Published online Oct 21, 2015. doi: 10.3748/wjg.v21.i39.11160
Peer-review started: April 2, 2015
First decision: June 4, 2015
Revised: July 27, 2015
Accepted: August 29, 2015
Article in press: August 29, 2015
Published online: October 21, 2015
Processing time: 200 Days and 11.1 Hours
AIM: To find risk factors of lymph node metastasis (LNM) in early gastric cancer (EGC) and to find proper endoscopic therapy indication in EGC.
METHODS: We retrospectively reviewed the 2270 patients who underwent curative operation for EGC from January 2001 to December 2008. EGC was defined as malignant lesions that do not invade beyond the submucosal layer of the stomach wall irrespective of presence of lymph node metastasis.
RESULTS: Among 2270 enrolled patients, LNM was observed in 217 (9%) patients. LNM in intramucosal (M) cancer and submucosal (SM) cancer was detected in 38 (2.8%, 38/1340) patients and 179 (19%, 179/930) patients, respectively. In univariate analysis, the risk factors for LNM in EGC were size of tumor, Lauren classification, ulcer, lymphatic invasion, vascular invasion, and depth of invasion. However, in multivariate analysis, size of tumor, lymphatic invasion, vascular invasion, and depth of invasion were risk factors for LNM in EGC. Size of tumor, lymphatic invasion, vascular invasion, and depth of invasion were risk factors for LNM in cases of intramucosal cancer and submucosal cancer. In particular, there was no lymph node metastasis in cases of well differentiated early gastric cancer below 1 cm in size without ulcer regardless of lymphovascular invasion.
CONCLUSION: Tumor size, perilymphatic-vascular invasion, and depth of invasion were risk factors for LNM in EGC. There was no LNM in EGC below 1 cm regardless risk factors.
Core tip: Although the depth of tumor infiltration, tumor size as a maximum tumor diameter, and perilymphovascular invasion are independent risk factors for lymph node metastasis (LNM) in early gastric cancer (EGC), there was no LNM in intramucosal cancer which was not signet ring cell type and was below 1 cm without ulceration regardless of lymphatic invasion. This means that endoscopic submucosal dissection can be the treatment of choice in patients with intramucosal cancer below 1 cm without ulceration. There was LN metastasis in EGC of extended criteria in this study. But, the possibility of LNM in intramucosal cancer of extended indication was below 1%.
- Citation: Park JH, Lee SH, Park JM, Park CS, Park KS, Kim ES, Cho KB. Prediction of the indication criteria for endoscopic resection of early gastric cancer. World J Gastroenterol 2015; 21(39): 11160-11167
- URL: https://www.wjgnet.com/1007-9327/full/v21/i39/11160.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i39.11160
Early detection in gastric cancer is increasing with screening endoscopy. Consequently early gastric cancer (EGC) which was resectable with endoscopic resection has increased. In gastric cancer, the most significant factor in endoscopic resection is the absence of lymph node metastasis (LNM) because it determines the treatment. For that reason, prediction of lymph node metastasis in EGC is very important.
The predictors of the absence of LNM in EGC were tumor size of 2 cm or smaller, histologically differentiated type, intramucosal cancer, and no lymphovascular (LV) invasion[1]. According to the risk factors of LNM, endoscopic submucosal dissection (ESD) is a standard treatment for differentiated-type adenocarcinoma without ulceration, of which the depth of invasion is up to muscularis mucosa and the diameter is below 2 cm (Japanese gastric cancer treatment guidelines 2010[1]). However, some recent studies have reported extended indications for endoscopic resection[2-6] in differentiated EGC without lymphatic or vascular involvement, including: (1) mucosal cancers with no ulcerative findings, regardless of tumor size; (2) mucosal cancers with ulcerative findings ≤ 30 mm; and (3) minute (≤ 500 μm from the muscularis mucosae) submucosal invasive cancers ≤ 30 mm.
However, evidence from these studies is limited in South Korea. Thus, the purpose of this study was to determine the risk factors of lymph node metastasis in EGC removed by gastrectomy and to determine the safety of extended criteria for endoscopic treatment of EGC in South Korea.
A total of 2270 patients who had undergone gastrectomy with lymph node dissection for EGC at Yeungnam University hospital and Keimyung University hospital and we retrospectively reviewed the patient who has been taken radiologic imaging study and upper gastrofibroscope, and confirmed pathological reports after operation.
The patient profiles were investigated, including sex, age, tumor location, size, ulceration, histological type, lymphovascular invasion, and depth of invasion. Well and moderately differentiated tubular adenocarcinoma and papillary adenocarcinoma were classified as differentiated lesions. Poorly differentiated adenocarcinoma, signet ring cell carcinoma, and mucinous carcinoma were categorized as undifferentiated types. Lesions with ulcer or ulcer scar within cancer were regarded as ulcerated lesions. The depth of submucosal invasion was checked from the muscularis mucosa to the point of deepest penetration. The depth of submucosal invasion was subclassified according to two groups: SM1 (≤ 500 μm penetration into submucosa) and SM2 (> 500 μm). The tumor size was measured by the results of the pathological report after surgical resection.
Statistical analysis was performed using the SPSS program. The relationship between lymph node metastasis and various factors was assessed using the simple χ2 test and multiple logistic regression analysis.
Male to female ratio was 1488:782 and mean age was 59.3 ± 11.7. The mean length of major axis was 25.9 ± 16.5 mm. The most common location of early gastric cancer was middle anterior wall of stomach.
Among 1340 patients with M cancers, 2.8% (39/1340) were diagnosed as LMN; 19.2% (179/930) in SM cancer, 14.5% (55/379) in SM1 lesion, and 22.3% (123/551) in SM2 lesion. The relationships between various clinical or histological factors and the risk of LNM are summarized in Table 1, Table 2 and Table 3. Tumor size, lymphatic or venule invasion, deeper vertical invasion, and ulceration were the risk factors of lymp node metastasis. Similar to the finding for cancer involving intramucosa, significant correlation was observed between tumor larger than 3 cm and lymphovascular invasion with an increased risk of LNM. Also, cancer with involvement deep into the submucosa showed greater association with LNM; and significant correlation was observed between tumor larger than 2 cm and lymphovascular invasion and LNM in submucosal cancer. This meant that the possibility of LNM in cancer with involvement deep into the submucosa was greater than in smaller sized tumor than in intramucosal cancer.
| OR | 95%CI | P value | |
| Tumor size ≤ 30 mm vs > 30 mm | 2.1 | 1.5-3.0 | < 0.001 |
| Depth of invasion | |||
| M vs SM1 | 1.6 | 1.0-2.4 | < 0.001 |
| M vs SM2 | 4.7 | 3.0-7.2 | 0.040 |
| Lymphatic invasion | 4.1 | 2.8-6.0 | < 0.001 |
| Vascular invasion | 4.7 | 3.1-7.1 | < 0.001 |
| Negative | Positive | P value | |
| Gender | 0.217 | ||
| Male/female | 843/458 | 29/10 | |
| Age | 58.5 ± 11.8 | 50.6 ± 14.5 | < 0.001 |
| Size of tumor (mm, mean ± SD) | 23.5 ± 15.7 | 37.9 ± 28.6 | < 0.001 |
| Tumor size | < 0.001 | ||
| ≤ 30 mm | 961 (81) | 18 (54) | |
| > 30 mm | 224 (19) | 15 (46) | |
| Tumor location | 0.052 | ||
| Upper | 192 (15) | 14 (36) | |
| Middle | 736 (59) | 19 (48) | |
| Lower | 324 (26) | 6 (16) | |
| Lauren | < 0.001 | ||
| Intestine type | 634 (61) | 11 (38) | |
| Diffuse type | 371 (36) | 16 (55) | |
| Mix type | 33 (3) | 2 (7) | |
| Histologic type | 0.715 | ||
| Differentiated | 799 (62) | 20 (53) | |
| Undifferentiated | 498 (38) | 18 (47) | |
| Ulcer finding | 0.757 | ||
| Absence/presence | 619/550 | 14/14 | |
| Lymphatic invasion | < 0.001 | ||
| Absence/presence | 1202/93 | 23/15 | |
| Vascular invasion | < 0.001 | ||
| Absence/presence | 1272/22 | 22/17 | |
| Perineural invasion | < 0.001 | ||
| Absence/presence | 1279/13 | 36/3 |
| Negative | Positive | P value | |
| Gender | 0.369 | ||
| Male/female | 497/254 | 119/60 | |
| Age | 61.0 ± 11.3 | 60.2 ± 11.4 | < 0.001 |
| Size of tumor (mm, mean ± SD) | 27.0 ± 14.8 | 36.1 ± 19.9 | < 0.001 |
| Tumor size | < 0.001 | ||
| ≤ 20 mm | 512 (73) | 94 (55) | |
| > 20 mm | 188 (27) | 76 (45) | |
| Tumor location | 0.651 | ||
| Upper | 157 (21) | 34 (20) | |
| Middle | 376 (50) | 93 (52) | |
| Lower | 223 (29) | 52 (28) | |
| Lauren | 0.514 | ||
| Intestine type | 343 (56) | 69 (47) | |
| Diffuse type | 215 (35) | 63 (43) | |
| Mix type | 52 (9) | 16 (10) | |
| Histologic type | 0.884 | ||
| Differentiated | 489 (65) | 113 (63) | |
| Undifferentiated | 258 (35) | 66 (37) | |
| Ulcer finding | 0.115 | ||
| Absence/presence | 255/388 | 51/104 | |
| Lymphatic invasion | < 0.001 | ||
| Absence/presence | 526/222 | 47/130 | |
| Vascular invasion | < 0.001 | ||
| Absence/presence | 665/82 | 104/71 | |
| Perineural invasion | 0.103 | ||
| Absence/presence | 693/56 | 157/22 |
According to extended criteria, 3 (0.8%, 3/378) differentiated intramucosal lesions without lymphovascular invasion and ulceration regardless of tumor size showed association with LNM. Two (0.9%, 2/230) differentiated intramucosal ulcerative lesions below 3 cm without lymphovascular invasion showed association with LNM. Three (2.7%, 3/113) differentiated submucosal (≤ 500 μm from the muscularis mucosae) lesions were below 3 cm without lymphovascular invasion (Table 4). Although there were few patients with LNM in cases reflected by extended criteria, the possibility of LNM in EGC remained.
| Ulcer | Differentiation | VI | LI | Size | |||
| ≤10 mm | > 10 mm | > 20 mm | > 30 mm | ||||
| M | |||||||
| Ulcer negative | Differentiated | No | No | 0/102 | 2/112 | 0/81 | 1/57 |
| Yes | 0/5 | 0/2 | 0/1 | 0/5 | |||
| Yes | No | 0 | 0 | 0 | 1/2 | ||
| Yes | 0 | 1/2 | 0/0 | 1/2 | |||
| Undifferentiated | No | No | 1/66 | 2/41 | 0/41 | 1/45 | |
| Yes | 0/3 | 0/3 | 0/1 | 0/2 | |||
| Yes | No | 0 | 0 | 0 | 0 | ||
| Yes | 0 | 0 | 0/1 | 3/3 | |||
| Ulcer positive | Differentiated | No | No | 1/59 | 1/104 | 0/67 | 1/47 |
| Yes | 0/5 | 1/12 | 0/1 | 1/5 | |||
| Yes | No | 0 | 0 | 0 | 0 | ||
| Yes | 0/2 | 0/4 | 0/0 | 1/1 | |||
| Undifferentiated | No | No | 0/40 | 0/50 | 1/42 | 0/46 | |
| Yes | 0/4 | 0/2 | 0/6 | 1/4 | |||
| Yes | No | 0 | 0 | 0 | 0 | ||
| Yes | 0/1 | 1/2 | 0/1 | 1/1 | |||
| SM1 | |||||||
| Ulcer negative | Differentiated | No | No | 0/10 | 1/19 | 0/12 | 1/13 |
| Yes | 0/3 | 0/3 | 1/2 | 0/1 | |||
| Yes | No | 0 | 0 | 1/1 | 2/2 | ||
| Yes | 0 | 1/3 | 0/0 | 1/3 | |||
| Undifferentiated | No | No | 0/7 | 0/6 | 1/8 | 0/6 | |
| Yes | 0 | 0/2 | 0/0 | 0/1 | |||
| Yes | No | 0 | 0 | 0 | 0 | ||
| Yes | 0 | 0/1 | 0/1 | 2/3 | |||
| Ulcer positive | Differentiated | No | No | 0/14 | 2/24 | 0/19 | 1/13 |
| Yes | 0/1 | 0/0 | 1/7 | 1/5 | |||
| Yes | No | 0 | 0/1 | 0/0 | 0 | ||
| Yes | 0 | 0/1 | 2/3 | 3/3 | |||
| Undifferentiated | No | No | 0/6 | 2/12 | 2/7 | 1/6 | |
| Yes | 0/2 | 1/7 | 0/3 | 1/7 | |||
| Yes | No | 0 | 0 | 0 | 1/1 | ||
| Yes | 1/2 | 3/5 | 1/4 | 1/1 | |||
| SM2 | |||||||
| Ulcer negative | Differentiated | No | No | 0/4 | 2/23 | 1/25 | 0/18 |
| Yes | 0 | 1/12 | 2/5 | 11/27 | |||
| Yes | No | 0/1 | 0/0 | 0/0 | 0 | ||
| Yes | 0/1 | 1/3 | 0/1 | 6/8 | |||
| Undifferentiated | No | No | 0/1 | 1/14 | 2/9 | 1/4 | |
| Yes | 0/2 | 1/1 | 0/2 | 3/7 | |||
| Yes | No | 0/1 | 0/0 | 0/0 | 0 | ||
| Yes | 0/1 | 0/0 | 5/6 | 1/3 | |||
| Ulcer positive | Differentiated | No | No | 3/16 | 2/33 | 1/31 | 3/31 |
| Yes | 0/3 | 5/15 | 12/27 | 13/31 | |||
| Yes | No | 0 | 0 | 1/3 | 0/3 | ||
| Yes | 0/1 | 1/2 | 2/5 | 4/5 | |||
| Undifferentiated | No | No | 0/4 | 2/14 | 1/19 | 4/18 | |
| Yes | 0/2 | 1/8 | 1/6 | 4/11 | |||
| Yes | No | 0 | 0 | 0/1 | 1/1 | ||
| Yes | 1/1 | 3/4 | 5/8 | 3/5 | |||
In our study, none (0/102) of the differentiated intramucosal lesions below 1 cm without lymphovascular invasion and ulceration showed association with LNM. In particular, there was no lymph node metastasis (0/127) in cases of well differentiated early gastric cancer below 1 cm in size without ulcer regardless of lymphovascular invasion. The undifferentiated intramucosal cancer below 1 cm in size with ulcer did not show association with metastasis. One (1.2%, 1/81) of the undifferentiated early gastric cancers below 1 cm without ulcer regardless of lymphovascular invasion showed association with LNM. The cell type of the patient in one case was signet ring. Thus, there was no LNM in patients with early gastric cancer below 1 cm in the without ulcer group without signet ring type cancer in cell differentiation (Table 4).
Of 406 patients with undifferentiated M cancer, 2.7% (11/406) were found to have LNM. Seven patients with perilymphatic-vascular invasion were confirmed LNM and four of them had no ulcerated lesion. In 11 patients with LNM, one case was below 10 mm in size (Table 4).
In 324 patients with undifferentiated submucosal cancer, 20.3% (66/324) were found to have LNM. Of the subgroup of 16 patients with undifferentiated SM1 lesion, 16.3% (16/98) were diagnosed as LNM. Yet, of 82 undifferentiated SM1 lesions without LNM, 31 cases had ulcerated lesion, no one had perivascular invasion, 32 cases showed perilymph invasion. And, of 16 undifferentiated SM1 lesions with LNM, no one had perivascular invasion, and perilymph invasion was detected in three cases. Seven patients had lymphatic and vascular invasion.
Based on these results, the treatment of choice of undifferentiated SM1 lesion is not endoscopic resection but surgical resection (Table 4).
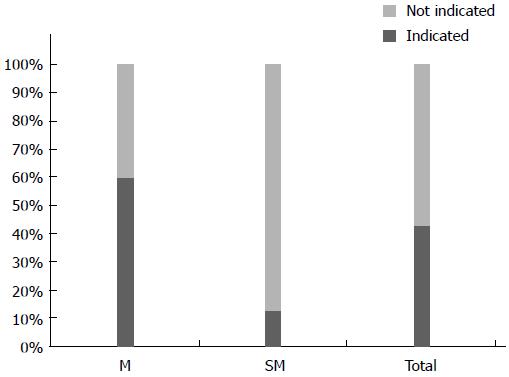
In Yeungnam and Keimyung university hospitals, 1340 patients with M cancer underwent surgery. Among them, 799 (59.6%) patients were confirmed as differentiated M cancer without LNM. Among 930 patients with SM invasion, 118 (12.6%) differentiated SM1 cancer patients without perilymphatic-vascular invasion were free from LNM; 40.3% (917/2270) of patients with EGC were overtreated (Figure 1).
The definition of EGC was suggested by the Japanese Gastroenterologic Endoscopic Society in 1962. In this definition, EGC is defined as gastric cancer which invades within submucosa regardless of lymph node metastasis[7].
The existence of LNM was association with bad prognosis[8]. The 5-year survival rate is > 90% in EGC, and the absence of lymph node metastasis is the most significant prognostic factor[9,10]. The 5-year survival rate was reported to be 87.3% in patients with regional lymph nodes metastasis and 94.2% in those without[11]. Maruyama et al[12] reported that the number of metastatic lymph nodes in patients with EGC was associated with the survival rate. EGC patients with LNM had a lower survival rate than patients without lymph node metastasis[13].
Because of an increased accuracy of diagnosis of EGC, which in turn leads to a better prognosis, increased interest has been focused on betterment of the quality of life and minimalization of invasive procedures. Furthermore, nowadays, various minimally invasive treatment modalities have been developed and endoscopic resection has enabled rapid restoration of patient’s health with lower risk of procedure, however, this endoscopic treatment also has risk of disease recurrence and distant metastasis.
Therefore, we suggested investigation of the relationship between various risk factors and LNM, and we think that our research will be helpful in development of more delicate criteria for endoscopic resection of EGC.
The overall incidence of a LNM in EGC ranges from 10% to 15%[9,10,14,15]. The incidence of lymph node invasion was reported upto 4.8% in mucosal cancers and 23.6% in SM cancers[9,10,16]. These results are similar to ours generally, and the incidence of LNM was similar to those in mucosal carcinoma reported by Yamao et al[17] and Tsujitani et al[18], and lower risk in submucosal carcinoma reported by other researchers[19].
So far, standard treatment of SM cancer is gastrectomy with lymphadenectomy[20]. But, endoscopic resection such as EMR or ESD is widely used standard treatment modality for mucosal cancer[9,21]. In studies analyzing the outcome of endoscopic resection in undifferentiated EGC, complete resection rate of undifferentiated EGC was relatively lower than that of differentiated EGC[22].
There were various attempts to clarify risk factors predicting LNM and relationship between these risk factors and prognosis. Maehara et al[23] found that large tumor, lymphatics involvement, and submucosal invasion were risk factors for LNM in EGC patients. Yamao et al[17] also reported that lymphatic invasion, histologic type, and large tumor size were independent risk factors for LNM in intramucosal EGC. In our study, univariate and multivariate analysis showed association of LNM in EGC with large tumor size, submucosal invasion, and perilymphatic-vascular invasion.
Some researchers have advocated that submucosal invasion is the most predictive risk factor for LNM. Otherwise, of risk factors for metastasis, the most important is perilymphatic invasion[24]. As for lymphatic vascular invasion, this may be the most important direct route to the regional lymph nodes[6]. Relation of prognostic factors, including gender, tumor size, depth of invasion, endoscopic findings, and lymphatic invasion to LNM in SM cancer has been demonstrated[20,25,26]. Besides pathologic type, Lauren classification and perineural involvement also showed significant and independent association with LNM.
However, Keita Nakahara et al[6] reported that the histological type was not a risk factor for LNM. In our results, histological type and presence of differentiation were unrelated to LNM. And, in the study by Nakahara et al[6], no significant difference in invasion depth was observed between SM1 and M cancers among EGC. Yet, in our studies, a statistically significant difference was observed between SM1 and M, and SM1 and SM2. With respect to size and ulceration, the possibility of SM invasion is higher and ulceration develops more readily in larger lesions. In contrast, there was no difference between ulcerated lesion and LNM in M, SM1, and SM2 (Table 1). Molecular biologically, proliferating cell nuclear antigen labeling index of greater than 25%, matrix metalloproteinase-9-positive tumors, tumor with gastric mucin phenotype, and vascular endothelial growth factor-C-positive tumors showed an association with LNM[27].
EMR has been widely used for treatment of EGC. However, current application of EMR is limited to differentiated EGC[28]. Endoscopic resection is basically contraindicated even in differentiated type submucosal gastric cancer. In fact, Kunisaki et al[29] reported that the incidence of LNM was 1.8% in patients with submucosal gastric cancer measuring less than 20 mm and without lymphovascular invasion, and the site of LNM was restricted to the paragastric lymph nodes.
Kurihara et al[19] reported that reoperation after an endoscopic resectio for EGC with SM1 invasion is unnecessary because most SM1 cancers < 20 mm do not have the lymph node metastasis. Gotoda et al[4] reported that none of 145 differentiated adenocarcinomas < 30 mm, a lack of lymphovascular invasion and submucosal penetration < 500 μm, had nodal metastasis[4]. Gotoda et al[4] proposed an extension of the indications for endoscopic treatment, and one of the extended indications was differentiated SM1 adenocarcinoma measuring less than 30 mm[4]. Abe et al[30] also reported that an EMR could be suitable for SM1 cancers and no lymphatic invasion. Abe et al[30] suggested that SM cancer with < 15 mm in diameter could be treated by EMR or a local resection. In our study, there were few patients with LNM in cases reflected by extended criteria. However, the possibility of LNM in EGC remained. In particular, there was no LNM in patients with EGC below 1 cm without ulcer in the group without signet ring type cancer in cell differentiation (Table 4).
Korenaga et al[31] reported that accurate assessment of depth of cancer invasion was difficult in lesions larger than 15 mm resected piecemeal. Clinically, it is difficult to determine whether lesions are confined to the mucosa or not[32,33]. Also, several studies reported that assessment of depth of cancer invasion after endoscpic resection is inaccurate in up to 20% of lesions[32,33]. Biopsies are too superficial to provide this information, but EMR provides a larger specimen, which allows assessment of depth of cancer invasion and lymphovascular invasion[4]. But, when resection if fragmented, the bruised margin makes it difficult to evaluate the stump, and the degree of radical treatment cannot be adequately evaluated. This issue is particularly important in lesions for which local treatment is indicated. When en bloc/total resection is technically impossible, indications of EMR should not be readily extended[6]. In addition, accurate evaluation of the presence of lymphatic vascular invasion preoperatively or before endoscopic resection is impossible, therefore, postoperative histological evaluation is essential.
Endoscopic ultrasonography, computed tomography etc. were widely used by staging of EGC. But, resected tissue by EMR or ESD and radiological examination could not exclude perfectively regional LNM. However, recently, minimal invasive surgery or stomach conserving therapy such as sentinel node navigation surgery, hybrid NOTES, and endoscopic submucosal dissection with sentinel node navigation surgery has been newly developed[34,35].
In addition, from the technical perspective of en block/total resection, a condition of 30 mm or less may be considered safe[6]. The new endoscopic technique or tools such as ESD and IT knife enable complete resection of large and ulcerated lesions.
Because of the high probability of LNM and tumor residual, patients with submucosal undifferentiated EGC are not candidates for treatment by EMR[28]. By our studies, 66 (20.3%) cases of 324 undifferentiated SM cancer were related to LNM.
In the Kunisaki et al[36] study, the incidence of LNM in patients with poorly differentiated type mucosal cancer was 2.2%. In this study, the incidence of LNM was 3.4% in patients with undifferentiated mucosal tumors. Therefore, endoscopic resection may be contraindicated in these patients.
As mentioned above, currently, exclusion of histologically poorly differentiated submucosal gastric cancer from the indications for endoscopic resection is mandatory, whereas histologically differentiated submucosal gastric cancer can be curatively resected endoscopically[36]. However, endoscopic resection may be reasonable for histologically undifferentiated mucosal gastric cancer below 10 mm in size and without lymphovascular invasion, for undifferentiated submucosal cancer less than 10 mm and without lymphovascular invasion in elderly patients with severe co-morbid disease, because the incidence of LNM is low in these patients.
In conclusion, we suggest that depth of tumor infiltration, tumor size as a maximum tumor diameter, and perilymphovascular invasion are independent risk factors for LNM in EGC.
Our study shows that there was no LNM in intramucosal cancer which was not signet ring cell type and was below 1 cm without ulceration regardless of lymphatic invasion. This means that ESD can be the treatment of choice in patients with intramucosal cancer below 1 cm without ulceration.
ESD has shown advantages over conventional EMR for removal of larger or ulcerated EGC lesions in an en bloc manner as well as for prevention of residual disease and local recurrence. Some reports showed LN metastasis in EGC of extended indication, particularly in submucosal invasive EGC. Our study shows LN metastasis in EGC of extended criteria, too. But, the possibility of LNM in intramucosal cancer of extended indication was below 1%. Many more cases of materials and more large scaled multicenter studies are essential for development of eligibility criteria for endoscopic treatment of EGC.
Recently endoscopic resection of early gastric cancer (EGC) has been widely performed. In endoscopic treatment, the presence of lymph node metastasis (LNM) is the most important issue. However, data on risk factors for LNM in South Korean EGC have been limited. The aims of this study were to find risk factors of LNM in EGC and to find proper endoscopic therapy indication in EGC.
According to the risk factors of LNM, endoscopic submucosal dissection (ESD) is a standard treatment for differentiated-type adenocarcinoma without ulceration, of which the depth of invasion is up to muscularis mucosa and the diameter is below 2 cm. However, recent studies have reported extended indications for endoscopic resection in differentiated EGC with no lymphatic or vascular involvement, including: (1) mucosal cancers without ulcerative findings, regardless of tumor size; (2) mucosal cancers with ulcerative findings ≤ 30 mm; and (3) minute (≤ 500 μm from the muscularis mucosae) submucosal invasive cancers ≤ 30 mm. However, evidence from these studies is limited in South Korea.
Although the depth of tumor infiltration, tumor size as a maximum tumor diameter, and perilymphovascular invasion are independent risk factors for LNM in EGC, there was no LNM in intramucosal cancer which was not signet ring cell type and was below 1 cm without ulceration regardless of lymphatic invasion. This means that ESD can be the treatment of choice in patients with intramucosal cancer below 1 cm without ulceration. Gotoda et al proposed an extension of the indications for endoscopic treatment, and one of the extended indications was histologically differentiated type SM1 tumor measuring less than 30 mm. But, there was LN metastasis in EGC of extended criteria in this study. However, the possibility of LNM in intramucosal cancer of extended indication was below 1%.
There was no LNM in intramucosal cancer which was not signet ring cell type and was below 1 cm without ulceration regardless of lymphatic invasion. This means that ESD can be the treatment of choice in patients with intramucosal cancer below 1 cm without ulceration.
The depth of submucosal invasion was subclassified according to two groups: SM1 (≤ 500 μm penetration into submucosa) and SM2 (> 500 μm). Extended indication criteria for endoscopic resection in differentiated EGC with no lymphatic or vascular involvement, includes: (1) mucosal cancers without ulcerative findings, regardless of tumor size; (2) mucosal cancers with ulcerative findings ≤ 30 mm; and (3) minute (≤ 500 μm from the muscularis mucosae) submucosal invasive cancers ≤ 30 mm.
The present paper is certainly well designed and conducted and draws solid and convincing conclusion that are mostly in line with similar studies from recent literature.
P- Reviewer: Guo XZ, Lorenzo-Zuniga V, Polistina F S- Editor: Ji FF L- Editor: A E- Editor: Liu XM
| 1. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011;14:113-123. [PubMed] |
| 2. | Soetikno R, Kaltenbach T, Yeh R, Gotoda T. Endoscopic mucosal resection for early cancers of the upper gastrointestinal tract. J Clin Oncol. 2005;23:4490-4498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 395] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 3. | Gotoda T. Endoscopic resection of early gastric cancer. Gastric Cancer. 2007;10:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 495] [Article Influence: 27.5] [Reference Citation Analysis (1)] |
| 4. | Gotoda T, Yanagisawa A, Sasako M, Ono H, Nakanishi Y, Shimoda T, Kato Y. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer. 2000;3:219-225. [PubMed] |
| 5. | Yamaguchi N, Isomoto H, Fukuda E, Ikeda K, Nishiyama H, Akiyama M, Ozawa E, Ohnita K, Hayashi T, Nakao K. Clinical outcomes of endoscopic submucosal dissection for early gastric cancer by indication criteria. Digestion. 2009;80:173-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 6. | Nakahara K, Tsuruta O, Tateishi H, Arima N, Takeda J, Toyonaga A, Sata M. Extended indication criteria for endoscopic mucosal resection of early gastric cancer with special reference to lymph node metastasis--examination by multivariate analysis. Kurume Med J. 2004;51:9-14. [PubMed] |
| 7. | Kajitani T. The general rules for the gastric cancer study in surgery and pathology. Part I. Clinical classification. Jpn J Surg. 1981;11:127-139. [PubMed] |
| 8. | Kitamura K, Yamaguchi T, Taniguchi H, Hagiwara A, Sawai K, Takahashi T. Analysis of lymph node metastasis in early gastric cancer: rationale of limited surgery. J Surg Oncol. 1997;64:42-47. [PubMed] |
| 9. | Roviello F, Rossi S, Marrelli D, Pedrazzani C, Corso G, Vindigni C, Morgagni P, Saragoni L, de Manzoni G, Tomezzoli A. Number of lymph node metastases and its prognostic significance in early gastric cancer: a multicenter Italian study. J Surg Oncol. 2006;94:275-280; discussion 274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 129] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 10. | Kim DY, Joo JK, Ryu SY, Kim YJ, Kim SK. Factors related to lymph node metastasis and surgical strategy used to treat early gastric carcinoma. World J Gastroenterol. 2004;10:737-740. [PubMed] |
| 11. | Noh SH, Hyung WJ, Cheong JH. Minimally invasive treatment for gastric cancer: approaches and selection process. J Surg Oncol. 2005;90:188-193; discussion 193-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Maruyama K, Okabayashi K, Kinoshita T. Progress in gastric cancer surgery in Japan and its limits of radicality. World J Surg. 1987;11:418-425. [PubMed] |
| 13. | Maehara Y, Orita H, Okuyama T, Moriguchi S, Tsujitani S, Korenaga D, Sugimachi K. Predictors of lymph node metastasis in early gastric cancer. Br J Surg. 1992;79:245-247. [PubMed] |
| 14. | Borie F, Millat B, Fingerhut A, Hay JM, Fagniez PL, De Saxce B. Lymphatic involvement in early gastric cancer: prevalence and prognosis in France. Arch Surg. 2000;135:1218-1223. [PubMed] |
| 15. | Pelz J, Merkel S, Horbach T, Papadopoulos T, Hohenberger W. Determination of nodal status and treatment in early gastric cancer. Eur J Surg Oncol. 2004;30:935-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Namieno T, Koito K, Higashi T, Sato N, Uchino J. General pattern of lymph node metastasis in early gastric carcinoma. World J Surg. 1996;20:996-1000. [PubMed] |
| 17. | Yamao T, Shirao K, Ono H, Kondo H, Saito D, Yamaguchi H, Sasako M, Sano T, Ochiai A, Yoshida S. Risk factors for lymph node metastasis from intramucosal gastric carcinoma. Cancer. 1996;77:602-606. [PubMed] |
| 18. | Tsujitani S, Oka S, Saito H, Kondo A, Ikeguchi M, Maeta M, Kaibara N. Less invasive surgery for early gastric cancer based on the low probability of lymph node metastasis. Surgery. 1999;125:148-154. [PubMed] |
| 19. | Kurihara N, Kubota T, Otani Y, Ohgami M, Kumai K, Sugiura H, Kitajima M. Lymph node metastasis of early gastric cancer with submucosal invasion. Br J Surg. 1998;85:835-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 88] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Itoh H, Oohata Y, Nakamura K, Nagata T, Mibu R, Nakayama F. Complete ten-year postgastrectomy follow-up of early gastric cancer. Am J Surg. 1989;158:14-16. [PubMed] |
| 21. | Adachi Y, Shiraishi N, Kitano S. Modern treatment of early gastric cancer: review of the Japanese experience. Dig Surg. 2002;19:333-339. [PubMed] |
| 22. | Kang HY, Kim SG, Kim JS, Jung HC, Song IS. Clinical outcomes of endoscopic submucosal dissection for undifferentiated early gastric cancer. Surg Endosc. 2010;24:509-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 83] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 23. | Maehara Y, Okuyama T, Oshiro T, Baba H, Anai H, Akazawa K, Sugimachi K. Early carcinoma of the stomach. Surg Gynecol Obstet. 1993;177:593-597. [PubMed] |
| 24. | An JY, Baik YH, Choi MG, Noh JH, Sohn TS, Kim S. Predictive factors for lymph node metastasis in early gastric cancer with submucosal invasion: analysis of a single institutional experience. Ann Surg. 2007;246:749-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 139] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 25. | Ichikura T, Uefuji K, Tomimatsu S, Okusa Y, Yahara T, Tamakuma S. Surgical strategy for patients with gastric carcinoma with submucosal invasion. A multivariate analysis. Cancer. 1995;76:935-940. [PubMed] |
| 26. | Park DJ, Lee HK, Lee HJ, Lee HS, Kim WH, Yang HK, Lee KU, Choe KJ. Lymph node metastasis in early gastric cancer with submucosal invasion: feasibility of minimally invasive surgery. World J Gastroenterol. 2004;10:3549-3552. [PubMed] |
| 27. | Kwee RM, Kwee TC. Predicting lymph node status in early gastric cancer. Gastric Cancer. 2008;11:134-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 129] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 28. | Li H, Lu P, Lu Y, Liu C, Xu H, Wang S, Chen J. Predictive factors of lymph node metastasis in undifferentiated early gastric cancers and application of endoscopic mucosal resection. Surg Oncol. 2010;19:221-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 29. | Kunisaki C, Shimada H, Nomura M, Akiyama H. Appropriate lymph node dissection for early gastric cancer based on lymph node metastases. Surgery. 2001;129:153-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 30. | Abe N, Sugiyama M, Masaki T, Ueki H, Yanagida O, Mori T, Watanabe T, Atomi Y. Predictive factors for lymph node metastasis of differentiated submucosally invasive gastric cancer. Gastrointest Endosc. 2004;60:242-245. [PubMed] |
| 31. | Korenaga D, Haraguchi M, Tsujitani S, Okamura T, Tamada R, Sugimachi K. Clinicopathological features of mucosal carcinoma of the stomach with lymph node metastasis in eleven patients. Br J Surg. 1986;73:431-433. [PubMed] |
| 32. | Sano T, Okuyama Y, Kobori O, Shimizu T, Morioka Y. Early gastric cancer. Endoscopic diagnosis of depth of invasion. Dig Dis Sci. 1990;35:1340-1344. [PubMed] |
| 33. | Ohashi S, Segawa K, Okamura S, Mitake M, Urano H, Shimodaira M, Takeda T, Kanamori S, Naito T, Takeda K. The utility of endoscopic ultrasonography and endoscopy in the endoscopic mucosal resection of early gastric cancer. Gut. 1999;45:599-604. [PubMed] |
| 34. | Cho WY, Kim YJ, Cho JY, Bok GH, Jin SY, Lee TH, Kim HG, Kim JO, Lee JS. Hybrid natural orifice transluminal endoscopic surgery: endoscopic full-thickness resection of early gastric cancer and laparoscopic regional lymph node dissection--14 human cases. Endoscopy. 2011;43:134-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 35. | Bok GH, Kim YJ, Jin SY, Chun CG, Lee TH, Kim HG, Jeon SR, Cho JY. Endoscopic submucosal dissection with sentinel node navigation surgery for early gastric cancer. Endoscopy. 2012;44:953-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 36. | Kunisaki C, Takahashi M, Nagahori Y, Fukushima T, Makino H, Takagawa R, Kosaka T, Ono HA, Akiyama H, Moriwaki Y. Risk factors for lymph node metastasis in histologically poorly differentiated type early gastric cancer. Endoscopy. 2009;41:498-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |