performance status, late recurrence, and most favorable localization allowing resection. Indeed, reported post-operative mortality and morbidity were very low in both resection of grafted liver and other organ metastases (e.g., lung, adrenal gland). Based on the current knowledge, surgery for HCC recurrence is a valuable option if performed in selected patients with curative intents, and, as recommended in a recent consensus conference[6], surgery should be attempted whenever feasible. On the contrary, little is known about re-transplantation for intra-hepatic recurrent HCC, and it is currently considered not appropriate[6].
Selected patients with unresectable but limited HCC recurrence may undergo loco-regional therapy including TACE, SIRT, and RFA, with potential improvement in survival[6]. These treatments appeared to be safe and well tolerated and may be repeated multiple times or combined in a multimodality approach[31,41,49,54,56,68,75,80].
When recurrent HCC is presenting as or becomes systemically spread, systemic treatments are warranted[6]. Among these, systemic chemotherapy (e.g., doxorubicin, or fluoropyrimidine and platinum) demonstrated very limited efficacy in both preventing and controlling HCC recurrence[84], confirming HCC as a low chemo-sensitivity tumor. Little is known about the efficacy of systemic chemotherapy combined with other immunosuppressive agents like mTOR.
In contrast, systemic therapy with Sorafenib has gained significant interest in the recent years and has been the object of several publications assessing its efficacy and safety for the treatment of HCC recurrence in LT patients. Some studies demonstrated that the administration of Sorafenib, with or without mTOR, is associated with improvement of patient survival[45,58,62,63,73,79,81]. However, several studies also reported adverse effects related to Sorafenib, which required dose reduction or discontinuation in a high percentage of patients[26,34,43,45,53,66,76]. Of note, Sorafenib has been associated with major adverse effects (Grade 3 and 4) including five cases of centrolobular hepatocellular necrosis with lymphoplasmacellular and granulocytic infiltration of the portal tracts (with or without eosinophilia)[26,45,76], 1 case of massive gastrointestinal bleeding[66], 1 case of sepsis and multi-organ failure[34], and 1 case of severe diarrhea[53]. The complication of these conditions led to death in 4 patients. Therefore, the risk/benefit and the cost/effectiveness ratios of Sorafenib remain unknown and should be further investigated[95]. However, pooling together the available data, it seems that most of the times, Sorafenib is able to stabilize the disease, even though complete responses are rare. Notwithstanding, a benefit in patient survival post-HCC recurrence has been reported in the majority of the study series[23,34,61,65,73,76,79,80], which was assessed at 12.1 mo for Sorafenib alone and at 18.2 mo for Sorafenib + mTOR. This is highly superior to the 3.3 mo of survival for patients receiving best supportive care[79]. A synergistic effect of Sorafenib + mTOR inhibitors has been advocated by some authors[23,45,79]; however, the current evidence is insufficient to draw definitive conclusions. Moreover, it was not possible to specifically assess the effects of Sorafenib combined with different ongoing immunosuppressive therapies (e.g., calcineurin inhibitors, Tacrolimus).
The use of mTOR has also been proposed as an immunosuppression paradigm to be applied at the time of HCC recurrence[93,96]. This is because mTOR seems to have a strong immunosuppressive activity with concomitant antineoplastic properties[97] that may prevent or control HCC recurrence as well as protect from de novo cancers[98].
Managing post-transplant recurrent HCC is a challenging field, as reflected by the highly heterogeneous conditions and treatment strategies encountered in the literature. Since the first report in 1995[30], novel therapies have been introduced; however, international guidelines are still lacking. The available evidence is of rather low quality to sort management paradigms with predictable outcomes. Waiting for a significant progress, the best treatment may likely remain the prevention of HCC recurrence by applying stringent selection criteria for LT candidates and relying on up-to-date imaging and biological assessments, which need to be repeated shortly before transplantation.
However, once HCC recurrence is diagnosed in LT patients, clinicians and surgeons face a difficult decision making process. Recently, Toso et al[98] proposed a management algorithm that applies different treatment strategies depending on the localization of the recurrence (hepatic vs extra-hepatic) and the feasibility of surgical resection. The present systematic review supports this paradigm, which can be summarized as follows: (1) consider to switch to mTOR or decrease overall immunosuppression; (2) apply surgery whenever feasible; (3) reserve more aggressive approaches for cases with better potential outcomes (e.g., late recurrence); and (4) recur to systemic treatments, namely Sorafenib, for unresectable multifocal disease and HCC re-recurrence.
The present systematic review has several limitations mainly related to the retrospective nature of the included studies, which displayed high heterogeneity in the clinical parameters and outcomes evaluated, missing data, and small sample size. Moreover, external validity of the present results cannot be assured due to the highly selected study populations and specialized centers in which the clinical trials have been performed.
The clinical management of HCC recurrence in LT patients is challenging and is associated with a poor prognosis independently of the type of treatment. Most of the time, a multimodal approach is required to slow down the disease progression. Although the administration of Sorafenib with or without mTOR appears promising, further clinical trials are needed to assess its efficacy and safety. Finally, an international consensus meeting should be advocated in order to assess guidelines and draw up future research directions in the field of HCC recurrence management in LT patients.
Hepatocellular carcinoma (HCC) recurrence after liver transplantation drastically affects patient survival. Management of HCC recurrence involves a wide range of local and systemic treatment modalities that lack established guidelines.
The present systematic review provides a better understanding of the clinical experience in the management of post-transplant HCC recurrence. It also highlights the need of developing standardized treatment strategies tailored for different clinical settings.
By means of the systematic approach, this review allows the reader to have an overview of the state of art in the management of post-transplant HCC recurrence, ranging from surgery to systemic therapy. However, this study also highlights that the overall evidence available are low and further well-conducted studies are needed.
The clinical management of HCC recurrence in transplanted patients remains challenging and is associated with a poor prognosis independently of the type of treatment. In the near future, research studies need to establish whether the treatment approach has to be chosen based on the underlying etiology of liver disease or HCC recurrence pattern; which are the indications and outcomes of multimodal approaches; which are the efficacy and safety of target therapy combined with immunosuppressant agents. Finally, an international consensus meeting is awaited in order to assess guidelines and clinical recommendations.
Sorafenib is an orally active multikinase inhibitor approved for the treatment of advanced HCC. mTOR (i.e., Mammalian target of rapamycin) inhibitors can be used as immunosuppressant agents in transplant patients as well as cancer chemotherapy.
This is an interesting systematic review describing the management of post transplant HCC recurrence.
P- Reviewer: He ST, Kapoor S S- Editor: Ji FF L- Editor: A E- Editor: Liu XM
| 1. | Thomas MB, Jaffe D, Choti MM, Belghiti J, Curley S, Fong Y, Gores G, Kerlan R, Merle P, O’Neil B. Hepatocellular carcinoma: consensus recommendations of the National Cancer Institute Clinical Trials Planning Meeting. J Clin Oncol. 2010;28:3994-4005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 323] [Article Influence: 21.5] [Reference Citation Analysis (1)] |
| 2. | Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D, Bray F. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3526] [Cited by in RCA: 3659] [Article Influence: 304.9] [Reference Citation Analysis (2)] |
| 3. | El-Serag HB. Hepatocellular carcinoma and hepatitis C in the United States. Hepatology. 2002;36:S74-S83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 129] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 4. | El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557-2576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3846] [Cited by in RCA: 4267] [Article Influence: 237.1] [Reference Citation Analysis (2)] |
| 5. | Mittal S, Sada YH, El-Serag HB, Kanwal F, Duan Z, Temple S, May SB, Kramer JR, Richardson PA, Davila JA. Temporal trends of nonalcoholic fatty liver disease-related hepatocellular carcinoma in the veteran affairs population. Clin Gastroenterol Hepatol. 2015;13:594-601.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 210] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 6. | Clavien PA, Lesurtel M, Bossuyt PM, Gores GJ, Langer B, Perrier A. Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol. 2012;13:e11-e22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 761] [Cited by in RCA: 785] [Article Influence: 60.4] [Reference Citation Analysis (1)] |
| 7. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5110] [Cited by in RCA: 5313] [Article Influence: 183.2] [Reference Citation Analysis (0)] |
| 8. | Schwartz M. Liver transplantation for hepatocellular carcinoma. Gastroenterology. 2004;127:S268-S276. [PubMed] |
| 9. | Yao FY, Ferrell L, Bass NM, Bacchetti P, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: comparison of the proposed UCSF criteria with the Milan criteria and the Pittsburgh modified TNM criteria. Liver Transpl. 2002;8:765-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 313] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 10. | Sutcliffe R, Maguire D, Portmann B, Rela M, Heaton N. Selection of patients with hepatocellular carcinoma for liver transplantation. Br J Surg. 2006;93:11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Hollebecque A, Decaens T, Boleslawski E, Mathurin P, Duvoux C, Pruvot FR, Dharancy S. Natural history and therapeutic management of recurrent hepatocellular carcinoma after liver transplantation. Gastroenterol Clin Biol. 2009;33:361-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Davis E, Wiesner R, Valdecasas J, Kita Y, Rossi M, Schwartz M. Treatment of recurrent hepatocellular carcinoma after liver transplantation. Liver Transpl. 2011;17 Suppl 2:S162-S166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 13. | Welker MW, Bechstein WO, Zeuzem S, Trojan J. Recurrent hepatocellular carcinoma after liver transplantation - an emerging clinical challenge. Transpl Int. 2013;26:109-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 14. | Duvoux C, Roudot-Thoraval F, Decaens T, Pessione F, Badran H, Piardi T, Francoz C, Compagnon P, Vanlemmens C, Dumortier J. Liver transplantation for hepatocellular carcinoma: a model including α-fetoprotein improves the performance of Milan criteria. Gastroenterology. 2012;143:986-994.e3; quiz e14-e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 561] [Cited by in RCA: 728] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 15. | DuBay D, Sandroussi C, Sandhu L, Cleary S, Guba M, Cattral MS, McGilvray I, Ghanekar A, Selzner M, Greig PD. Liver transplantation for advanced hepatocellular carcinoma using poor tumor differentiation on biopsy as an exclusion criterion. Ann Surg. 2011;253:166-172. [PubMed] |
| 16. | Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, Camerini T, Roayaie S, Schwartz ME, Grazi GL. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1574] [Article Influence: 92.6] [Reference Citation Analysis (1)] |
| 17. | Toso C, Asthana S, Bigam DL, Shapiro AM, Kneteman NM. Reassessing selection criteria prior to liver transplantation for hepatocellular carcinoma utilizing the Scientific Registry of Transplant Recipients database. Hepatology. 2009;49:832-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 286] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 18. | Rubin J, Ayoub N, Kaldas F, Saab S. Management of recurrent hepatocellular carcinoma in liver transplant recipients: a systematic review. Exp Clin Transplant. 2012;10:531-543. [PubMed] |
| 19. | Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52948] [Cited by in RCA: 47201] [Article Influence: 2950.1] [Reference Citation Analysis (0)] |
| 20. | Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18487] [Cited by in RCA: 24869] [Article Influence: 1776.4] [Reference Citation Analysis (3)] |
| 21. | Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8858] [Cited by in RCA: 12674] [Article Influence: 844.9] [Reference Citation Analysis (0)] |
| 22. | Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11058] [Cited by in RCA: 14940] [Article Influence: 878.8] [Reference Citation Analysis (0)] |
| 23. | Bhoori S, Toffanin S, Sposito C, Germini A, Pellegrinelli A, Lampis A, Mazzaferro V. Personalized molecular targeted therapy in advanced, recurrent hepatocellular carcinoma after liver transplantation: a proof of principle. J Hepatol. 2010;52:771-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 24. | Castroagudín JF, González-Quintela A, Martínez J, Tomé S, Forteza J, Varo E. Bilateral adrenal metastases from hepatocellular carcinoma after liver transplantation. Hepatogastroenterology. 2002;49:249-251. [PubMed] |
| 25. | Gringeri E, Boetto R, Bassi D, D’Amico FE, Polacco M, Romano M, Neri D, Feltracco P, Zanus G, Cillo U. Laparoscopic microwave thermal ablation for late recurrence of local hepatocellular carcinoma after liver transplant: case report. Prog Transplant. 2014;24:142-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Herden U, Fischer L, Schäfer H, Nashan B, von Baehr V, Sterneck M. Sorafenib-induced severe acute hepatitis in a stable liver transplant recipient. Transplantation. 2010;90:98-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 27. | Ho CK, Chapman WC, Brown DB. Radiofrequency ablation of recurrent hepatocellular carcinoma in a patient after liver transplantation: two-year follow-up. J Vasc Interv Radiol. 2007;18:1451-1453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Kim R, Aucejo F. Radiologic complete response with sirolimus and sorafenib in a hepatocellular carcinoma patient who relapsed after orthotopic liver transplantation. J Gastrointest Cancer. 2011;42:50-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Mazloom A, Hezel AF, Katz AW. Stereotactic body radiation therapy as a bridge to transplantation and for recurrent disease in the transplanted liver of a patient with hepatocellular carcinoma. Case Rep Oncol. 2014;7:18-22. [PubMed] |
| 30. | Ringe B, Böker K, Schlitt HJ, Sproviero J, Hundrieser J, Tillmann HL, Chavan A, Flemming P, Galanski M, Pichlmayr R. Recurrence of hepatitis B virus cirrhosis and hepatocellular carcinoma: an indication for retransplantation? Clin Transplant. 1995;9:190-196. [PubMed] |
| 31. | Rivera L, Giap H, Miller W, Fisher J, Hillebrand DJ, Marsh C, Schaffer RL. Hepatic intra-arterial infusion of yttrium-90 microspheres in the treatment of recurrent hepatocellular carcinoma after liver transplantation: a case report. World J Gastroenterol. 2006;12:5729-5732. [PubMed] |
| 32. | Stippel DL, Kasper HU, Schleimer K, Töx U, Bangard C, Hölscher AH, Beckurts KT. Successful use of sirolimus in a patient with bulky ovarian metastasis of hepatocellular carcinoma after liver transplantation. Transplant Proc. 2005;37:2185-2187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 33. | Takahara T, Nitta H, Hasegawa Y, Itou N, Takahashi M, Wakabayashi G. Using sorafenib for recurrent hepatocellular carcinoma after liver transplantation--interactions between calcineurin inhibitor: two case reports. Transplant Proc. 2011;43:2800-2805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 34. | Waidmann O, Hofmann WP, Zeuzem S, Trojan J. mTOR inhibitors and sorafenib for recurrent heptocellular carcinoma after orthotopic liver transplantation. J Hepatol. 2011;54:396-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 35. | Yeganeh M, Finn RS, Saab S. Apparent remission of a solitary metastatic pulmonary lesion in a liver transplant recipient treated with sorafenib. Am J Transplant. 2009;9:2851-2854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 36. | Alamo JM, Barrera L, Casado MD, Bernal C, Marin LM, Suarez G, Sanchez-Moreno L, Jimenez R, Suarez-Grau JM, Sousa JM, Cordero E, Gomez-Bravo MA. Efficacy, tolerance, and safety of mammalian target of rapamycin inhibitors as rescue immunosuppressants in liver transplantation. Transplant Proc. 2009;41:2181-2183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 37. | Wang Y, Speeg KV, Washburn WK, Halff G. Sirolimus plus sorafenib in treating HCC recurrence after liver transplantation: a case report. World J Gastroenterol. 2010;16:5518-5522. [PubMed] |
| 38. | Bates MJ, Farkas E, Taylor D, McFadden PM. Pulmonary resection of metastatic hepatocellular carcinoma after liver transplantation. Ann Thorac Surg. 2008;85:412-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 39. | Carr BI. Hepatic artery chemoembolization for hepatocellular carcinoma recurrence confined to the transplanted liver. Case Rep Oncol. 2012;5:506-510. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 40. | Catalano G, Urbani L, Biancofiore G, Bindi L, Boldrini A, Consani G, Bisà M, Campatelli A, Petruzzi P, Cioni R. Hepatic resection after liver transplantation as a graft-saving procedure: indication criteria, timing and outcome. Transplant Proc. 2004;36:545-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 41. | Cheng YC, Chen TW, Fan HL, Yu CY, Chang HC, Hsieh CB. Transarterial chemoembolization for intrahepatic multiple recurrent HCC after liver resection or transplantation. Ann Transplant. 2014;19:309-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 42. | Chok KS, Chan SC, Cheung TT, Chan AC, Fan ST, Lo CM. Late recurrence of hepatocellular carcinoma after liver transplantation. World J Surg. 2011;35:2058-2062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 43. | De Simone P, Crocetti L, Pezzati D, Bargellini I, Ghinolfi D, Carrai P, Leonardi G, Della Pina C, Cioni D, Pollina L. Efficacy and safety of combination therapy with everolimus and sorafenib for recurrence of hepatocellular carcinoma after liver transplantation. Transplant Proc. 2014;46:241-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 44. | Escartin A, Sapisochin G, Bilbao I, Vilallonga R, Bueno J, Castells L, Dopazo C, Castro E, Caralt M, Balsells J. Recurrence of hepatocellular carcinoma after liver transplantation. Transplant Proc. 2007;39:2308-2310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 78] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 45. | Gomez-Martin C, Bustamante J, Castroagudin JF, Salcedo M, Garralda E, Testillano M, Herrero I, Matilla A, Sangro B. Efficacy and safety of sorafenib in combination with mammalian target of rapamycin inhibitors for recurrent hepatocellular carcinoma after liver transplantation. Liver Transpl. 2012;18:45-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 126] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 46. | Gunay Y, Guler N, Akyildiz M, Yaprak O, Dayangac M, Yuzer Y, Tokat Y. Management of patients with recurrent hepatocellular carcinoma following living donor liver transplantation: a single center experience. Gulf J Oncolog. 2014;1:12-18. [PubMed] |
| 47. | Kim HR, Cheon SH, Rha SY, Lee S, Han KH, Chon CY, Lee JD, Sung JS, Chung HC. Treatment of recurrent hepatocellular carcinoma after liver transplantation. Asia Pac J Clin Oncol. 2011;7:258-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 48. | Kim R, El-Gazzaz G, Tan A, Elson P, Byrne M, Chang YD, Aucejo F. Safety and feasibility of using sorafenib in recurrent hepatocellular carcinoma after orthotopic liver transplantation. Oncology. 2010;79:62-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 49. | Ko HK, Ko GY, Yoon HK, Sung KB. Tumor response to transcatheter arterial chemoembolization in recurrent hepatocellular carcinoma after living donor liver transplantation. Korean J Radiol. 2007;8:320-327. [PubMed] |
| 50. | Kornberg A, Küpper B, Tannapfel A, Katenkamp K, Thrum K, Habrecht O, Wilberg J. Long-term survival after recurrent hepatocellular carcinoma in liver transplant patients: clinical patterns and outcome variables. Eur J Surg Oncol. 2010;36:275-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 51. | Lee CH, Brubaker LM, Gerber DA, Ku YM, Kim YH, Shin SS, Semelka RC. MRI findings of recurrent hepatocellular carcinoma after liver transplantation: preliminary results. J Magn Reson Imaging. 2011;33:1399-1405. [PubMed] |
| 52. | Marangoni G, Faraj W, Sethi H, Rela M, Muiesan P, Heaton N. Liver resection in liver transplant recipients. Hepatobiliary Pancreat Dis Int. 2008;7:590-594. [PubMed] |
| 53. | Perricone G, Mancuso A, Belli LS, Mazzarelli C, Zavaglia C. Sorafenib for the treatment of recurrent hepatocellular carcinoma after liver transplantation: does mTOR inhibitors association augment toxicity? Eur J Gastroenterol Hepatol. 2014;26:577-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 54. | Pfiffer TE, Seehofer D, Nicolaou A, Neuhaus R, Riess H, Trappe RU. Recurrent hepatocellular carcinoma in liver transplant recipients: parameters affecting time to recurrence, treatment options and survival in the sorafenib era. Tumori. 2011;97:436-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 55. | Roayaie S, Schwartz JD, Sung MW, Emre SH, Miller CM, Gondolesi GE, Krieger NR, Schwartz ME. Recurrence of hepatocellular carcinoma after liver transplant: patterns and prognosis. Liver Transpl. 2004;10:534-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 346] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 56. | Roh YN, David Kwon CH, Song S, Shin M, Man Kim J, Kim S, Joh JW, Lee SK. The prognosis and treatment outcomes of patients with recurrent hepatocellular carcinoma after liver transplantation. Clin Transplant. 2014;28:141-148. [PubMed] |
| 57. | Shin WY, Suh KS, Lee HW, Kim J, Kim T, Yi NJ, Lee KU. Prognostic factors affecting survival after recurrence in adult living donor liver transplantation for hepatocellular carcinoma. Liver Transpl. 2010;16:678-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (1)] |
| 58. | Sotiropoulos GC, Nowak KW, Fouzas I, Vernadakis S, Kykalos S, Klein CG, Paul A. Sorafenib treatment for recurrent hepatocellular carcinoma after liver transplantation. Transplant Proc. 2012;44:2754-2756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 59. | Taketomi A, Fukuhara T, Morita K, Kayashima H, Ninomiya M, Yamashita Y, Ikegami T, Uchiyama H, Yoshizumi T, Soejima Y. Improved results of a surgical resection for the recurrence of hepatocellular carcinoma after living donor liver transplantation. Ann Surg Oncol. 2010;17:2283-2289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 60. | Toso C, Cader S, Mentha-Dugerdil A, Meeberg G, Majno P, Morard I, Giostra E, Berney T, Morel P, Mentha G. Factors predicting survival after post-transplant hepatocellular carcinoma recurrence. J Hepatobiliary Pancreat Sci. 2013;20:342-347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 61. | Valdivieso A, Bustamante J, Gastaca M, Uriarte JG, Ventoso A, Ruiz P, Fernandez JR, Pijoan I, Testillano M, Suarez MJ. Management of hepatocellular carcinoma recurrence after liver transplantation. Transplant Proc. 2010;42:660-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 62. | Vitale A, Boccagni P, Kertusha X, Zanus G, D’Amico F, Lodo E, Pastorelli D, Ramirez Morales R, Lombardi G, Senzolo M. Sorafenib for the treatment of recurrent hepatocellular carcinoma after liver transplantation? Transplant Proc. 2012;44:1989-1991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 63. | Weinmann A, Niederle IM, Koch S, Hoppe-Lotichius M, Heise M, Düber C, Schuchmann M, Otto G, Galle PR, Wörns MA. Sorafenib for recurrence of hepatocellular carcinoma after liver transplantation. Dig Liver Dis. 2012;44:432-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 64. | Yamagami T, Yoshimatsu R, Ishikawa M, Kajiwara K, Aikata H, Tashiro H, Kakizawa H, Toyoda N, Ohdan H, Awai K. Transcatheter arterial chemoembolization with an interventional-CT system for recurrent hepatocellular carcinoma after living donor liver transplantation. Hepatogastroenterology. 2014;61:1387-1392. [PubMed] |
| 65. | Yoon DH, Ryoo BY, Ryu MH, Lee SG, Hwang S, Suh DJ, Lee HC, Kim TW, Ahn CS, Kim KH. Sorafenib for recurrent hepatocellular carcinoma after liver transplantation. Jpn J Clin Oncol. 2010;40:768-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 66. | Zavaglia C, Airoldi A, Mancuso A, Vangeli M, Viganò R, Cordone G, Gentiluomo M, Belli LS. Adverse events affect sorafenib efficacy in patients with recurrent hepatocellular carcinoma after liver transplantation: experience at a single center and review of the literature. Eur J Gastroenterol Hepatol. 2013;25:180-186. [PubMed] |
| 67. | Zhang FJ, Li CX, Zhang L, Wu PH, Jiao DC, Duan GF. Short- to mid-term evaluation of CT-guided 125I brachytherapy on intra-hepatic recurrent tumors and/or extra-hepatic metastases after liver transplantation for hepatocellular carcinoma. Cancer Biol Ther. 2009;8:585-590. [PubMed] |
| 68. | Zhou B, Shan H, Zhu KS, Jiang ZB, Guan SH, Meng XC, Zeng XC. Chemoembolization with lobaplatin mixed with iodized oil for unresectable recurrent hepatocellular carcinoma after orthotopic liver transplantation. J Vasc Interv Radiol. 2010;21:333-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 69. | Han KN, Kim YT, Yoon JH, Suh KS, Song JY, Kang CH, Sung SW, Kim JH. Role of surgical resection for pulmonary metastasis of hepatocellular carcinoma. Lung Cancer. 2010;70:295-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 70. | Kitano K, Murayama T, Sakamoto M, Nagayama K, Ueno K, Murakawa T, Nakajima J. Outcome and survival analysis of pulmonary metastasectomy for hepatocellular carcinoma. Eur J Cardiothorac Surg. 2012;41:376-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 71. | Kwon JB, Park K, Kim YD, Seo JH, Moon SW, Cho DG, Kim YW, Kim DG, Yoon SK, Lim HW. Clinical outcome after pulmonary metastasectomy from primary hepatocellular carcinoma: analysis of prognostic factors. World J Gastroenterol. 2008;14:5717-5722. [PubMed] |
| 72. | Sapisochin G, Goldaracena N, Astete S, Laurence JM, Davidson D, Rafael E, Castells L, Sandroussi C, Bilbao I, Dopazo C. Benefit of Treating Hepatocellular Carcinoma Recurrence after Liver Transplantation and Analysis of Prognostic Factors for Survival in a Large Euro-American Series. Ann Surg Oncol. 2015;22:2286-2294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 134] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 73. | Alsina AE, Makris A, Nenos V, Sucre E, Arrobas J, Franco E, Kemmer N. Can sorafenib increase survival for recurrent hepatocellular carcinoma after liver transplantation? A pilot study. Am Surg. 2014;80:680-684. [PubMed] |
| 74. | Sommacale D, Dondero F, Sauvanet A, Francoz C, Durand F, Farges O, Kianmanesh R, Belghiti J. Liver resection in transplanted patients: a single-center Western experience. Transplant Proc. 2013;45:2726-2728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 75. | Regalia E, Fassati LR, Valente U, Pulvirenti A, Damilano I, Dardano G, Montalto F, Coppa J, Mazzaferro V. Pattern and management of recurrent hepatocellular carcinoma after liver transplantation. J Hepatobiliary Pancreat Surg. 1998;5:29-34. [PubMed] |
| 76. | Staufer K, Fischer L, Seegers B, Vettorazzi E, Nashan B, Sterneck M. High toxicity of sorafenib for recurrent hepatocellular carcinoma after liver transplantation. Transpl Int. 2012;25:1158-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 77. | Chen WT, Yu CY, Huang TL, Chen TY, Tsang LLC, Ou HY, Chen CL, Cheng YF. Does transarterial embolization improve survival for recurrent hepatocellular carcinoma after living donor liver transplantation? Chin J Radiology (Taiwan). 2012;37:101-104. |
| 78. | Pfeiffenberger J, Koschny R, Hoffmann K, Mehrabi A, Schmitz A, Radeleff B, Stremmel W, Schemmer P, Ganten TM. Sorafenib treatment is save and may affect survival of recurrent hepatocellular carcinoma after liver transplantation. Langenbecks Arch Surg. 2013;398:1123-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 79. | Sposito C, Mariani L, Germini A, Flores Reyes M, Bongini M, Grossi G, Bhoori S, Mazzaferro V. Comparative efficacy of sorafenib versus best supportive care in recurrent hepatocellular carcinoma after liver transplantation: a case-control study. J Hepatol. 2013;59:59-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 127] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 80. | Tan WF, Qiu ZQ, Yu Y, Ran RZ, Yi B, Lau WY, Liu C, Qiu YH, Feng FL, Wang JH. Sorafenib extends the survival time of patients with multiple recurrences of hepatocellular carcinoma after liver transplantation. Acta Pharmacol Sin. 2010;31:1643-1648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 81. | Waghray A, Balci B, El-Gazzaz G, Kim R, Pelley R, Narayanan Menon KV, Estfan B, Romero-Marrero C, Aucejo F. Safety and efficacy of sorafenib for the treatment of recurrent hepatocellular carcinoma after liver transplantation. Clin Transplant. 2013;27:555-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 82. | Yoon YC, Hong TH, You YK, Kim DG. Clinical analysis of recurrent hepatocellular carcinoma after living donor liver transplantation. Clin Transplant. 2013;27:E192-E198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 83. | Hwang S, Kim YH, Kim DK, Ahn CS, Moon DB, Kim KH, Ha TY, Song GW, Jung DH, Kim HR. Resection of pulmonary metastases from hepatocellular carcinoma following liver transplantation. World J Surg. 2012;36:1592-1602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 84. | Lee JO, Kim DY, Lim JH, Seo MD, Yi HG, Oh DY, Im SA, Kim TY, Bang YJ. Palliative chemotherapy for patients with recurrent hepatocellular carcinoma after liver transplantation. J Gastroenterol Hepatol. 2009;24:800-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 85. | Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15860] [Cited by in RCA: 21638] [Article Influence: 1352.4] [Reference Citation Analysis (1)] |
| 86. | Kim KS, Jung HS, Choi WC, Eo WK, Cheon SH. A case of recurred hepatocellular carcinoma refractory to doxorubicin after liver transplantation showing response to herbal medicine product, Rhus verniciflua Stokes extract. Integr Cancer Ther. 2010;9:100-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 87. | Zimmerman MA, Ghobrial RM, Tong MJ, Hiatt JR, Cameron AM, Hong J, Busuttil RW. Recurrence of hepatocellular carcinoma following liver transplantation: a review of preoperative and postoperative prognostic indicators. Arch Surg. 2008;143:182-188; discussion 188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 216] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 88. | Vagefi PA, Dodge JL, Yao FY, Roberts JP. Potential role of the donor in hepatocellular carcinoma recurrence after liver transplantation. Liver Transpl. 2015;21:187-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 89. | Toso C, Mentha G, Majno P. Liver transplantation for hepatocellular carcinoma: five steps to prevent recurrence. Am J Transplant. 2011;11:2031-2035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 90. | Hojo M, Morimoto T, Maluccio M, Asano T, Morimoto K, Lagman M, Shimbo T, Suthanthiran M. Cyclosporine induces cancer progression by a cell-autonomous mechanism. Nature. 1999;397:530-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 852] [Cited by in RCA: 827] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 91. | Vivarelli M, Cucchetti A, Piscaglia F, La Barba G, Bolondi L, Cavallari A, Pinna AD. Analysis of risk factors for tumor recurrence after liver transplantation for hepatocellular carcinoma: key role of immunosuppression. Liver Transpl. 2005;11:497-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 168] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 92. | Chinnakotla S, Davis GL, Vasani S, Kim P, Tomiyama K, Sanchez E, Onaca N, Goldstein R, Levy M, Klintmalm GB. Impact of sirolimus on the recurrence of hepatocellular carcinoma after liver transplantation. Liver Transpl. 2009;15:1834-1842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 115] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 93. | Toso C, Merani S, Bigam DL, Shapiro AM, Kneteman NM. Sirolimus-based immunosuppression is associated with increased survival after liver transplantation for hepatocellular carcinoma. Hepatology. 2010;51:1237-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 253] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 94. | Trevisani F, Garuti F, Cucchetti A, Lenzi B, Bernardi M. De novo hepatocellular carcinoma of liver allograft: a neglected issue. Cancer Lett. 2015;357:47-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 95. | Mancuso A, Mazzarelli C, Perricone G, Zavaglia C. Sorafenib efficacy for treatment of HCC recurrence after liver transplantation is an open issue. J Hepatol. 2014;60:681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 96. | Vivarelli M, Dazzi A, Zanello M, Cucchetti A, Cescon M, Ravaioli M, Del Gaudio M, Lauro A, Grazi GL, Pinna AD. Effect of different immunosuppressive schedules on recurrence-free survival after liver transplantation for hepatocellular carcinoma. Transplantation. 2010;89:227-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 97. | Soll C, Clavien PA. Inhibition of mammalian target of rapamycin: two goals with one shot? J Hepatol. 2011;54:182-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 98. | Toso C, Mentha G, Majno P. Integrating sorafenib into an algorithm for the management of post-transplant hepatocellular carcinoma recurrence. J Hepatol. 2013;59:3-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |