Published online Oct 21, 2015. doi: 10.3748/wjg.v21.i39.11127
Peer-review started: May 20, 2015
First decision: June 19, 2015
Revised: July 8, 2015
Accepted: August 30, 2015
Article in press: August 30, 2015
Published online: October 21, 2015
Processing time: 153 Days and 14.3 Hours
AIM: To elucidate the role of fibulin-5 (FBLN-5) as a suppressor of hepatocellular carcinoma (HCC) cell metastasis via integrin.
METHODS: The expression of FBLN-5 was determined by immunohistochemistry in 140 HCC samples and matched normal tissues, and was further confirmed by RT-PCR and Western blot analyses in various cell lines. Recombinant FBLN-5 was expressed in Escherichia coli BL21(DE3), purified and used in cell attachment assays. Expression of a specific plasmid or a specific siRNA in HCC cells resulted in the overexpression or knockdown of FBLN-5, respectively. Further, the migration and invasion of HCC cells were investigated using the Boyden chamber and transwell assays. The concentration of secreted matrix metalloproteinase 7 (MMP-7) was determined using ELISA.
RESULTS: FBLN-5 expression was found to be downregulated in HCC. Its expression was significantly correlated with advanced tumor metastasis; this was indicative of poor 5-year overall survival. Recombinant full-length human FBLN-5 promoted the attachment of HCC cells via integrins: it inhibited HCC cell adhesion and migration to fibronectin in a concentration-dependent manner. It also inhibited HCC cell migration and invasion through an integrin-binding arginine-glycine-aspartic acid (RGD) motif by downregulating MMP-7.
CONCLUSION: These results suggest that lower FBLN-5 expression is an important indicator of poor survival and that FBLN-5 inhibits HCC motility via an integrin-dependent mechanism. RGD-dependent suppression of MMP-7 by FBLN-5 might contribute to the development of new therapeutic strategies for HCC.
Core tip: Fibulin-5 (FBLN-5) is a matricellular protein that contains an arginine-glycine-aspartic acid motif, the role of which is to bind certain integrins and thereby mediate cancer cell motility. Several studies have revealed that FBLN-5 may promote or suppress tumor progression through its interaction with integrins in various human tumors in a context-specific manner, which might be a crucial event in the invasiveness of malignant tumor cells.
-
Citation: Tang JC, Liu JH, Liu XL, Liang X, Cai XJ. Effect of fibulin-5 on adhesion, migration and invasion of hepatocellular carcinoma cells
via an integrin-dependent mechanism. World J Gastroenterol 2015; 21(39): 11127-11140 - URL: https://www.wjgnet.com/1007-9327/full/v21/i39/11127.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i39.11127
Hepatocellular carcinoma (HCC) is one of the most common cancers worldwide, and it is associated with a considerably high mortality rate. At present, more than 0.7 million people have been diagnosed with HCC, which corresponds to an incidence of 16 per 0.1 million people[1]. In 90% of the cases, HCC is a result of chronic liver damage and is therefore a typical inflammation-related cancer characterized by the close association between the tumor microenvironment and tumor cells.
Changes in the interaction between the extracellular matrix and tumor cells play an important role in tumorigenesis and metastasis. Fibulin-5 (FBLN-5) is an extracellular matrix glycoprotein that has been shown to be expressed in elastin-rich tissues; it mediates changes in the matrix structures via its interaction with several extracellular proteins such as tropoelastin, elastin monomers, lysyl oxidase-like proteins, and latent TGF-β-binding proteins[2]. FBLN-5 contains an arginine-glycine-aspartic acid (RGD) motif, the function of which is to bind to certain integrins and mediate the adhesion of endothelial cells[3,4]. Moreover, FBLN-5 also inhibits the expression of matrix metalloproteinase 7 (MMP-7) in lung cancer cells via an integrindependent mechanism and downregulation of the ERK pathway[5]. Experiments on FBLN-5-null mice have provided evidence for the role of FBLN-5 as an angiogenesis inhibitor and its roles in the proliferation, migration and invasion of certain tumors. The effect of FBLN-5 on tumorigenesis appears to be largely context-dependent, and may involve the inhibitory effect of fibulin-5 on angiogenesis; however, the exact mechanisms are still under investigation[2].
In this study, we investigated the expression of FBLN-5 and its relationship with clinicopathological features in order to understand its role in HCC progression, and to identify the molecular mechanisms responsible for its functions.
Site-directed mutagenesis of eukaryotically expressed FBLN-5 was performed, wherein Asp56 was substituted with Glu within the integrin-binding RGD motif, so as to prevent integrin binding[6,7]. The KOD Hot start DNA polymerase kit (Millpore, United States) was used according to the manufacturer’s recommendations.
The primers that were used for site-directed mutation PCR were: forward, 5′-TCC CCG AGG CCT GCC GAG GAG AAA TGA TGT GTG TTA ACC AAA ATG-3′ and reverse, 5′-CAT TTT GGT TAA CAC ACA TCA TTT CTC CTC GGC AGG CCT CGG GGA-3′.
One hundred and forty HCC samples and normal tissue samples were collected from patients with HCC (115 males and 25 females) who underwent surgery at Sir Run Run Shaw Hospital of Zhejiang University between January 2006 and December 2010. The procedures and data collection were approved by the human research committee of Sir Run Run Shaw Hospital, and written informed consent was obtained from all patients before the study was started. Clinicopathological data including age, gender, tumor size, nodal status, physiological and biochemical indicators, Barcelona clinic liver cancer (BCLC) tumor stage, and tumor-node-metastasis (TNM) stage were obtained retrospectively from the patients’ clinical records and pathology reports. Before the surgery was performed, the patients underwent staging examinations, including blood routine examination, liver function tests, abdominal ultrasonography and/or magnetic resonance imaging. Based on the Union for International Cancer Control (UICC) 1987 and BCLC system, 18 patients were found to have stage Icancer; 84, stage II; 30, stage III; and 8, stage IV. Based on the BCLC tumor staging system alone, 14 patients were found to have stage 0 cancer; 79, stage A; 28, stage B; 16, stage C; and 3, stage D. The survival rates were determined based on the data from the cancer registry of our hospital, or the required information was collected from the patients’ attending physicians.
Normal and HCC tissue samples were selected based on the diagnosis and microscopic observation of the tumor tissue. Immunohistochemistry was performed with mouse antibodies against FBLN-5 (Abcam, United Kingdom) as previously described[8].
The immunostaining results were examined independently by two pathologists who were blinded to the clinical data. FBLN-5 staining was scored from 0 to +3: 0 indicated that none of the cells were stained; +1, less than 5% of the cells were stained; +2, 5%-50% of the cells were stained; and +3, more than 50% of the cells were stained. Further, the intensity of staining was graded from 0 to 3 as previously described[9].
PCR amplification of FBLN-5 mRNA (forward: 5′-CCAAACTATCCCACGATC-3′; reverse: 5′-CAGGAACATTCGCACAG-3′) and GAPDH mRNA (forward: 5′-ACAGTCAGCCGCATCTTCTT-3′; reverse: 5′-TGGAAGATGGTGATGGGATT-3′) was performed using SsoFast EvaGreen Supermix with the Low ROX Kit (BioRad, United States), as previously described[10]. For methylation-specific PCR (MSP), the methylated FBLN-5 promoter was amplified using the primers reported previously[5]. MSP products were analyzed by electrophoresis on 2% agarose gels.
Cell samples were homogenized in RIPA lysis buffer [50 mmol/L Tris-HCl (pH 7.5), 150 mmol/L NaCl, 1% NP-40, 0.5% Na-deoxycholate, and 0.1% SDS] for protein extraction. Western blot analysis was performed as described in a previous study[10]. Antibodies against V5 (Cell Signaling Technology, United States), FBLN-5 (Abcam, United Kingdom), MMP-7 (Cell Signaling Technology, United States) and GAPDH (Sigma, Germany) were used. The blots were visualized using Image Quant LAS-4000 (Fujifilm, Japan).
We used four cell lines: the human immortalized normal hepatocyte cell line LO2 and three HCC cell lines (MHCC97L, MHCC97H and HCC-LM3). These cell lines were obtained from the Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences, Shanghai, China. The cells were maintained in complete Dulbecco’s modified Eagle medium (DMEM, Gibco, United States). Two other HCC cell lines (HepG2 and Hep3B) obtained from American Type Culture Collection (ATCC, United States) were cultured in minimum essential medium (MEM, Gibco, United States). All the media contained 10% (v/v) fetal bovine serum (FBS; Gibco, United States) and 1% (v/v) penicillin/streptomycin. The cells were incubated at 37 °C in a humidified atmosphere containing 5% CO2.
The plasmids (pcDNA3.1-FBLN-5, pcDNA3.1-FBLN-5 with the RGD mutation, and pcDNA3.1) or small interfering RNA (siRNA against FBLN-5) was transiently transfected into cells with Lipofectamine 2000 or the RNAi MAX reagent according to the manufacturer’s instructions (Invitrogen, United States).
Proteins fused with glutathione S-transferase (GST) in the pGEX vector were expressed in Escherichia coli BL21(DE3), induced with 1 mmol/L isopropyl-α-d-thiogalactoside (IPTG; Promega, United States), and purified according to the manufacturer’s instructions for glutathione sepharose beads TM 4B (GE, United States). Purification experiments for the GST fusion protein were carried out as previously described[11]. Unless otherwise stated, FBLN-5 expressed by the prokaryotic system represented the recombinant protein with GST and full-length FBLN-5, and prokaryotically expressed FBLN-5-RGE represented the recombinant protein with GST and full-length FBLN-5 D56E.
The concentration of secreted MMP-7 was determined in triplicate by ELISA using the Human Total MMP-7 Quantikine ELISA kit (R&D Systems, United States) according to the manufacturer’s protocol.
The synthetic peptides GRGDS and SDGRG (Sangon Biotech, China) were used to determine whether FBLN-5 binds to the HCC cells via integrin-binding to the RDG domain. Recombinant full-length FBLN-5 and FBLN-5-RGE were diluted to 20-400 nmol/L in DPBS+ and incubated overnight in 96-well microplates at 4 °C in order to facilitate adsorption onto the wells. Non-specific binding was blocked using 10 mg/mL heat-denatured bovine serum albumin (BSA) for 1 h. BSA was aspirated, and 100 μL of HCC-LM3 or Hep3B cell suspension (5 × 105 cells/mL) was added to the wells and incubated for about 30 min at 37 °C in a 5% (v/v) CO2 atmosphere. In order to determine the number of cells that had adhered, a known number of cells was added to the wells and fixed with 10 μL of 4% paraformaldehyde. Each well was washed three times with 200 μL of DPBS+, and the cells were stained with 100 μL of 0.1% (w/v) crystal violet for 1 h. The dye that was taken up by cells was solubilized in 100 μL of 10% (v/v) acetic acid, and absorbance was measured at 590 nm. The absorbance of the cells present in the wells was considered as the percentage of cell attachment.
Each well was coated with 200 nmol/L of FBLN-5, FBLN-5-RGE or fibronectin (FN) in DPBS+ overnight at 4 °C. Then, the cells were incubated with 10 mg/mL heat-denatured BSA for 1 h at 20 °C to block non-specific binding. The effect of FBLN-5 on the migration of HCC cells and on the response of the cells to TGF-β was determined as follows: (1) the HCC-LM3 cells that had adhered to the wells were trypsinized, neutralized, seeded at confluence (5 × 105 cells/well), and incubated at 37 °C in a 5% CO2 incubator for up to 12 h, until a confluent monolayer was formed. The cells were then serum-starved overnight and washed twice with serum-free DMEM before wounding with the tip of a sterile P10 pipette. The effect of FBLN-5 on the migration of HCC-LM3 cells was assessed at the time of wounding. After 48 h, the wound was photographed in order to determine the number of cells that had migrated into the cleared wound; and (2) cell migration was evaluated by counting the number of HCC-LM3 cells that had migrated on three independent membranes under a 100 × phase-contrast microscope; the number was then normalized using the number of the control cells (in transwell chambers coated with BSA) in order to determine the relative ratio. After the excess dye was washed out and dried, the crystal violet adsorbed by the cell nuclei was extracted with 10% acetic acid. Absorption of the destained cells at 590 nm was measured.
Invasion assays were performed in 24-well transwell units with 8-μm filters coated with 1:6 diluted matrigel (BD Biosciences, United States); the assays were performed in triplicate. Each well contained approximately 2 × 106 cells. After 36 h of incubation, the cells that passed through the filters into the bottom wells were fixed in formalin and stained with crystal violet. The number of cells in 10 randomly selected fields (× 100) from each well was counted under a microscope.
Unless otherwise stated, data are expressed as mean ± SD and analyzed using the Student’s t-test (GraphPad Prism 5.02); differences were considered significant when P value was less than 0.05.
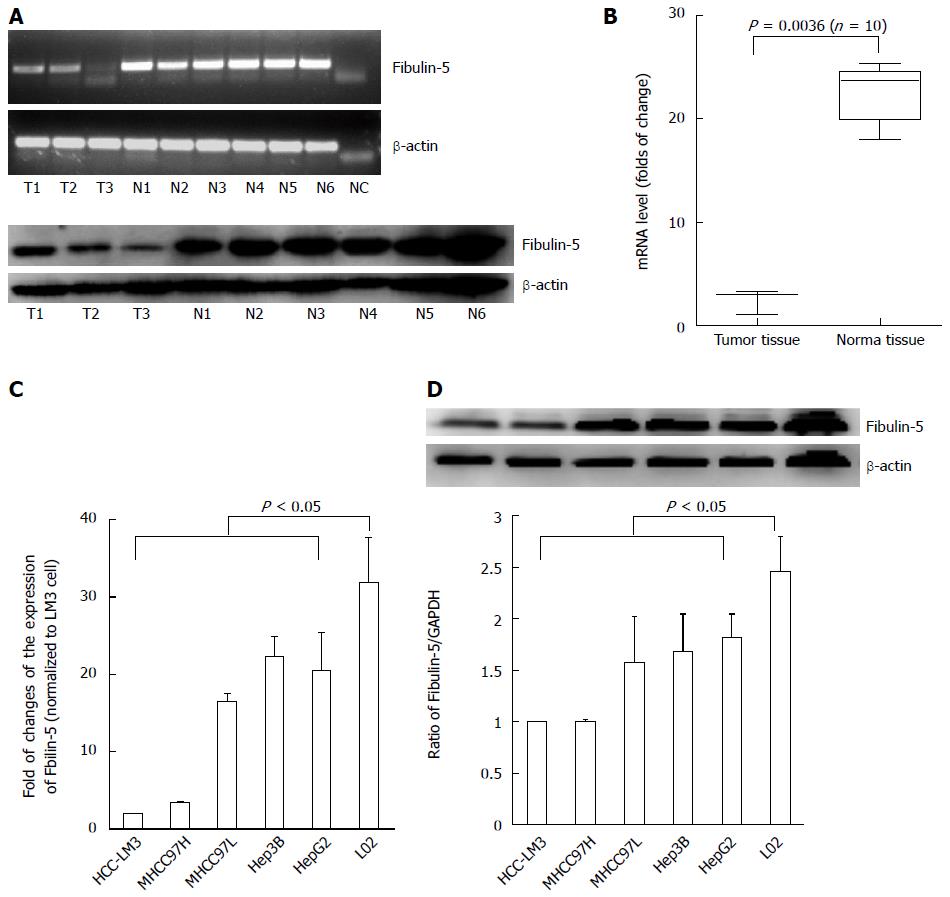
To determine whether FBLN-5 plays a role in the pathogenesis of HCC, semi-quantitative reverse transcription-PCR (RT-PCR) and Western blot analyses were performed on three HCC and six normal tissue samples. FBLN-5 expression was low in the tumor specimens and high in the normal tissues (Figure 1A). Next, mRNA expression of FBLN-5 in another batch of 10 matched sets of frozen tumor and normal samples was analyzed using qRT-PCR; the results were found to be consistent with those of mRNA expression of FBLN-5 (P < 0.05) (Figure 1B).
The downregulation of FBLN-5 was further confirmed by qRT-PCR and Western blot in HCC cells including MHCC97L, MHCC97H, HCC-LM3, HepG2 and Hep3B cells, and cells of the immortalized normal hepatocyte cell line LO2 (P < 0.05, Figure 1C and D). Moreover, to investigate whether the downregulation of FBLN-5 is due to epigenetic silencing, the above-mentioned 10 matched tissue samples were analyzed using the MSP assay, and methylation of the FBLN-5 promoter was not found in any of the tumor samples (0%). Similar phenomena were also observed in HCC-LM3, MHCC97H, MHCC97L, HepG2 and Hep3B cells (Figure S1). Collectively, these findings indicate that FBLN-5 expression is downregulated in both HCC tissues and cells, and suggest that FBLN-5 might participate in the pathogenesis of HCC.
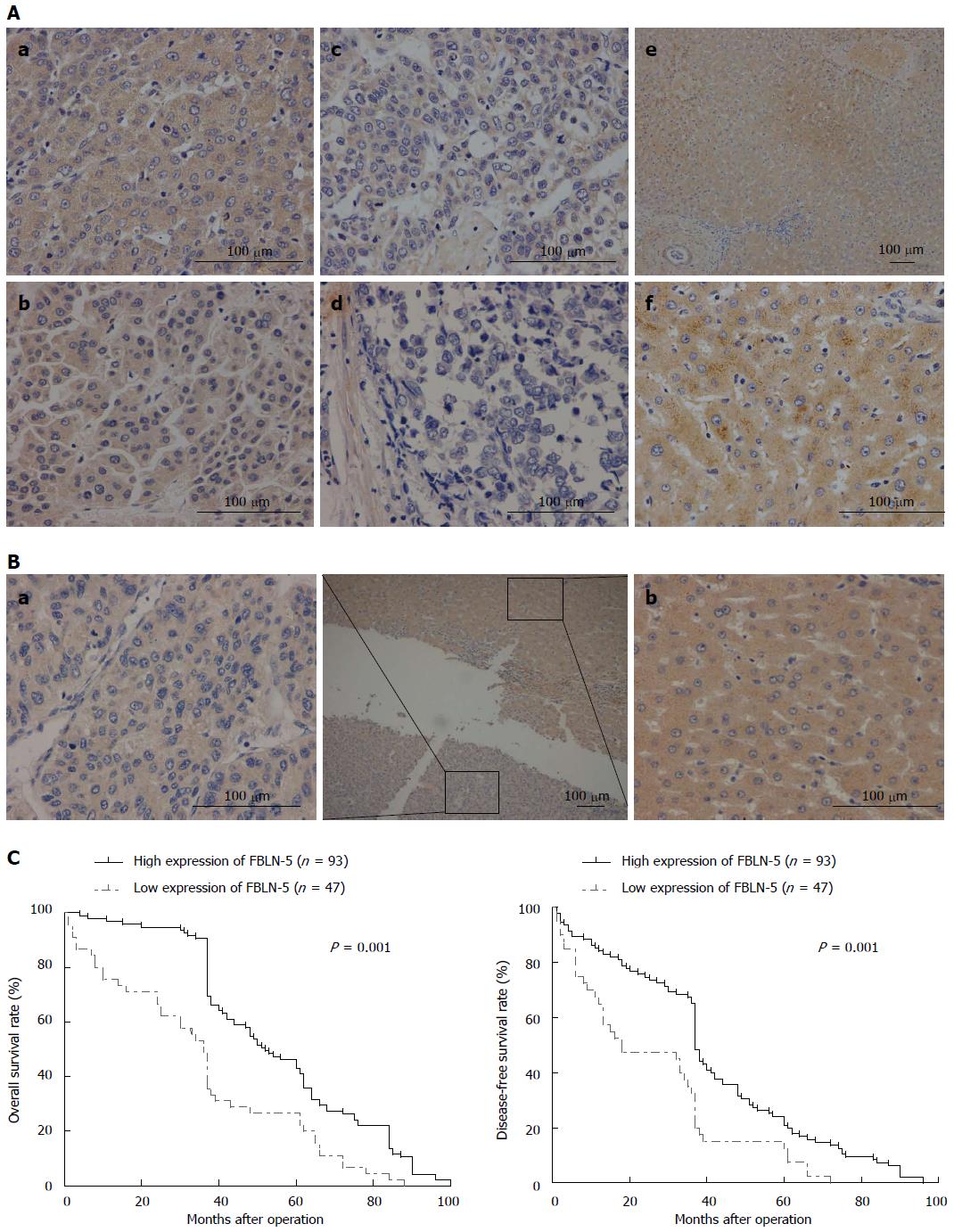
To investigate whether downregulation of FBLN-5 expression is associated with certain prognostic factors, we classified the patients into groups based on their immunohistochemistry findings. As shown in Table 1, patients with a higher number of tumor nodules (n≥ 2) and BCLC stages B-D and TNM stages III-IV tumor had significantly lower FBLN-5 expression than patients with a single tumor nodule (P = 0.023) and BCLC stages 0-A (P = 0.001) and TNM stages I-II (P = 0.001) tumor. No significant difference was found in the FBLN-5 level according to age, gender, tumor size, presence of HBV, serum AFP level, or histological type at the time of diagnosis.
| Parameter | Total No. of patients(n = 140) | FBLN-5 expression | P value1 | ||
| High (n= 93) | Low (n = 47) | ||||
| Age (yr) | < 50 | 54 | 40 | 14 | 0.211 |
| ≥ 50 | 86 | 55 | 31 | ||
| Gender | Male | 113 | 79 | 34 | 0.287 |
| Female | 27 | 16 | 11 | ||
| Tumor size (cm) | < 5 | 82 | 57 | 25 | 0.618 |
| ≥ 5 | 58 | 38 | 20 | ||
| No. of tumor nodules | 1 | 122 | 87 | 35 | 0.0232 |
| ≥ 2 | 18 | 8 | 10 | ||
| Recurrent Cancer | Absent | 87 | 64 | 23 | 0.064 |
| Present | 53 | 31 | 22 | ||
| Cirrhosis | Absent | 50 | 29 | 21 | 0.063 |
| Present | 90 | 66 | 24 | ||
| Serum AFP level | Negative | 79 | 52 | 27 | 0.558 |
| Positive | 61 | 43 | 18 | ||
| HBV | HBsAg (-) | 32 | 22 | 10 | 0.902 |
| HBsAg (+) | 108 | 73 | 35 | ||
| TNM tumor stage | I + II | 102 | 79 | 23 | 0.0012 |
| III + IV | 38 | 16 | 22 | ||
| BCLC tumor stage | 0 + A | 93 | 74 | 19 | 0.0012 |
| B + C + D | 47 | 21 | 26 | ||
Disease-specific survival analysis using Cox regression revealed that the prognosis of patients with low expression of FBLN-5 in the tumor cells was significantly poorer than that of patients with high expression of FBLN-5 (P = 0.001; Table 2). FBLN-5 expression was indeed associated with poorer overall survival and disease-free survival in HCC patients (P < 0.05, respectively; Figure 2). These results clearly indicate that FBLN-5 may act as a potent biomarker of prognosis of HCC patients.
| Parameter | No. | No. of events | HR (95%CI)1 | P value2 | |
| Age (yr) | < 50 | 54 | 18 | 0.373 (0.169-0.823) | 0.158 |
| ≥ 50 | 86 | 24 | |||
| Gender | Male | 113 | 30 | 0.864 (0.324-2.306) | 0.771 |
| Female | 27 | 12 | |||
| Tumor size (cm) | < 5 | 82 | 23 | 1.874 (0.902-3.895) | 0.093 |
| ≥ 5 | 58 | 19 | |||
| No. of tumor nodules | 1 | 122 | 32 | 3.634 (1.481-8.916) | 0.0053 |
| ≥ 2 | 18 | 10 | |||
| Recurrent cancer | Absent | 87 | 15 | 0.587 (0.231-1.491) | 0.263 |
| Present | 53 | 28 | |||
| Cirrhosis | Absent | 50 | 14 | 0.924 (0.426-2.004) | 0.841 |
| Present | 90 | 29 | |||
| Serum AFP level | Negative | 79 | 21 | 0.615 (0.280-1.352) | 0.227 |
| Positive | 61 | 21 | |||
| HBV | HBsAg (-) | 32 | 11 | 1.487 (0.670-3.302) | 0.329 |
| HBsAg (+) | 108 | 31 | |||
| TNM tumor stage | I + II | 102 | 11 | 7.478 (2.571-21.753) | 0.0013 |
| III + IV | 38 | 31 | |||
| BCLC tumor stage | 0 + A | 93 | 8 | 5.257 (1.707-16.186) | 0.0043 |
| B + C + D | 47 | 34 | |||
| FBLN-5 Expression | High | 95 | 13 | 4.276 (2.033-8.995) | 0.0013 |
| Low | 45 | 29 | |||
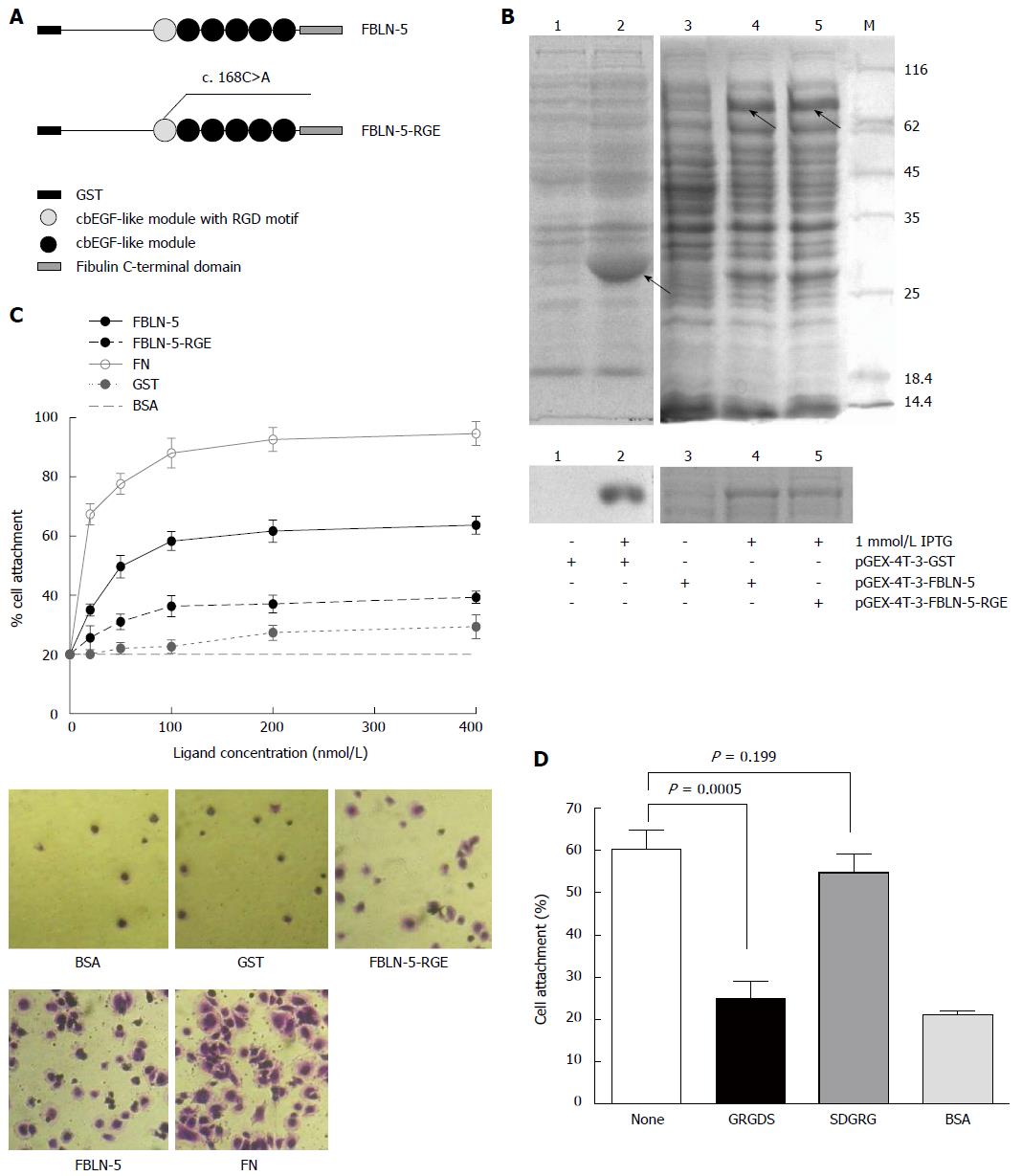
To investigate the mechanism of integrin regulation by FBLN-5, we tried to determine the functional role of its RGD motif. A site-mutated (D56E) RGD motif in pGEX-FBLN-5 was constructed and named FBLN-5-RGE (Figure 3A). GST, GST-FBLN-5 and GST-FBLN-5-RGE were purified and pulled down using glutathione sepharose beads, and monitored using SDS/PAGE and Coomassie blue staining (Figure 3B). Since FN promotes integrin-mediated cell attachment via its RGD motif[12], in cell attachment assays, human plasma FN diluted to 10-400 nmol/L in DPBS+ was used as the positive control. HCC cells in BSA-blocked wells were used as a negative control, and full-length FBLN-5 and FBLN-5-RGE were used in all the subsequent experiments.
Compared to FBLN-5, FBLN-5-RGE supported the adhesion of a much lower number of HCC-LM3 cells (Figure 3C; 35% at 200 nmol/L). We also found that Hep3B, another typical type of HCC cell, resulted in a higher percentage of attachment to FBLN-5 than to FBLN-5-RGE (Figure S2A; 65% and 40%, respectively, at 400 nmol/L). The findings revealed that the RGD motif was necessary for the binding of FBLN-5 to HCC cells. FBLN-5 or FN at 200 nmol/L in DPBS+ and at 400 nmol/L resulted in the attachment of a similar number of HCC cells, with the former concentration showing minimal endotoxicity. According to the above results, the concentration of 200 nmol/L of FBLN-5 or FN in DPBS+ was selected for further experiments, as this seems to be the minimum concentration necessary for the in vitro adhesion of HCC cells.
Moreover, pre-incubation of HCC cells with the synthetic peptide GRGDS resulted in significant block of FBLN-5 adhesion (P < 0.001 compared with untreated HCC-LM3 cells exposed to FBLN-5), while incubation with the control peptide SDGRG did not result in inhibition of cell attachment both in HCC-LM3 cells and Hep3B cells (Figure 3D, S2B). These results indicate that HCC cells bind to FBLN-5 via an integrin-dependent mechanism.
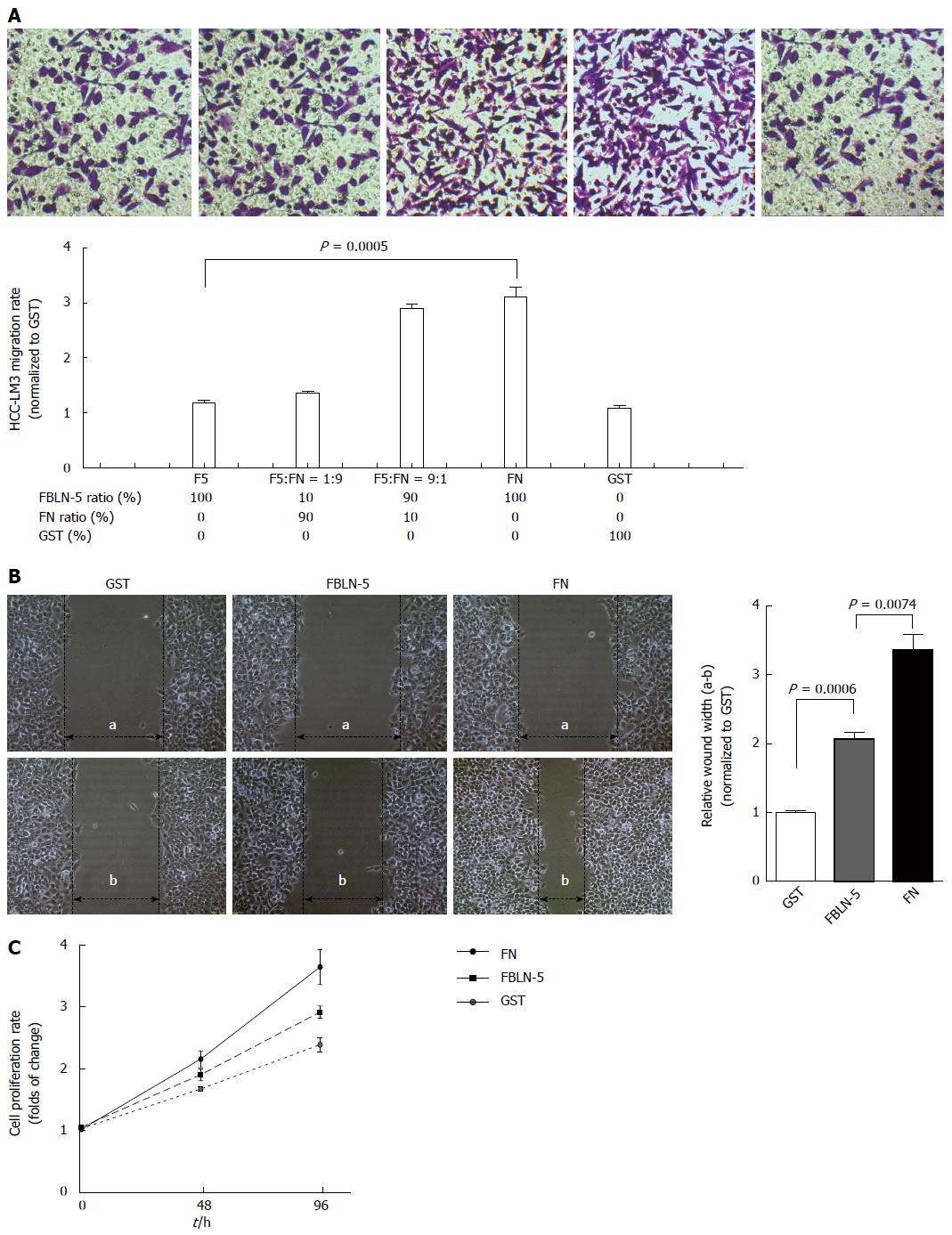
FBLN-5 and FN both ligate integrin[13], so the possibility of FBLN-5 modulation of FN-mediated adhesion and migration of HCC-LM3 cells was investigated. To determine whether the adhesion of HCC-LM3 cells to FBLN-5 influences migration, 50000 HCC-LM3 cells were added to each chamber of the transwell, the bottom of which contained membranes coated with BSA or FBLN-5 and FN in a range of ratios (F5:FN). Compared to groups with high FN or high FN/FBLN-5 levels, the number of migrated HCC-LM3 cells was significantly lower in the groups with FBLN-5 or lower FN/FBLN-5 ratios (P < 0.001; Figure 4A). Moreover, HCC-LM3 cells were plated at confluence on 200 nmol/L of GST, FBLN-5 or FN. The wound healing assays revealed that HCC-LM3 cells migrated to a lesser extent in the presence of FBLN-5 than FN (P < 0.001; Figure 4B). To investigate whether the adhesion of HCC-LM3 cells to FBLN-5 influences proliferation, the cells (2 × 103/well) were plated on 200 nmol/L FBLN-5 or FN and were counted on 2 and 4 d. Coincidentally, the proliferation of HCC-LM3 cells plated on FBLN-5 was significantly less than that of cells plated on FN (Figure 4C). These results show that in the presence of elevated FBLN-5/FN levels, FBLN-5 directly inhibits FN-mediated migration and proliferation.
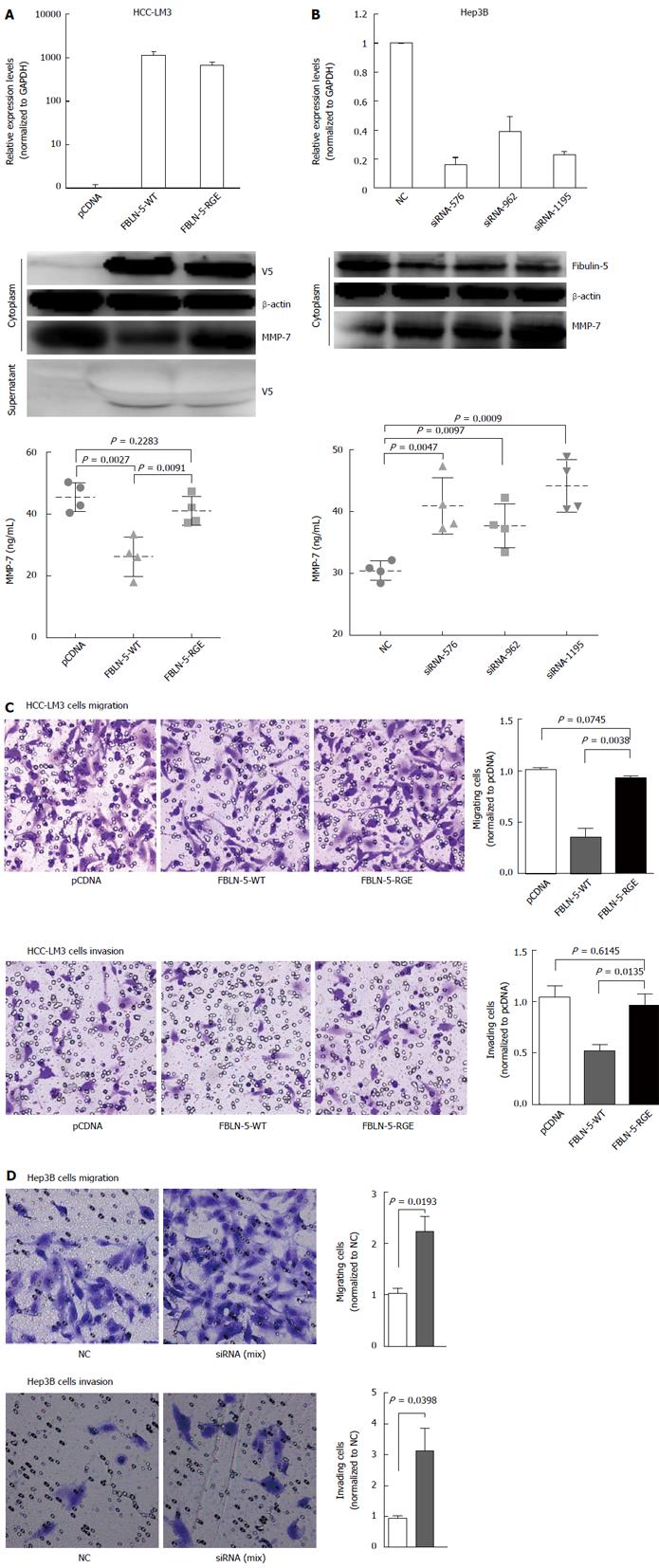
Based on the biochemical properties of FBLN-5 and the presence of the RGD motif[14], we hypothesized that it is involved in regulating cancer cell migration and invasion. The inverse correlation between FBLN-5 and MMP-7 expression has been reported previously in several types of cancer[5,15]. HCC-LM3 cells transfected with EV, FBLN-5-WT or FBLN-RGE were subjected to immunoblot analysis and ELISA to determine the levels of MMP-7 protein. FBLN-5-WT containing the RGD motif suppressed MMP-7 protein expression. In contrast, the FBLN-5-RGE mutant restored most of the MMP-7 expression and 40% of the level of secreted MMP-7 (P < 0.05; Figure 5A), which suggests that the inhibition of MMP-7 by FBLN-5 is mediated by the RGD motif through integrin signaling. Furthermore, knockdown of FBLN-5 resulted in an obvious increase in the level of MMP-7 protein in Hep3B cells (P < 0.05; Figure 5B).
We next evaluated the effect of FBLN-5 on HCC cell invasion and migration in transwells with or without matrigel. The representative fields showing cell migration and invasion are shown (Figure 5C and D). Overexpression of FBLN-5 in HCC-LM3 cells caused a significant decrease in cell migration and invasion (P < 0.05, respectively; Figure 5C), while knockdown of FBLN-5 resulted in an obvious increase in Hep3B cell migration and invasion (P < 0.05, respectively; Figure 5D). These findings suggest that the RGD motif of FBLN-5 plays an important role in human HCC cell migration and invasion via inhibition of MMP-7 expression.
Metastasis, which differs from tumor initiation, is the most lethal characteristic of HCC and is the main cause of HCC-related death. Matricellular proteins participate in matrix-cell interactions and exert regulatory roles via a variety of molecular mechanisms[16]. The importance of FBLN-5 in many human malignancies, such as nasopharyngeal carcinoma, lung and breast cancers, has been shown in mouse models and studies of various diseases[2], and recently, Tu et al[15] demonstrated that FBLN-5 inhibited the migration and invasion of HCC cells via downregulation of MMP-7 expression in 86 HCC tissues samples. However, as an extracellular matrix protein, precisely how FBLN-5 interacts with HCC cells and influences their functions remains unclear. Besides, more clinical tissue samples are required to confirm loss of FBLN-5 expression in HCC cells. In this study, we examined the interaction of FBLN-5 with HCC cells, and how these interactions regulate their behavior. We found that HCC cells adhere to FBLN-5 via integrin binding, and that their migration and invasion are inhibited by downregulation of MMP-7 via an integrin-binding RGD motif-dependent mechanism. We present the results of a systematic survey of FBLN-5 expression in 140 samples of HCC tissues, which is twice the number of samples studied in Tu’s paper[15].
Our results indicate that the mRNA and protein levels of FBLN-5 are decreased in HCC tissues. However, the MSP assay showed that methylation of the FBLN-5 promoter was not found in any of the tumor samples or HCC cell lines, which indicates that the downregulation of FBLN-5 might not be related with any epigenetic alterations. Reduced expression of FBLN-5 was associated with clinically aggressive HCC and correlated with the number of tumor nodules, BCLC tumor stage, and TNM stage. Univariate analysis showed that the 5-year survival rate of HCC patients with low FBLN-5 expression in tumor cells was significantly lower than that in HCC patients with high FBLN-5 expression. These results suggest that reduced expression of FBLN-5 can be used as a predictor of poor prognosis of HCC and may play a role as a tumor suppressor in human HCC.
The ability of cancer cells to migrate and invade the basement membrane and the surrounding tissues, blood, and lymphatic vessels is an important characteristic of cancer and is a prerequisite for progression and metastasis of tumors[17]. Here, we found that exogenous FBLN-5 in HCC cells or HCC cells stimulated with the FBLN-5 protein could inhibit the proliferation, migration and invasion of the cells in culture systems. Conversely, after transfection with FBLN-5 siRNA, the number of cells migrating through or invading the filter significantly increased; this suggests that FBLN-5 downregulates human HCC cell proliferation, migration, and invasion. Compared with other studies[5,9,12,15,18-20], the current study indicates that FBLN-5 functions as a tumor suppressor or oncogene in various cancers in a context-specific manner.
Several studies have shown that the integrin family of transmembrane proteins is involved in the adhesion of cells with their extracellular matrix, which includes FN; moreover, integrins are known to play important roles in multiple features of cell physiology, including proliferation, migration and invasion[21,22]. In this study, we found that although FLBN-5 is ligated to the same integrins as FN, it exhibits considerably different cellular effects from FN and can modulate FN-mediated adhesion and migration. These results explain how FBLN-5 inhibits HCC migration and invasion as a component of the extracellular matrix. It has been reported that the effects of FBLN-5 on HCC cells are mediated by the inhibition of MMP-7, which is up-regulated and associated with poor prognosis of HCC[23,24]. In our study, we found that this molecular mechanism underlying the interaction between FBLN-5 and MMP-7 is mediated by integrin. The RGD motif of FBLN-5 binds to the integrins αvβ3, αvβ5, and α9β1 in CHO cells[3], but the specific integrin subunit(s) that mediate the inhibitory effect of FBLN-5 on MMP-7 in HCC still need to be identified.
FBLN-5 has been identified as a novel target gene for TGF-β in fibroblasts[18], endothelial cells[18,25] and epithelial cells[9]. We found that TGF-β results in significant upregulation of FBLN-5 expression in HCC cells, while the tumor-promoting function of TGF-β is suppressed by FBLN-5 both in HCC-LM3 and Hep3B cells. Further investigation is required to examine the molecular connections potentially linking FBLN-5 to the acquisition of oncogenic signaling by TGF-β.
In summary, our findings suggest that downregulation of FBLN-5 is a common abnormality in HCC and is correlated with HCC progression and poor survival. Recombinant full-length human FBLN-5 promoted the attachment of HCC cells via integrins, and inhibited HCC cell adhesion and migration to fibronectin in a concentration-dependent manner. Our results further indicate that FBLN-5 functions as a suppressor of HCC metastasis via an integrin-dependent mechanism by downregulating MMP-7. Although much work needs to be carried out to fully understand the function of FBLN-5 in the control of HCC progression, the validation of its relevance in vitro is an intriguing finding for cancer therapy.
The authors would like to thank the members of the Pathology Department, Clinical Laboratory of Sir Run Run Shaw Hospital, Zhejiang University, for their technical support.
Fibulin-5 (FBLN-5) is characterized by an integrin-binding arginine-glycine-aspartic acid (RGD) motif that can bind certain integrins and regulate cell motility; it regulates the expression of several matrix metalloproteinases and tissue inhibitors of metalloproteinase in various cancers in a context-specific manner. However, the roles of FBLN-5 in hepatocellular carcinoma (HCC) remain to be fully elucidated.
Previous experiments have revealed that the effects of FBLN-5 on HCC cells are mediated via the inhibition of matrix metalloproteinase 7 (MMP-7). Here, the authors showed that although FLBN-5 is ligated to the same integrins as FN, it has considerably different cellular effects and can modulate FN-mediated adhesion and migration. These results explain how FBLN-5 inhibits HCC migration and invasion as a component of the extracellular matrix.
This is the first study to demonstrate that recombinant full-length human FBLN-5 promotes the attachment of HCC cells via integrins. FBLN-5 inhibits HCC cell adhesion and migration to fibronectin in a concentration-dependent manner. In contrast, after transfection with FBLN-5 siRNA, the number of cells that migrated through or invaded the filter significantly increased; this suggests that FBLN-5 downregulates cancer cell proliferation, migration, and invasion in human HCC. These results provide further evidence for the role of FBLN-5 as a suppressor of HCC metastasis via an integrin-dependent mechanism involving downregulation of MMP-7.
This systematic survey of FBLN-5 expression in 140 samples of HCC tissues demonstrated that lower FBLN-5 expression is an important indicator of poor survival and that FBLN-5 inhibits HCC motility via an integrin-dependent mechanism; further, RGD-dependent suppression of MMP-7 by FBLN-5 might contribute to the development of new therapeutic strategies for HCC treatment.
The aim of this work was clear, and the authors performed plenty of experiments. It would be better to explain the reason for replacement of Asp56 within the integrin-binding RGD motif with Glu. It was assumed that this mutation weakened tumor suppression of FBNL-5. It would be better to introduce the relation between FBLN-5 and cell migration. Based on the results of this study, down-regulation of FBLN-5 seemed to promote cell motility and proliferation.
P- Reviewer: Tomizawa M S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Zhang DN
| 1. | Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3249] [Cited by in RCA: 3594] [Article Influence: 276.5] [Reference Citation Analysis (4)] |
| 2. | Tang JC, Xie AY, Cai XJ. [Diverse functions of fibulin-5 in tumors]. Mol Biol (Mosk). 2014;48:875-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Nakamura T, Lozano PR, Ikeda Y, Iwanaga Y, Hinek A, Minamisawa S, Cheng CF, Kobuke K, Dalton N, Takada Y. Fibulin-5/DANCE is essential for elastogenesis in vivo. Nature. 2002;415:171-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 470] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 4. | Nakamura T, Ruiz-Lozano P, Lindner V, Yabe D, Taniwaki M, Furukawa Y, Kobuke K, Tashiro K, Lu Z, Andon NL. DANCE, a novel secreted RGD protein expressed in developing, atherosclerotic, and balloon-injured arteries. J Biol Chem. 1999;274:22476-22483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 142] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 5. | Yue W, Sun Q, Landreneau R, Wu C, Siegfried JM, Yu J, Zhang L. Fibulin-5 suppresses lung cancer invasion by inhibiting matrix metalloproteinase-7 expression. Cancer Res. 2009;69:6339-6346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 83] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 6. | Smith LL, Giachelli CM. Structural requirements for alpha 9 beta 1-mediated adhesion and migration to thrombin-cleaved osteopontin. Exp Cell Res. 1998;242:351-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 85] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 7. | Schluterman MK, Chapman SL, Korpanty G, Ozumi K, Fukai T, Yanagisawa H, Brekken RA. Loss of fibulin-5 binding to beta1 integrins inhibits tumor growth by increasing the level of ROS. Dis Model Mech. 2010;3:333-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Hu X, Sui X, Li L, Huang X, Rong R, Su X, Shi Q, Mo L, Shu X, Kuang Y. Protocadherin 17 acts as a tumour suppressor inducing tumour cell apoptosis and autophagy, and is frequently methylated in gastric and colorectal cancers. J Pathol. 2013;229:62-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 9. | Lee YH, Albig AR, Regner M, Schiemann BJ, Schiemann WP. Fibulin-5 initiates epithelial-mesenchymal transition (EMT) and enhances EMT induced by TGF-beta in mammary epithelial cells via a MMP-dependent mechanism. Carcinogenesis. 2008;29:2243-2251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 110] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 10. | Liang X, Tang J, Liang Y, Jin R, Cai X. Suppression of autophagy by chloroquine sensitizes 5-fluorouracil-mediated cell death in gallbladder carcinoma cells. Cell Biosci. 2014;4:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 11. | Feng Y, Wu H, Xu Y, Zhang Z, Liu T, Lin X, Feng XH. Zinc finger protein 451 is a novel Smad corepressor in transforming growth factor-β signaling. J Biol Chem. 2014;289:2072-2083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Wang R, Clark RA, Mosher DF, Ren XD. Fibronectin’s central cell-binding domain supports focal adhesion formation and Rho signal transduction. J Biol Chem. 2005;280:28803-28810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Lomas AC, Mellody KT, Freeman LJ, Bax DV, Shuttleworth CA, Kielty CM. Fibulin-5 binds human smooth-muscle cells through alpha5beta1 and alpha4beta1 integrins, but does not support receptor activation. Biochem J. 2007;405:417-428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 68] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 14. | Albig AR, Schiemann WP. Fibulin-5 function during tumorigenesis. Future Oncol. 2005;1:23-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Tu K, Dou C, Zheng X, Li C, Yang W, Yao Y, Liu Q. Fibulin-5 inhibits hepatocellular carcinoma cell migration and invasion by down-regulating matrix metalloproteinase-7 expression. BMC Cancer. 2014;14:938. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Geiger TR, Peeper DS. Metastasis mechanisms. Biochim Biophys Acta. 2009;1796:293-308. [PubMed] |
| 17. | Chong HC, Tan CK, Huang RL, Tan NS. Matricellular proteins: a sticky affair with cancers. J Oncol. 2012;2012:351089. [PubMed] |
| 18. | Schiemann WP, Blobe GC, Kalume DE, Pandey A, Lodish HF. Context-specific effects of fibulin-5 (DANCE/EVEC) on cell proliferation, motility, and invasion. Fibulin-5 is induced by transforming growth factor-beta and affects protein kinase cascades. J Biol Chem. 2002;277:27367-27377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 127] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 19. | Wlazlinski A, Engers R, Hoffmann MJ, Hader C, Jung V, Müller M, Schulz WA. Downregulation of several fibulin genes in prostate cancer. Prostate. 2007;67:1770-1780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 87] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 20. | Hwang CF, Shiu LY, Su LJ, Yu-Fang Yin WS, Huang SC, Chiu TJ, Huang CC, Zhen YY, Tsai HT, Fang FM. Oncogenic fibulin-5 promotes nasopharyngeal carcinoma cell metastasis through the FLJ10540/AKT pathway and correlates with poor prognosis. PLoS One. 2013;8:e84218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 21. | Winograd-Katz SE, Fässler R, Geiger B, Legate KR. The integrin adhesome: from genes and proteins to human disease. Nat Rev Mol Cell Biol. 2014;15:273-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 422] [Cited by in RCA: 475] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 22. | García JR, García AJ. Cellular mechanotransduction: sensing rigidity. Nat Mater. 2014;13:539-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Wang J, Su H, Han X, Xu K. Inhibition of fibroblast growth factor receptor signaling impairs metastasis of hepatocellular carcinoma. Tumour Biol. 2014;35:11005-11011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Chen L, Li M, Li Q, Wang CJ, Xie SQ. DKK1 promotes hepatocellular carcinoma cell migration and invasion through β-catenin/MMP7 signaling pathway. Mol Cancer. 2013;12:157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 118] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 25. | Albig AR, Schiemann WP. Fibulin-5 antagonizes vascular endothelial growth factor (VEGF) signaling and angiogenic sprouting by endothelial cells. DNA Cell Biol. 2004;23:367-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 101] [Article Influence: 4.8] [Reference Citation Analysis (0)] |