Published online Oct 21, 2015. doi: 10.3748/wjg.v21.i39.11168
Peer-review started: July 6, 2015
First decision: July 19, 2015
Revised: August 2, 2015
Accepted: August 30, 2015
Article in press: August 30, 2015
Published online: October 21, 2015
Processing time: 108 Days and 18.3 Hours
AIM: To investigate the association of plasma levels of interleukin (IL)-6 and -8 with Wilms’ tumor 1 (WT1)-specific immune responses and clinical outcomes in patients with pancreatic ductal adenocarcinoma (PDA) treated with dendritic cells (DCs) pulsed with three types of major histocompatibility complex class I and II-restricted WT1 peptides combined with chemotherapy.
METHODS: During the entire treatment period, plasma levels of IL-6 and -8 were analyzed by ELISA. The induction of WT1-specific immune responses was assessed using the WT1 peptide-specific delayed-type hypersensitivity (DTH) test.
RESULTS: Three of 7 patients displayed strong WT1-DTH reactions throughout long-term vaccination with significantly decreased levels of IL-6/-8 after vaccinations compared with the levels prior to treatment. Moreover, overall survival (OS) was significantly longer in PDA patients with low plasma IL-6 levels (< 2 pg/mL) after 5 vaccinations than in patients with high plasma IL-6 levels (≥ 2 pg/mL) (P = 0.025). After disease progression, WT1-DTH reactions decreased severely and were ultimately negative at the terminal stage of cancer. The decreased levels of IL-6/-8 observed throughout long-term vaccination were associated with WT1-specific DTH reactions and long-term OS.
CONCLUSION: Prolonged low levels of plasma IL-6/-8 in PDA patients may be a prognostic marker for the clinical outcomes of chemoimmunotherapy.
Core tip: We recently reported a phase 1 clinical study in pancreatic cancer patients using dendritic cells (DCs) pulsed with multiple major histocompatibility complex class I and II-restricted Wilms’ tumor 1 (WT1) epitopes (DC/WT1-I/II) in combination with chemotherapy. Little is known about the prognostic markers for the clinical outcomes of chemoimmunotherapy. We examined the association of plasma levels of interleukin (IL)-6/-8 with WT1-specific immune responses and clinical outcomes in pancreatic cancer patients treated with chemotherapy combined with DC/WT1-I/II. The study demonstrates that prolonged low levels of plasma IL-6/-8 in pancreatic ductal adenocarcinoma patients may be a prognostic marker for the clinical outcomes of chemoimmunotherapy.
- Citation: Tsukinaga S, Kajihara M, Takakura K, Ito Z, Kanai T, Saito K, Takami S, Kobayashi H, Matsumoto Y, Odahara S, Uchiyama K, Arakawa H, Okamoto M, Sugiyama H, Sumiyama K, Ohkusa T, Koido S. Prognostic significance of plasma interleukin-6/-8 in pancreatic cancer patients receiving chemoimmunotherapy. World J Gastroenterol 2015; 21(39): 11168-11178
- URL: https://www.wjgnet.com/1007-9327/full/v21/i39/11168.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i39.11168
Patients with pancreatic ductal adenocarcinoma (PDA) have a particularly poor prognosis, with a 5-year survival rate of < 1%[1]. Thus, PDA remains one of the deadliest human tumors, characterized by high mortality, rapid progression, and resistance to chemotherapy and radiation therapy. Compared with single chemotherapy with standard agents such as gemcitabine, multi chemotherapy regimens such as FOLFIRINOX (consisting of 5-fluorouracil/folinic acid/oxaliplatin/irinotecan) and gemcitabine/nab-paclitaxel have been associated with significant improvement in median overall survival (OS) from 6 to 11 mo[2,3]. New therapeutic approaches for PDA are urgently needed. PDA cells express tumor-associated antigens (TAAs), including Wilms’ Tumor gene 1 (WT1)[4]. Therefore, immunotherapy targeting PDA-associated antigens may be an alternative approach in patients with PDA.
Dendritic cells (DCs) are potent antigen-presenting cells (APCs) extensively used for the development of anticancer immunotherapies[5,6]. DCs capture and process TAAs into peptides and present these fragments through major histocompatibility complex (MHC) class I and II pathways, thus simultaneously stimulating both CD4+ and CD8+ T cells[5,6]. TAAs are recognized by CD8+ cytotoxic T-lymphocytes (CTLs) in the context of MHC class I molecules, whereas CD4+ T cells recognize antigenic peptides in association with MHC class II molecules. CD8+ CTLs recognize MHC class I-peptide complexes on cancer cells and destroy these cells through effector molecules such as granzyme B and perforin[7]. DCs have been pulsed with various MHC class I-restricted antigenic peptides for the treatment of patients with PDA in clinical studies; however, the antitumor effects of these vaccines targeting only CD8+ CTLs are not as vigorous in clinical trials[7]. Increasing evidence has suggested that CD4+ T cells prime and maintain antigen-specific CD8+ CTLs[8] and play a direct role in the destruction of tumor cells[9]. Therefore, the stimulation of both CD4+ and CD8+ T cells is an efficient strategy for treating patients with advanced cancer. We recently conducted a phase 1 clinical study in patients with PDA to examine the clinical and immunological responses to DCs pulsed with multiple MHC class I and II-restricted WT1 epitopes (DC/WT1-I/II) in combination with chemotherapy[10,11]. The vaccination of PDA patients with DC/WT1-I/II simultaneously induced WT1-specific CD4+ and CD8+ T cell responses in vivo and in vitro[10,11]. WT1-specific delayed-type hypersensitivity (DTH) induced by combination therapy was associated with maintenance of WT1-specific memory CTLs, resulting in long-term clinical responses[10]. Moreover, we previously reported that the post-treatment neutrophil to lymphocyte (N/L) ratio is a treatment-related prognostic factor for improved survival of PDA patients after DC/WT1-I/II treatment[11]. In the present study, we analyzed the association of plasma interleukin (IL)-6 and IL-8 levels and WT1 peptide-specific DTH in PDA patients during long-term chemoimmunotherapy using DC/WT1-I/II. The value of plasma IL-6 and IL-8 levels as prognostic markers was also assessed.
The study was reviewed and approved by the ethics committee of the Jikei Institutional Review Board, Jikei University School of Medicine (Tokyo, Japan), and the clinical study committee of Jikei University Kashiwa Hospital [No. 14-60 (3209) and 21-204 (6082)]. All 7 PDA patients provided written informed consent and underwent chemoimmunotherapy with DC/WT1-I/II vaccination and chemotherapy[10]. All procedures were performed in accordance with the Helsinki Declaration.
Autologous peripheral blood mononuclear cells (PBMCs) were isolated from peripheral blood by Ficoll-Plaque Premium (GE Healthcare Bio-Sciences, Piscataway, NJ, United States) density gradient solution. Adherent PBMCs were cultured for 5 d in AIM-V medium (Gibco Life Technologies, New York, United States) containing granulocyte macrophage colony-stimulating factor (50 ng/mL, Primmune Corp. Kobe, Japan) and IL-4 (50 ng/mL, R&D Systems, Minneapolis, MN, United States) to generate immature DCs. The immature DCs were activated with penicillin-killed and lyophilized preparations of a low-virulence strain (Su) of Streptococcus pyogenes (OK-432; 10 mg/mL, Chugai Pharmaceutical Co, Ltd, Tokyo, Japan) and prostaglandin E2 (PGE2; 50 ng/mL, Daiichi Fine Chemical Co, Ltd, Toyama, Japan) for an additional 24 h. The mature DCs were pulsed with a mixture of three WT1 peptide types restricted to HLA-A*02:01, A*02:06 (126-134: RMFPNAPYL), A*24:02 (235-243: CYTWNQMNL) and MHC-class II (332-347: KRYFKLSHLQMHSRKH; NeoMPS Inc., San Diego, CA, United States) as previously described[10].
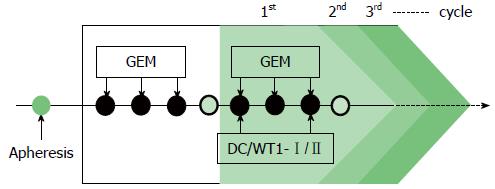
Gemcitabine was intravenously administered at a dose of 1000 mg/m2 on days 1, 8, and 15 of a 28-d cycle. After the first cycle of gemcitabine administration, the patients were treated with a combination of gemcitabine and DC/WT1-I/II. The DC/WT1-I/II vaccine (usually 1 × 107 cells/dose) was intradermally administered biweekly, regardless of the chemotherapy regimen (Figure 1). In Japan, the oral 5-fluorouracil (FU) S-1 is used to treat patients with gemcitabine-refractory PDA[12]. Therefore, some patients with gemcitabine-refractory PDA received S-1 during chemoimmunotherapy. Patients without early progressive disease received chemoimmunotherapy until the occurrence of disease progression, unacceptable adverse events, or withdrawal of patient consent.
Computed tomography was performed every 4 to 8 wk during treatment until disease progression. The clinical response was determined according to Response Evaluation Criteria in Solid Tumors. Stable disease (SD) was defined as disease that was stable for more than 8 wk after the start of treatment. Overall survival (OS) and progression-free survival (PFS) were calculated from the date of treatment to the date of death or final follow-up and the date of disease progression, respectively.
To determine the induction of WT1-specific immune responses during chemoimmunotherapy, the WT1 peptide-specific DTH test was performed before treatment and after every vaccination. Briefly, 30 μg of the three types of WT1 peptides in saline or saline alone was intradermally injected separately into the forearm. The maximum diameter of erythema and induration were measured 48 h after WT1 peptide injection. WT1-specific DTH positivity was defined as erythema and induration greater than 2 mm in the maximum diameter. We selected the value of 5-mm erythema and induration to discriminate between weak (2-5 mm) and strong (> 5 mm) WT1-specific DTH reactions. The DTH test was performed prior to treatment and during long-term treatment.
Throughout the vaccination period, plasma was collected and immediately frozen at -140 °C until further use. To investigate the quantitative relationship between IL-6 or IL-8 plasma levels and WT1-specific DTH reactions during vaccinations, the stored plasma was tested for IL-6 or IL-8 every 5 vaccinations using Enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems) according to the manufacturer’s instructions. Background cytokine levels were subtracted from each sample.
Statistical analyses of prognostic factors for OS and PFS were performed according to the Kaplan-Meier method and evaluated using the log-rank test. IL-6 or IL-8 levels were evaluated in a t-test analysis. A P value of < 0.05 was considered statistically significant.
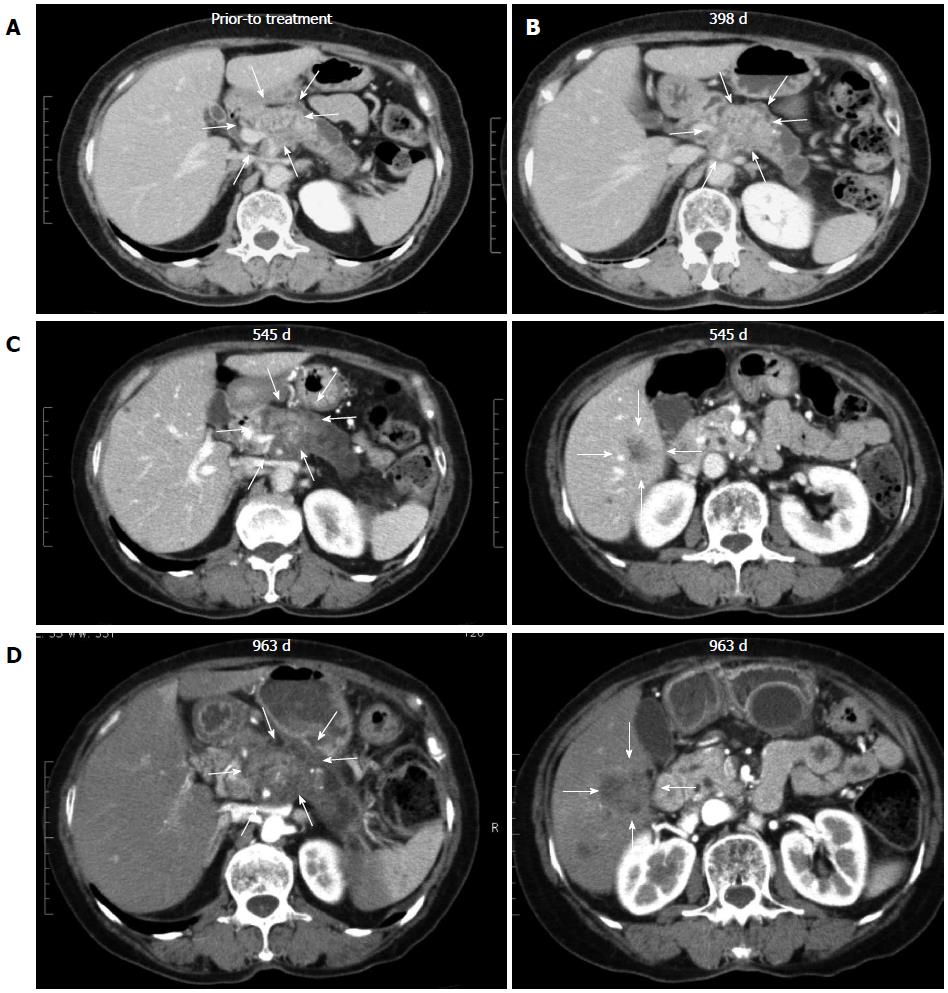
All 7 patients with PDA received gemcitabine followed by a combination of gemcitabine and biweekly vaccinations with DC/WT1-I/II (Figure 1). The clinical characteristics of all PDA patients are presented in Table 1. All patients had disease stage IV and HLA types of A (A*02:01, A*02:06, or A*24:02), DR (DRB1*04:05, DRB1*08:03, DRB1*15:01, or DRB1*15:02) or DP (DPB1*05:01, or DPB1*09:01). Treatment with the DC/WT1-I/II vaccine was non-toxic and safe[10]. None of the 7 PDA patients achieved complete or partial response, and 6 (85.7%) exhibited SD. As OS of ≥ 1 year in advanced PDA patients generally indicates that treatment has been beneficial[13], the treated PDA patients were classified into 2 groups: OS ≥ 1 and < 1 year. Three of 7 patients (No. 1, 2, and 6) exhibited OS of ≥ 1 year, and the remaining 4 patients (No. 3, 4, 5, 7) exhibited OS of < 1 year (Table 1). These 3 patients (No. 1, 2, and 6) had long-term SD, resulting in long-term survival (OS ≥ 1 year). From the beginning of treatment, one patient (No. 6) received biweekly 1000 mg/m2 gemcitabine combined with DC/WT1-I/II vaccination because of neutropenia. Despite receiving insufficient doses of gemcitabine, the local pancreatic lesions in the patient were stable for more than 1 year (Figure 2A-C, left panel); however, we identified liver metastases at 545 d after the first treatment (Figure 2C, right panel). Therefore, the patient continued treatment with S-1, an oral fluoropyrimidine, or gemcitabine/nab-paclitaxel combined with the DC/WT1-I/II vaccine. At 545 d after the first treatment, the patient maintained stable primary pancreatic cancer (Figure 2D, left panel) with slightly enlarged liver metastases (Figure 2D, right panel), and survived for more than 1000 d with 100% Karnofsky Performance Status (KPS).
| No. | Sex | Age (yr) | Location | Size (mm) | Metastases | UICC stage | Vaccine (times) | OS (d) | PFS (d) | HLA type | Best overall tumor response | |||||
| HLA-A | DRB1 | DPB1 | ||||||||||||||
| 1 | M | 70 | Body | 22 | Peritonitis | IV | 35 | 582 | 440 | 02:01 | 24:02 | 04:05 | 15:02 | 05:01 | 09:01 | Stable disease |
| 2 | M | 68 | Body | 15 | Liver, lymph nodes | IV | 46 | 717 | 208 | 24:02 | 33:03 | 08:03 | 13:02 | 02:02 | 04:01 | Stable disease |
| 3 | F | 49 | Head | 18 | Liver, peritonitis, lymph nodes | IV | 7 | 133 | 26 | 02:01 | 24:02 | 04:05 | 09:01 | 02:02 | 05:01 | Progressive disease |
| 4 | M | 35 | Body | 25 | Liver, lymph nodes | IV | 6 | 283 | 147 | 02:01 | - | 09:01 | 15:01 | 02:01 | 05:01 | Stable disease |
| 5 | F | 72 | Body | 22 | Peritonitis, lymph nodes | IV | 14 | 215 | 109 | 02:06 | 24:02 | 08:02 | 12:01 | 02:01 | 05:01 | Stable disease |
| 6 | F | 69 | Body-tail | 45 | Lymph nodes | IV | 71+ | 1050+ | 545 | 24:02 | 33:03 | 13:02 | 15:01 | 04:01 | 13:01 | Stable disease |
| 7 | M | 39 | Head-body | 30 | Peritonitis | IV | 20 | 325 | 290 | 02:10 | 24:02 | 15:01 | 15:02 | 02:02 | 09:01 | Stable disease |
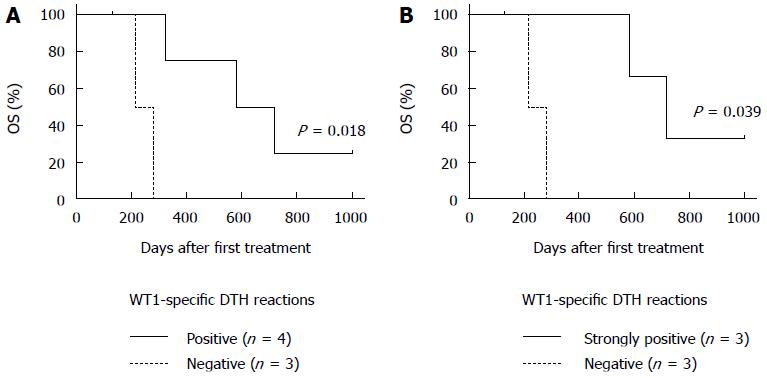
WT1-specific DTH reactions were not detected prior to treatment in all patients. Chemoimmunotherapy induced strong WT1-specific DTH reactions in all 3 patients (No. 1, 2, and 6) with long-term OS (≥ 1 year) after receiving only one dose of the DC/WT1-I/II vaccine (Table 2). Moreover, the strong WT1-specific DTH reactions in the 3 super-responders were efficiently maintained during the entire treatment period with at least 30 DC/WT1-I/II vaccinations (Table 2). However, the DTH reactions in 2 (No. 1 and 2) of the super-responders were severely decreased and became negative at the terminal stage of cancer (35 and 45 vaccinations, respectively). Interestingly, one super-responder (No. 6) remained alive more than 1000 d and received more than 71 vaccinations, resulting in the induction of strong WT1-specific DTH reactions throughout the vaccination period (Table 2). Moreover, a clinical response in terms of SD was achieved in all 3 patients (No. 1, 2, and 6) with strong WT1-specific DTH responses. These patients also maintained 100% KPS during treatment. By contrast, strong WT1-specific DTH reactions were not observed in all 4 nonsuper-responders with OS < 1 year (No. 3, 4, 5, and 7). In the 4 nonsuper-responders, one patient (No. 7) exhibited stable disease and weakly positive DTH reactions against WT1 peptides after 10 to 15 vaccinations; however, the DTH reactions became negative after 20 vaccinations, and the patient died at 325 d after the first treatment. Importantly, all 4 PDA patients with WT1-specific DTH reactions (No. 1, 2, 6, and 7) displayed significantly improved OS compared with the negative control patients (No. 3, 4, and 5) (P = 0.018) (Figure 3A). In particular, all 3 PDA patients with strong DTH reactions (No. 1, 2, and 6) survived more than 1 year, with significantly longer OS compared to the negative control patients (No. 1, 2, and 6) (P = 0.039) (Figure 3B). In addition, the WT1-specific DTH reactions observed in this clinical trial were HLA restricted (Table 2).
| Patient | Gemcitabine | HLA-A*02:01 | HLA-A*24:02 | HLA-DRB1/DPB1 | |||||||||||||||||||||||||||||||
| No. | Vaccine times | Vaccine times | Vaccine times | ||||||||||||||||||||||||||||||||
| Pre | Post | 1 | 5 | 10 | 15 | 20 | 25 | 30 | 35 | 40 | 45 | 70 | 1 | 5 | 10 | 15 | 20 | 25 | 30 | 35 | 40 | 45 | 70 | 1 | 5 | 10 | 15 | 20 | 25 | 30 | 35 | 40 | 45 | 70 | |
| 1 | - | - | + | + | ++ | ++ | ++ | ++ | + | - | ND | + | + | ++ | ++ | ++ | ++ | + | - | ND | + | + | ++ | ++ | ++ | ++ | + | - | ND | ||||||
| 2 | - | - | - | - | - | - | - | - | - | - | - | - | ND | + | + | ++ | ++ | + | + | + | + | + | - | ND | + | + | ++ | ++ | + | + | + | + | + | - | ND |
| 3 | - | - | - | - | ND | - | - | ND | - | - | ND | ||||||||||||||||||||||||
| 4 | - | - | - | - | ND | - | - | ND | - | - | ND | ||||||||||||||||||||||||
| 5 | - | - | - | - | - | ND | - | - | - | ND | - | - | - | ND | |||||||||||||||||||||
| 6 | - | - | - | - | - | - | - | - | - | - | - | - | - | + | + | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | + | + | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| 7 | - | - | - | - | - | - | - | ND | - | - | + | + | - | ND | - | + | + | - | ND | ||||||||||||||||
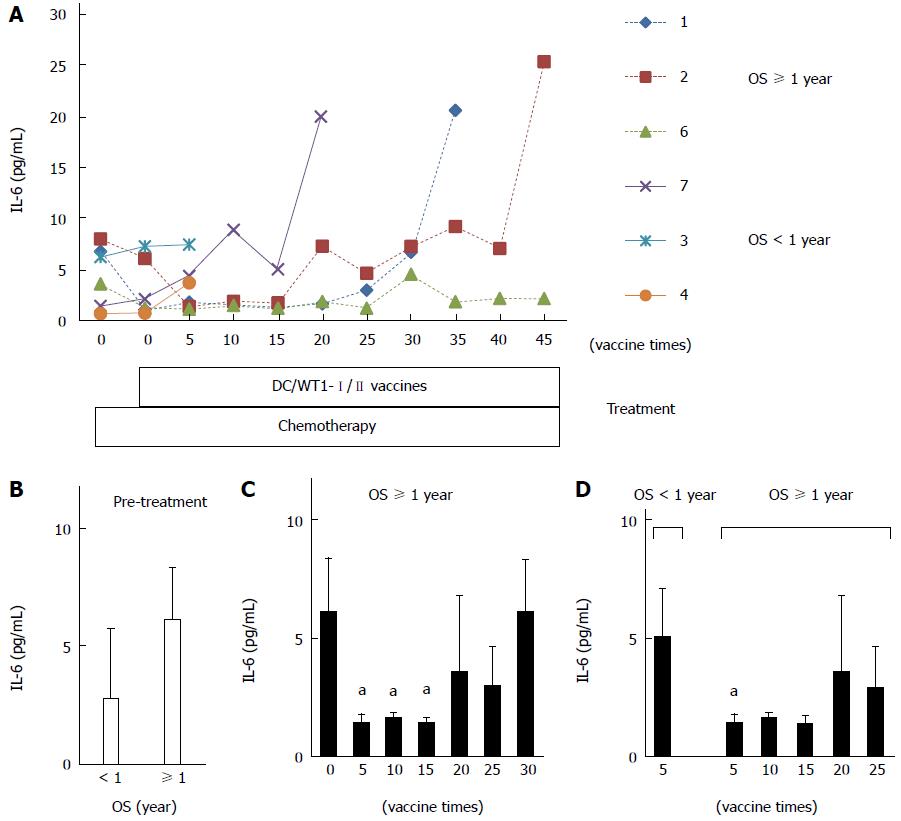
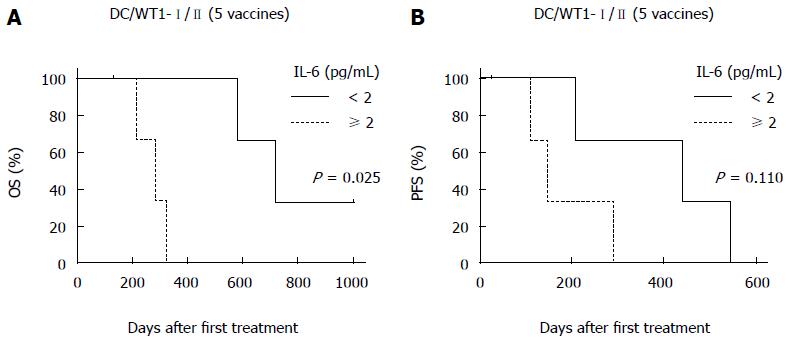
To assess the prognostic significance of plasma IL-6 levels in PDA patients receiving chemoimmunotherapy using DC/WT1-I/II vaccines, we analyzed plasma IL-6 levels by quantitative ELISA during treatment (Figure 4A). Prior to treatment, there was no difference in plasma IL-6 levels between super-responders (OS ≥ 1 year) and nonsuper-responders (OS < 1 year) (Figure 4B). In one nonsuper-responder (No. 5), plasma IL-6 levels were extremely high prior to treatment and continuously increased during treatment (60.25-274.5 pg/mL), and we excluded these data from the assessment of the relationship between plasma IL-6 levels and OS. After 1 course of gemcitabine alone, plasma IL-6 levels decreased in some patients but not significantly compared with prior to treatment (Figure 4A). All 3 super-responders (No. 1, 2, and 6) had long-term SD (440, 208, and 545 d, respectively), and plasma IL-6 levels in these patients were significantly decreased compared with the levels prior to treatment after 5 DC/WT1-I/II vaccinations. This significant decrease continued for 15 vaccinations (Figure 4C). Plasma IL-6 levels in 2 super-responders (No. 1 and 2) were increased after 35 and 45 vaccinations, respectively. However, 1 super-responder (No. 6) maintained low levels of IL-6 for at least 45 vaccinations, resulting in long-term OS. Because of early disease progression in nonsuper-responders (No. 3, 4, 5, and 7), these patients received at least 6 (7, 6, 14, and 20 times, respectively) DC/WT1-I/II vaccinations. Therefore, we also compared plasma IL-6 levels in super-responders and nonsuper-responders after 5 DC/WT1-I/II vaccinations. Compared with nonsuper-responders, plasma IL-6 levels in super-responders were significantly lower after 5 vaccinations (Figure 4D), indicating that plasma IL-6 levels decreased in super-responders after the initial vaccinations. In addition, IL-6 levels were remarkably increased at the terminal stage of cancer. Moreover, we assessed the association of IL-6 levels and OS and PFS after 5 DC/WT1-I/II vaccinations. The PDA patients with low levels of plasma IL-6 (< 2 pg/mL) after 5 vaccinations displayed significantly improved OS (P = 0.025) but not PFS (P = 0.110) compared with those patients with plasma IL-6 ≥ 2 pg/mL (Figure 5A and B).
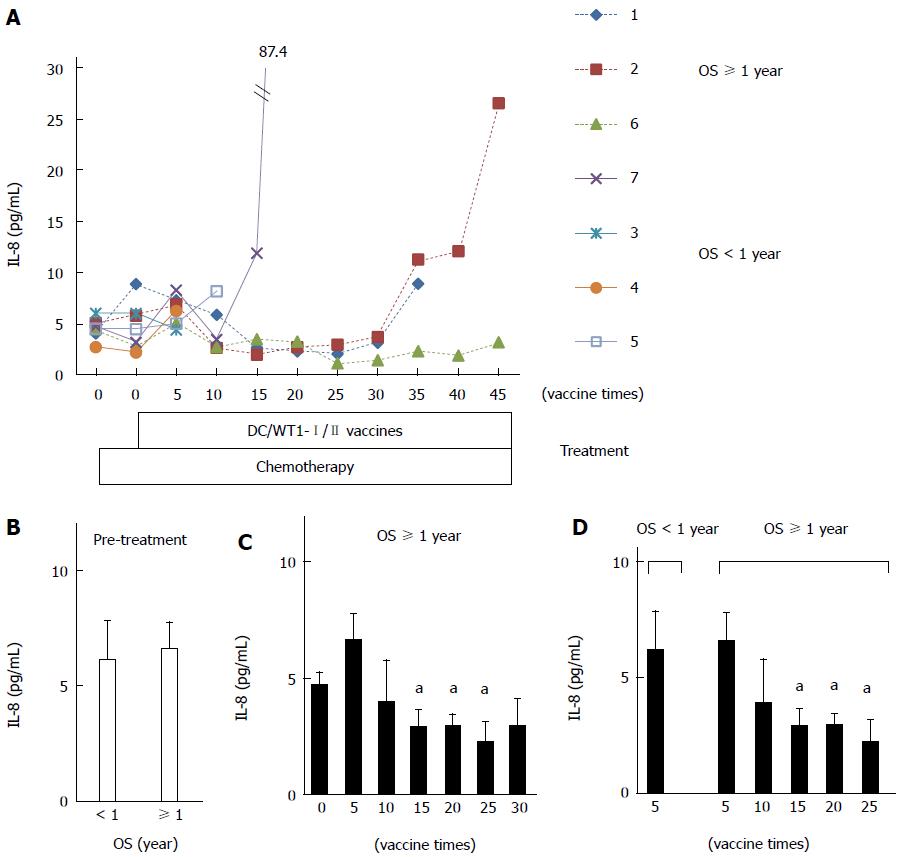
Plasma IL-8 levels in PDA patients after chemoimmunotherapy using DC/WT1-I/II vaccines were also analyzed by quantitative ELISA (Figure 6A). Prior to treatment, there was no difference in plasma IL-8 levels between super-responders (No. 1, 2, and 6) and nonsuper-responders (No. 3, 4, 5, and 7) (Figure 6B). Importantly, plasma IL-8 levels in all 3 super-responders with long-term SD were significantly decreased after 15 vaccinations compared with the levels observed prior to treatment, and this decrease continued after 25 vaccinations (Figure 6C). However, plasma IL-8 levels in 2 super-responders (No. 1 and 2) were increased after 35 vaccinations. The patients died 582 and 717 d, respectively (Table 1). However, one super-responder (No. 6) maintained low levels of IL-8 until at least 45 vaccinations and survived more than 1000 d. All 3 patients (No. 3, 4, and 5) with negative-DTH reactions against WT1 peptides received less than 15 DC/WT1-I/II vaccinations (7, 6, and 14 vaccinations, respectively) due to disease progression. Therefore, we also compared plasma IL-8 levels in super-responders at various vaccination periods and nonsuper-responders after 5 DC/WT1-I/II vaccinations. As shown in Figure 6D, plasma IL-8 levels were significantly higher in nonsuper-responders after 5 DC/WT1-I/II vaccinations than in super-responders after 15, 20 and 25 vaccinations. In addition, plasma IL-8 levels were remarkably increased at the terminal stage of cancer.
In the present study, we analyzed plasma IL-6 and IL-8 levels and assessed WT1-specific DTH reactions in PDA patients receiving DC/WT1-I/II vaccines. The data presented herein suggest that long-term decreases in plasma IL-6 and IL-8 levels compared to the early treatment period are associated with the induction of WT1-specific DTH reactions. Significantly prolonged OS was observed in PAD patients with strong WT1-specific DTH reactions. Thus, long-term low levels of plasma IL-6 and IL-8 during chemoimmunotherapy may be prognostic markers of clinical outcomes.
In a recent clinical trial, to simultaneously activate WT1-specific CD4+ and CD8+ T cells, mature DCs were pulsed with a mixture of three types of WT1 peptides, including MHC class I and II-restricted epitopes, and injected into one site biweekly as a cancer vaccine[10]. DC/WT1-I/II vaccinations not only induced WT1-specific CD4+ and CD8 T cells but also maintained long-term WT1-specific memory CD8+ T cells[10]. The phase 1 study indicated that WT1-specific DTH reactions induced after treatment with DC/WT1-I/II combined with chemotherapy were associated with long-term disease stability in advanced PDA. Moreover, the WT1-specific CTL responses continued throughout long-term vaccination and were associated with long-term OS[10]. With chemotherapy alone, SD is often transient and not considered indicative of true antitumor activity. Because, cancer vaccines are not as rapidly effective as cytotoxic agents, SD is considered an indicator of a meaningful therapeutic effect[14]. Long-term SD in patients receiving immunotherapy may be unique to cancer vaccines and is considered evidence of activity[10]. In the present study, we assessed immunological responses in 7 patients with stage IV PDA who received DC/WT1-I/II combined with chemotherapy. Three patients displayed strong WT1-specific DTH reactions in long-term chemoimmunotherapy, resulting in long-term OS ≥ 1 year. Antigenic peptide-specific DTH is an inflammatory reaction that is primarily mediated through CD4+ effector-memory T cells primed by the cancer vaccines[15]. A significant correlation between favorable clinical outcomes and the induction of a cancer vaccine-related antigen-specific DTH reaction has been observed[15]. Therefore, the long-term maintenance of strong WT1-specific DTH reactions in these 3 patients might be associated with long-term SD, resulting in prolonged survival. Indeed, one patient had long-term SD and continuously strong WT1-specific DTH, surviving at least 1000 d with 100% KPS.
The plasma levels of circulating IL-6/-8 were also analyzed by ELISA, a more quantitative analysis than the multiplexed measurement system of flow cytometry, and the cytokines levels were compared with the clinical outcomes. In the present study, none of the 7 PDA patients achieved complete or partial response, and 6 (85.7%) exhibited SD. Three patients (No. 1, 2, and 6) exhibited long-term SD, resulting in long-term survival. These 3 patients displayed significantly decreased levels of plasma IL-6 after 5 to 15 vaccinations. IL-6 is secreted from a variety of cells, primarily lymphocytes, macrophages, monocytes, fibroblasts, endothelial cells, and keratinocytes[16]. Some tumor cells also secrete IL-6[16]. One histological hallmark of PDA is the presence of a highly desmoplastic stroma, including several inflammatory cell populations, such as fibroblast, stellate, endothelial, endocrine, and immune cells, all of which produce different inflammatory cytokines[17]. The multifunctional inflammatory cytokine IL-6 plays a role in the development and progression of PDA by directly affecting tumorigenesis[18]. Moreover, IL-6 regulates the secretion of vascular endothelial growth factor in PDA cells, thereby stimulating angiogenesis and tumor vascularization resulting in lymphatic and distant metastasis and disease progression[18-20]. Therefore, the maintenance of low plasma IL-6 levels in 3 patients receiving chemoimmunotherapy using DC/WT-1-I/II at early vaccination periods (5 vaccines) and its continuation for 15 vaccinations may be responsible for the prolonged survival of the PDA patients. Importantly, these patients also maintained long-term strong WT1-specific DTH reactions. By contrast, extremely high levels of IL-6 and IFN-γ (data not shown) were detected in a nonsuper-responder (No. 5). The expression of IL-6 is potentiated by IFN-γvia prolonged NF-κB activation[21]. Three super-responders exhibited slightly increased IFN-γ production from CD4+ and CD8+ T cells upon stimulation with WT1 peptides in vitro[10]. The low levels of plasma IL-6 in the super-responders patients did not interfere with the induction of WT1-specific antitumor immune responses and were associated with a prolonged survival period.
After chemoimmunotherapy, significantly decreased plasma IL-8 levels were also obvious in all 3 super-responders with strong WT1-specific DTH reactions. Compared with IL-6, significantly decreased levels of plasma IL-8 were observed in the patients after longer periods of treatment (from 15 to 25 vaccinations). Although tumor-associated macrophages and monocytes are the most likely source of IL-8, this cytokine is also overexpressed in most human PDA cells under inflammatory conditions[22]. Several cell types within the tumor microenvironment produce a variety of cytokines. Differences in cells producing inflammatory cytokines such as IL-6 and IL-8 may underlie periods of decreased plasma IL-6 and IL-8 levels. In the tumor environment, these cytokines interact with other cell types in a complex network. Importantly, IL-8 plays a major role in the progression of PDA by promoting proliferation, migration, angiogenesis and metastasis[23]. Elevated levels of circulating IL-8 are associated with poor clinical outcome in patients with PDA and have been suggested as a prognosis marker[24,25]. Therefore, a significant decrease in plasma IL-8 levels might also be associated with good prognosis in PDA patients receiving the chemoimmunotherapy using DC/WT1-I/II. Indeed, all 3 super-responders with strong WT1- DTH reactions maintained low levels of IL-8 after 25 vaccinations and exhibited prolonged SD. However, plasma IL-8 and IL-6 levels in 2 super-responders (No. 1 and 2) were increased at the terminal stage of cancer, and these patients had WT1-negative DTH reactions. However, one super-responder (No. 6) maintained low levels of IL-8 and IL-6 and displayed strong WT1-specific DTH reactions after at least 45 vaccinations. Recently, we reported that IL-8 secretion from tumor cells enhances the generation and activation of CD163-positive M2 macrophages producing IL-10, leading to poor clinical outcomes in patients with cancer[26]. That finding is consistent with the results of the present study demonstrating that long-term low levels of circulating IL-8 in PDA patients receiving DC/WT1-I/II is associated with the maintenance of strong WT1-specific DTH reactions, resulting in good clinical outcomes.
In conclusion, both IL-6 and IL-8 were maintained at low levels in all 3 PDA patients with strong WT1-specific DTH reactions who received DC/WT1-I/II combined with chemotherapy. All 3 PDA patients exhibited long-term SD and prolonged survival times (582 to more than 1000 d). One patient with long-term strong WT1-specific DTH reactions maintained decreased levels of plasma IL-6 and IL-8 during therapy and maintained long-term SD, resulting in survival for more than 1000 d. Therefore, the maintenance of low plasma IL-6 and IL-8 levels may be associated with immunogenic changes in the desmoplastic stroma. Low levels of plasma IL-6 and IL-8 with strong WT1-DTH reactions may be a prognostic factor for PDA patients following chemoimmunotherapy using DC/WT1-I/II. These cytokine interactions are associated with tumor growth and progression, invasion and metastasis, angiogenesis and immune evasion. Although targeting IL-6 and IL-8 may improve not only clinical outcome but also the response to treatment in PDA patients, it is not clear if IL-6/-8-signaling inhibitors will translate into clinical benefits for PDA[19]. A limitation of the present study is the relatively small sample size. Further studies are needed to evaluate the clinical significance of circulating IL-6/-8 levels in PDA patients treated with DC/WT1-I/II combined with chemotherapy.
CD4+ T cells prime and maintain antigen-specific CD8+ CTLs and play a direct role in the destruction of tumor cells. Therefore, the stimulation of both CD4+ and CD8+ T cells is an efficient strategy for treating patients with pancreatic ductal adenocarcinoma (PDA). The authors had conducted a phase 1 clinical study in PAD patients to examine the clinical and immunological responses to dendritic cells (DCs) pulsed with multiple major histocompatibility complex class I and II-restricted Wilms’ tumor 1 (WT1) epitopes (DC/WT1-I/II) in combination with chemotherapy. The combination of DC/WT1-I/II and chemotherapy were associated with disease stability in PAD patients.
Authors investigate the association of plasma levels of interleukin (IL)-6 and -8 with WT1-specific immune responses and clinical outcomes in patients with PDA treated with DC/WT1-I/II combined with standard chemotherapy. The authors reported the association of plasma IL-6 and -8 levels and WT1 peptide-specific DTH in PDA patients during long-term chemoimmunotherapy.
Authors showed that plasma IL-6/-8 levels in PDA patients during long-term treatments. Prolonged low levels of plasma IL-6/-8 may be a prognostic marker for the clinical outcomes of chemoimmunotherapy.
The study’s results suggest that plasma IL-6/-8 in PDA patients during chemoimmunotherapy using DC/WT1-I/II is a prognostic biological marker for assessing the induction of WT1-specific immune responses and long-term overall survival.
The WT1 is highly expressed in various types of cancers, including PDA and has been found to be highly immunogenic. Thus, WT1 is one of the excellent tumor-associated antigens for the target of cancer immunotherapy.
The authors reported that prolonged low levels of plasma IL-6/-8 in PDA patients may be a prognostic marker for the clinical outcomes of chemoimmunotherapy. The data are well represented, organized, and clear.
P- Reviewer: Celio G Freire-De-Lima, Seong SY S- Editor: Yu J L- Editor: A E- Editor: Liu XM
| 1. | Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10002] [Cited by in RCA: 10453] [Article Influence: 696.9] [Reference Citation Analysis (0)] |
| 2. | Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4838] [Cited by in RCA: 5628] [Article Influence: 402.0] [Reference Citation Analysis (1)] |
| 3. | Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4035] [Cited by in RCA: 4877] [Article Influence: 406.4] [Reference Citation Analysis (0)] |
| 4. | Sugiyama H. Cancer immunotherapy targeting Wilms’ tumor gene WT1 product. Expert Rev Vaccines. 2005;4:503-512. [PubMed] |
| 5. | Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271-296. [PubMed] |
| 6. | Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419-426. [PubMed] |
| 7. | Koido S, Homma S, Takahara A, Namiki Y, Tsukinaga S, Mitobe J, Odahara S, Yukawa T, Matsudaira H, Nagatsuma K. Current immunotherapeutic approaches in pancreatic cancer. Clin Dev Immunol. 2011;2011:267539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 8. | Hu HM, Winter H, Urba WJ, Fox BA. Divergent roles for CD4+ T cells in the priming and effector/memory phases of adoptive immunotherapy. J Immunol. 2000;165:4246-4253. [PubMed] |
| 9. | Hung K, Hayashi R, Lafond-Walker A, Lowenstein C, Pardoll D, Levitsky H. The central role of CD4(+) T cells in the antitumor immune response. J Exp Med. 1998;188:2357-2368. [PubMed] |
| 10. | Koido S, Homma S, Okamoto M, Takakura K, Mori M, Yoshizaki S, Tsukinaga S, Odahara S, Koyama S, Imazu H. Treatment with chemotherapy and dendritic cells pulsed with multiple Wilms’ tumor 1 (WT1)-specific MHC class I/II-restricted epitopes for pancreatic cancer. Clin Cancer Res. 2014;20:4228-4239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 97] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 11. | Takakura K, Koido S, Kan S, Yoshida K, Mori M, Hirano Y, Ito Z, Kobayashi H, Takami S, Matsumoto Y. Prognostic markers for patient outcome following vaccination with multiple MHC Class I/II-restricted WT1 peptide-pulsed dendritic cells plus chemotherapy for pancreatic cancer. Anticancer Res. 2015;35:555-562. [PubMed] |
| 12. | Ueno H, Ioka T, Ikeda M, Ohkawa S, Yanagimoto H, Boku N, Fukutomi A, Sugimori K, Baba H, Yamao K. Randomized phase III study of gemcitabine plus S-1, S-1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer in Japan and Taiwan: GEST study. J Clin Oncol. 2013;31:1640-1648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 435] [Cited by in RCA: 472] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 13. | Stathis A, Moore MJ. Advanced pancreatic carcinoma: current treatment and future challenges. Nat Rev Clin Oncol. 2010;7:163-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 555] [Cited by in RCA: 628] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 14. | Wolchok JD, Hoos A, O’Day S, Weber JS, Hamid O, Lebbé C, Maio M, Binder M, Bohnsack O, Nichol G. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412-7420. [PubMed] |
| 15. | Aarntzen EH, Figdor CG, Adema GJ, Punt CJ, de Vries IJ. Dendritic cell vaccination and immune monitoring. Cancer Immunol Immunother. 2008;57:1559-1568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 71] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 16. | Yao X, Huang J, Zhong H, Shen N, Faggioni R, Fung M, Yao Y. Targeting interleukin-6 in inflammatory autoimmune diseases and cancers. Pharmacol Ther. 2014;141:125-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 489] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 17. | Lippitz BE. Cytokine patterns in patients with cancer: a systematic review. Lancet Oncol. 2013;14:e218-e228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 555] [Cited by in RCA: 680] [Article Influence: 56.7] [Reference Citation Analysis (0)] |
| 18. | Roshani R, McCarthy F, Hagemann T. Inflammatory cytokines in human pancreatic cancer. Cancer Lett. 2014;345:157-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 106] [Article Influence: 8.8] [Reference Citation Analysis (1)] |
| 19. | Codony-Servat J, Marín-Aguilera M, Visa L, García-Albéniz X, Pineda E, Fernández PL, Filella X, Gascón P, Mellado B. Nuclear factor-kappa B and interleukin-6 related docetaxel resistance in castration-resistant prostate cancer. Prostate. 2013;73:512-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 20. | Tang RF, Wang SX, Zhang FR, Peng L, Wang SX, Xiao Y, Zhang M. Interleukin-1alpha, 6 regulate the secretion of vascular endothelial growth factor A, C in pancreatic cancer. Hepatobiliary Pancreat Dis Int. 2005;4:460-463. [PubMed] |
| 21. | McLoughlin RM, Witowski J, Robson RL, Wilkinson TS, Hurst SM, Williams AS, Williams JD, Rose-John S, Jones SA, Topley N. Interplay between IFN-gamma and IL-6 signaling governs neutrophil trafficking and apoptosis during acute inflammation. J Clin Invest. 2003;112:598-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 202] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 22. | Kuwada Y, Sasaki T, Morinaka K, Kitadai Y, Mukaida N, Chayama K. Potential involvement of IL-8 and its receptors in the invasiveness of pancreatic cancer cells. Int J Oncol. 2003;22:765-771. [PubMed] |
| 23. | Li M, Zhang Y, Feurino LW, Wang H, Fisher WE, Brunicardi FC, Chen C, Yao Q. Interleukin-8 increases vascular endothelial growth factor and neuropilin expression and stimulates ERK activation in human pancreatic cancer. Cancer Sci. 2008;99:733-737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 24. | Bellone G, Smirne C, Mauri FA, Tonel E, Carbone A, Buffolino A, Dughera L, Robecchi A, Pirisi M, Emanuelli G. Cytokine expression profile in human pancreatic carcinoma cells and in surgical specimens: implications for survival. Cancer Immunol Immunother. 2006;55:684-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 162] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 25. | Chen Y, Shi M, Yu GZ, Qin XR, Jin G, Chen P, Zhu MH. Interleukin-8, a promising predictor for prognosis of pancreatic cancer. World J Gastroenterol. 2012;18:1123-1129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 76] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (2)] |
| 26. | Fujita Y, Okamoto M, Goda H, Tano T, Nakashiro K, Sugita A, Fujita T, Koido S, Homma S, Kawakami Y. Prognostic significance of interleukin-8 and CD163-positive cell-infiltration in tumor tissues in patients with oral squamous cell carcinoma. PLoS One. 2014;9:e110378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |