Published online Sep 28, 2015. doi: 10.3748/wjg.v21.i36.10400
Peer-review started: March 18, 2015
First decision: April 23, 2015
Revised: May 8, 2015
Accepted: July 15, 2015
Article in press: July 15, 2015
Published online: September 28, 2015
Processing time: 194 Days and 12.3 Hours
AIM: To present our extensive experience of hepatectomy for hepatocellular carcinoma using a microwave tissue coagulator to demonstrate the effectiveness of this device.
METHODS: A total of 1118 cases (1990-2013) were reviewed, with an emphasis on intraoperative blood loss, postoperative bile leakage and fluid/abscess formation, and adaptability to anatomical resection and hepatectomy with hilar dissection.
RESULTS: The median intraoperative blood loss was 250 mL; postoperative bile leakage and fluid/abscess formation were seen in 3.0% and 3.3% of cases, respectively. Anatomical resection was performed in 275 cases, including 103 cases of hilar dissection that required application of microwave coagulation near the hepatic hilum. There was no clinically relevant biliary tract stricture or any vascular problems due to heat injury. Regarding the influence of cirrhosis on intraoperative blood loss, no significant difference was seen between cirrhotic and non-cirrhotic patients (P = 0.38), although cirrhotic patients tended to have smaller tumors and underwent less invasive operations.
CONCLUSION: This study demonstrated outcomes of an extensive experience of hepatectomy using heat coagulative necrosis by microwave tissue coagulator.
Core tip: This study represented the perioperative results of 1118 cases of hepatectomy for hepatocellular carcinoma by microwave tissue coagulator in a single institute. Although this study did not include comparative evaluation of two liver parenchyma transection techniques, the precise analysis of more than 1000 cases of hepatectomy over two decades could provide significant information for the readers.
- Citation: Sasaki K, Matsuda M, Hashimoto M, Watanabe G. Liver resection for hepatocellular carcinoma using a microwave tissue coagulator: Experience of 1118 cases. World J Gastroenterol 2015; 21(36): 10400-10408
- URL: https://www.wjgnet.com/1007-9327/full/v21/i36/10400.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i36.10400
Hepatectomy using heat coagulative necrosis (HCN) has been widely adopted, since Curro et al[1] reported promising data on the use of a radiofrequency energy device (Habib 4X, RITA Medical System, Mountain View, United Kingdom) to induce HCN in liver parenchyma along the intended line of liver resection[2,3]. Several reports of hepatectomy using radiofrequency energy-induced HCN confirmed that there was decreased operative blood loss and operative time[4-6]. However, it is not a new method in Japan and Southeast Asia; Tabuse et al[7] published a report on hepatectomy using a microwave tissue coagulator (MTC; Microtaze, Alfresa-pharma, Tokyo, Japan) to induce HCN in 1981. The basic concept and estimated clinical benefits are virtually the same between hepatectomies using radiofrequency- or microwave energy-induced HCN and percutaneous thermal tumor ablation using radiofrequency energy and microwave energy. Hepatectomies using MTC have increased in Western countries because new microwave energy devices for liver resection have been developed, and some of these devices have been approved by certification bodies in Europe and the United States such as CE marking and Center for Devices and Radiological Health[8].
In Japan, there have been several small-scale reports on clinical outcomes of hepatectomy using MTC that showed notable results in reducing intraoperative blood loss compared with traditional methods[9,10]. However, liver resection for hepatocellular carcinoma (HCC) using MTC has not become a gold standard procedure. A possible reason could be that the use of MTC is difficult in hepatectomy cases needing meticulous hilar dissection because of the fear of heat injury to major vasculature and intrahepatic bile ducts. Moreover, the risk of postoperative bile leakage and intra-abdominal fluid/abscess formation - due to insufficient closure of small intrahepatic bile ducts by HCN and the presence of remnants of the coagulated tissue - was thought to be high, although there was no solid evidence of an increased incidence of these complications. These concerns are problems that are common to thermal devices used for hepatectomy.
During the past three decades, we performed hepatectomies using MTC in more than 1000 cases of HCC, which included major and minor hepatectomies. Here, we present our extensive surgical experience, with an emphasis on intraoperative blood loss, adaptability to hepatectomies that needed hepatic hilar dissection, and postoperative complications of postoperative bile leakage and intra-abdominal fluid/abscess formation.
The records of 1175 patients who underwent hepatectomies using HCN induced by MTC from 1990 to 2013 were reviewed. Of these patients, 42 patients who underwent laparoscopic liver resection were excluded, and 15 patients with missing data on preoperative treatment history, operative results, and major histopathologic characteristics such as tumor size and number were also excluded from the study. A total of 1118 patients were included in this retrospective, cohort study and data on their clinicopathological characteristics were collected. The study protocol was approved by the Human Ethics Review Committee of Toranomon Hospital.
The indications for hepatectomy were basically the same as those recommended in the Consensus-Based Clinical Practice Manual of the Japan Society of Hepatology[11]. Patients with hepatitis B infection were defined as those who were seropositive for hepatitis B virus surface antigen, and patients with hepatitis C were defined as those who were seropositive for hepatitis C virus antibody.
Whether a curative hepatectomy was achieved or not was evaluated immediately after the operation. Resection was deemed curative if macroscopic tumor clearance was achieved (R0 and R1). Non-curative cases were those with R2 resection and the following resections: (1) hepatectomy with simultaneous local ablation of HCC that could not be removed surgically; and (2) hepatectomy with portal and/or IVC thrombus. The operative procedures and liver segment were defined according to the Brisbane 2000 system of nomenclature for hepatic anatomy and resections[12]. As an operative procedure, anatomical liver resection (AR) was defined as the resection of HCC together with related portal veins and corresponding territory. For tumors located centrally and/or those close to the major vessels, we performed an AR as appropriate. For tumors located peripherally or those with extrahepatic growths, we preferred limited non-anatomical resection (NAR). In our institute, there was a time period in which fresh frozen plasma (FFP) was routinely administered to patients after hepatectomy for HCC; we therefore divided the analysis of blood transfusion requirement into two time periods (1990-2003 and 2004-2013).
Postoperative complications were basically graded according to the Clavien-Dindo classification, and every case with a grade II or higher complication was recorded as having a postoperative complication[13]. In this study, perioperative blood transfusion was not recorded as a class II complication and was reported separately because of the above-mentioned reason. Postoperative bile leakage was defined as the presence of an intra-abdominal fluid collection that was identified as bile macroscopically. Intra-abdominal fluid/abscess formation was defined as a fluid collection with an inflammatory reaction without obvious bile leakage.
Operative results such as intraoperative blood loss, the need for a blood transfusion, and postoperative complications including death, bile leakage and intra-abdominal fluid/abscess formation in two time periods (1990-2003 vs 2004-2013) were compared, as well as the presence of cirrhosis, and the resection procedure (AR vs NAR). The cumulative recurrence-free and overall survival rates of 749 patients who underwent primary curative hepatectomy without previous treatment were investigated to determine whether the use of MTC influenced long-term prognosis.
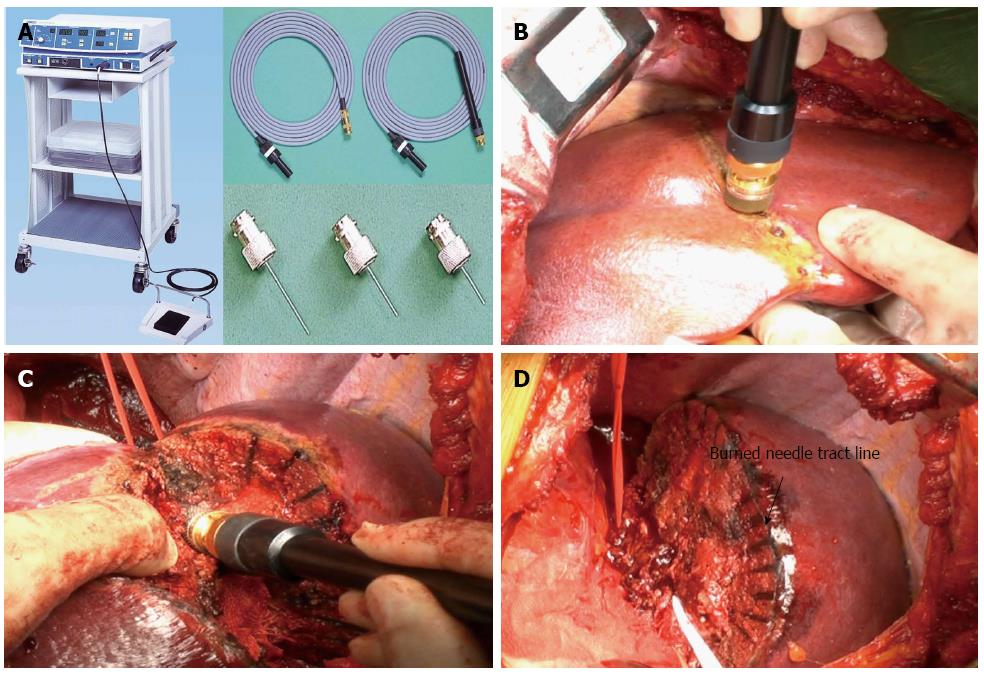
Basic information on the MTC has been reported in detail by Tabuse who is the medical pioneer in the surgical applications of MTC[14]. The MTC system consists of a microwave generator, a handpiece and a reusable needle antenna that can be adjusted in length from 10 mm to 45 mm (Figure 1A). There is an attachment device that changes the antenna angle to 90 degrees. This surgical tool is based on the principle that microwave irradiation of tissue with a frequency of 2450 Mhz (corresponding wavelength of 12 cm) via a monopolar antenna produces heat due to energy produced by the vibration of polar molecules in protein and water. The generation of heat will be limited to the electromagnetic field around the antenna, and the coagulation field is determined by the relationship between antenna length and tissue permittivity. In our experience, an area of liver parenchyma with a radius of approximately 5 mm around the antenna was coagulated in one session. Each coagulation session consisted of 30 to 45 s of coagulation and 5 s of dissociation.
After laparotomy, the liver was mobilized according to the size and site of the lesion. An intraoperative ultrasonography was always performed before liver resection to reveal the presence of previously undetected lesions and the relationship between tumor, major structures such as Glisson’s sheath, and the hepatic vein. The resection line was determined using ultrasonography and marked. Basically, the resection margin width was 10 mm from the tumor edge and it was altered according to the tumor characteristics and remnant liver function. The liver tissue was then coagulated by repeated insertion of the monopolar MTC needle electrode along the intended resection line (Figure 1B). The depth from the liver surface to the major Glisson’s sheath and the hepatic vein was precisely determined by ultrasonography and direct puncture of these structures was avoided. The tip of the antenna is blunt, and the surgeon can easily sense the contact of the tip with Glisson’s sheath or major vasculature, so that the antenna can be pulled out and the insertion angle changed. We inserted the antenna 5 to 10 mm away from major vasculature and bile duct according to the precise vascular map obtained by intraoperative ultrasonography to avoid heat injury to these structures. The coagulated liver parenchyma was divided by forceps and scissors, and additional HCNs were performed when the parenchymal transection reached the non-coagulated parenchyma as appropriate (Figure 1C). The major and approximately 3-5 mm or more size Glisson’s sheath and hepatic vein greater than 5 mm were not coagulated unless the antenna was directly inserted into these structures. When those structures were exposed, they was ligated or sealed by ultrasonically activated scalpel or the vessel sealing system.
The Pringle maneuver was not employed routinely; it was mainly used in major hepatectomies or for controlling bleeding from the transection surface.
Data were analyzed using SPSS software version 21 (IBM SPSS, Chicago, IL, United States). All clinical and pathological features were categorized as either continuous or categorical variables. Continuous variables were summarized as medians and ranges. The Mann-Whitney U test was used to compare continuous variables between the two groups. The χ2 or Fisher’s exact test was used to compare categorical variables as appropriate. Cumulative overall survival and recurrence-free survival were determined by the Kaplan-Meier method. A P value of < 0.05 was considered statistically significant.
The clinicopathological characteristics of the 1118 patients are shown in Table 1. In regards to patient characteristics, 79% were men and the median age at the time of hepatectomy was 63 years old (range 28-83 years). Of the 1118 patients, 976 (87%) underwent a primary hepatectomy, 123 (11%) underwent a second hepatectomy, and 19 (2%) underwent a third or more hepatectomies. The main etiologies of HCC were hepatitis C virus infection (60%), hepatitis B virus infection (25%) or alcohol abuse (3%). Regarding baseline liver function, most patients had Child-Pugh grade A liver function (88%) and 131 patients had grade B (12%). Only two Child-Pugh grade C patients underwent a hepatectomy. Histologically proven liver cirrhosis was seen in 59% of patients, and 74% showed F3 or F4 stage liver fibrosis. With respect to tumor-related characteristics, the median maximum tumor diameter was 22 mm (mean, 28.0 ± 21.0 mm). Eighty-two percent of patient underwent hepatectomy for a solitary tumor and 85% of patients met the Milan criteria (945/1118). A poor histological differentiation grade was seen in 21% of cases and microscopic vascular invasion was seen in 24%.
| Characteristics | n (%) |
| Patient-related factors | |
| Sex (M/F) | 882/236 |
| Age (range) (yr) | 63 (28-87) |
| Etiology | |
| Hepatitis B infection | 294 (26) |
| Hepatitis C infection | 648 (58) |
| Hepatitis B and C infection | 12 (1) |
| Alcohol abuse | 34 (3) |
| Others | 136 (12) |
| Operative situation | |
| Primary curative hepatectomy without previous treatment | 749 (67) |
| Primary curative hepatectomy with previous treatment | 108 (10) |
| Primary non-curative hepatectomy | 119 (11) |
| Second hepatectomy | 123 (11) |
| Three or more hepatectomies | 19 (2) |
| Baseline liver function | |
| Serum platelet count < 105 (μL) | 309 (28) |
| Child-Pugh grade A | 985 (88) |
| Child-Pugh grade B/C | 131/2 (12) |
| Liver cirrhosis1 | 656 (59) |
| Pre-operative AFP value (ng/mL) | |
| < 20 | 612 (55) |
| ≥ 20, < 100 | 244 (22) |
| ≥ 100, < 400 | 116 (10) |
| ≥ 400 | 131 (12) |
| Tumor-related factors | |
| Tumor size (mm) | 22 (2-250) |
| ≤ 2 | 502 (45) |
| < 2, ≤ 5 | 519 (46) |
| > 5 | 97 (9) |
| Solitary tumor | 922 (82) |
| Poorly differentiated | 233 (21) |
| Microscopic vascular invasion | 270 (24) |
| Macroscopic vascular invasion | 25 (2) |
Information on the type of operation and weight of the resected specimen is shown in Table 2. Major hepatectomy was performed in only 4% of cases. One hundred and three cases needed hepatic hilar dissection and AR was performed in 275 patients. Seventy-five percent of operations were NAR, and as a result, 49% of the cases had less than 50 g of tissue resected.
| n (%) | |
| Operative procedure | |
| Major hepatectomy | 42 (4) |
| Right hepatectomy | 15 |
| Left hepatectomy | 20 |
| Right trisectionectomy | 2 |
| Left trisectionectomy | 2 |
| Central bisectionectomy | 3 |
| Minor hepatectomy | |
| Right anterior sectionectomy | 26 |
| Right posterior sectionectomy | 23 |
| Left medial sectionectomy | 12 |
| Left lateral sectionectomy | 47 |
| Bisegmentectomy | 2 |
| Segmentectomy | 123 (11) |
| 2 | 4 |
| 3 | 11 |
| 5 | 22 |
| 6 | 27 |
| 7 | 12 |
| 8 | 29 |
| Sectionectomy or segmentectomy + limited resection | 18 |
| Limited resection | 843 (75) |
| Multiple site | 126 |
| Single site | 717 |
| Weight of the resected specimen (g)1 | |
| < 50 | 542 (49) |
| ≥ 50, < 100 | 243 (22) |
| ≥ 100, < 250 | 195 (18) |
| ≥ 250, < 500 | 87 (8) |
| ≥ 500 | 35 (3) |
The intra- and postoperative results are shown in Table 3. The median operative time was 165 min (range, 40-685 min; mean, 178 ± 76 min), and the median operative blood loss was 250 mL (range, 5-58515 mL; mean, 497 ± 1900 mL). The Pringle maneuver was required in 18% of all cases and 14% of cases with cirrhosis, and the median occlusion time was 13 min (range, 1-80 min). A positive resection margin in the pathological specimen (R1 resection) was seen in 11% of cases. Intra- and postoperative blood transfusions were required in 26% and 32% of cases, respectively. The overall perioperative blood transfusion rate reached 39%. However, when the study period was divided into two time periods, the rate of perioperative transfusion decreased from 66% (1990-2003) to 18% (2004-2013) (Table 4). Postoperative complications other than requirement of blood transfusion occurred in 19% of patients. The overall mortality rate was 0.9% (10/1118). Three patients died of postoperative liver failure, three died of postoperative liver abscess, two died of liver failure that was induced by postoperative pneumonia, one patient died of rupture of varices, and one patient died of intraoperative massive bleeding. The most common complication was surgical wound infection, which occurred in 5% of cases. Postoperative bile leakage occurred in 33 patients (3.0%) and intra-abdominal fluid/abscess formation was seen in 37 patients (3.3%). The complication rate in the group of 749 cases with no previous treatment that underwent a primary curative operation was 20%, while death, bile leakage, and intra-abdominal fluid/abscess formation occurred in 1.1%, 2.8%, and 3.5% of cases, respectively.
| Intraoperative results | |
| Operative time (min) | |
| median (range) | 165 (40-685) |
| mean ± SD | 180 ± 77 |
| Intraoperative blood loss (mL) | |
| median (range) | 255 (5-58515) |
| mean ± SD | 504 ± 1890 |
| Required Pringle maneuver | 200 (18%) |
| Required intraoperative blood transfusion | 300 (26%) |
| Red blood cell transfusion | 88 (9%) |
| Plasma transfusion | 281 (45%) |
| Postoperative results | |
| Required postoperative blood transfusion | 369 (32%) |
| Red blood cell transfusion | 111 (10%) |
| Plasma transfusion | 356 (31%) |
| Complications | |
| Class II1 | 120 |
| Class III | 72 |
| Class IV | 14 |
| Class V | 10 |
| Perioperative blood transfusion | 438 |
| Surgical wound infection | 58 |
| Intra-abdominal fluid collection and/or abscess formation | 37 |
| Uncontrollable ascites | 36 |
| Bile leakage | 33 |
| Pneumonia and/or atelectasis | 23 |
| Postoperative bleeding | 15 |
| Uncontrollable pleural effusion | 14 |
| Time period | P value | Presence of cirrhosis | P value | Operative procedure | P value | ||||
| 1990-2003 | 2004-2013 | LC | Non-LC | AR | NAR | ||||
| n | 511 | 607 | 656 | 462 | 275 | 843 | |||
| Preoperative factors | |||||||||
| Presence of cirrhosis (%) | 64 | 54 | < 0.01 | NA | NA | 38 | 65 | < 0.01 | |
| Tumor > 2 cm (%) | 51 | 58 | 0.02 | 48 | 66 | < 0.01 | 83 | 46 | |
| Intraoperative results | |||||||||
| Operative time (min) | 175 | 155 | < 0.01 | 160 | 173 | < 0.01 | 220 | 153 | < 0.01 |
| Intraoperative blood loss (mL) | 272 | 242 | 0.11 | 250 | 270 | 0.38 | 528 | 203 | < 0.01 |
| Anatomical resection | 23 | 25 | 1.00 | 16 | 37 | < 0.01 | NA | NA | |
| R1 resection (%) | 12 | 11 | 0.78 | 12 | 10 | 0.39 | 9 | 12 | 0.33 |
| Intraoperative BT required (%) | 45 | 10 | < 0.01 | 29 | 21 | < 0.01 | 34 | 5 | < 0.01 |
| Red blood cell transfusion (%) | 8 | 7 | 0.18 | 8 | 7 | 0.73 | 16 | 5 | < 0.01 |
| Plasma transfusion (%) | 44 | 7 | < 0.01 | 27 | 19 | < 0.01 | 31 | 21 | < 0.01 |
| Postoperative results | |||||||||
| Postoperative BT required | 56 | 12 | < 0.01 | 37 | 24 | < 0.01 | 39 | 29 | < 0.01 |
| Red blood cell transfusion | 15 | 4 | < 0.01 | 11 | 7 | 0.04 | 15 | 7 | < 0.01 |
| Plasma transfusion | 55 | 10 | < 0.01 | 36 | 23 | < 0.01 | 38 | 28 | < 0.01 |
| All complications1 | 18 | 14 | 0.08 | 17 | 16 | 0.63 | 20 | 15 | 0.10 |
| Death | 1.4 | 0.5 | 0.20 | 1.4 | 0.2 | 0.053 | 1.4 | 0.7 | 0.27 |
| Bile leakage | 3.7 | 2.3 | 0.21 | 2.3 | 3.9 | 0.72 | 4.4 | 2.5 | 0.15 |
| Intra-abdominal abscess formation | 4.3 | 2.4 | 0.10 | 3.2 | 3.5 | 0.06 | 4.4 | 3.0 | 0.25 |
The results of subgroup analyses that closely examined the characteristics of hepatectomy using MTC are shown in Table 4. Comparison of the two time periods revealed that the proportion of cases requiring intra- and postoperative blood transfusion significantly decreased in the later period except for those requiring intra-operative red blood cell transfusions. Regarding the influence of cirrhosis on intraoperative blood loss, no significant difference was seen between cirrhotic and non-cirrhotic patients, although cirrhotic patients tended to have smaller tumors and underwent less invasive operations. AR was performed in 275 patients (25%) and the median intraoperative blood loss was 528 mL, which was approximately double the amount for NAR. Postoperative bile leakage and intraoperative fluid/abscess formation occurred in 4.4% and 4.4% of cases, respectively. Although this group included 103 cases that underwent hepatic hilum dissection that had HCN near the hepatic hilum (Figure 1D), there was no clinical relevant postoperative biliary tract stricture or vessel aneurysm due to heat injury to Glisson’s sheath or the major hepatic vein.
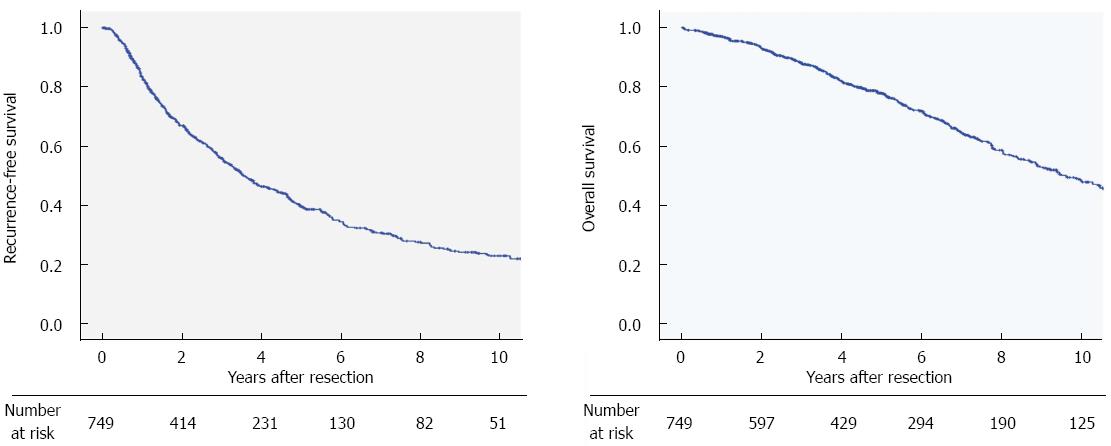
The recurrence-free survival and overall survival curves of the 749 patients who had no previous treatment and underwent primary curative resection are shown in Figure 2. The 1-, 3-, and 5-year recurrence-free survival rates were 84%, 56%, and 40%, respectively. The 3-, 5-, and 10-year overall survival rates were 88%, 78%, and 49%, respectively. The local recurrence rate in primary curative hepatectomy was 3.4% in R0 resection, and 8.4% in R1 resection.
MTC has been used in hepatectomies extensively in Japan and Southeast Asian countries because this device can reduce intraoperative blood loss remarkably without using an inflow control. The excellent operative results without using an inflow control benefit patients with underlying liver dysfunction and those with anemia, low serum platelet count, and coagulopathy.
Although microwave energy has been used in tumor ablation treatment, a microwave energy device for hepatectomy had not been available in Western countries until recently. Recent successful liver resections using radiofrequency energy to induce HCN encouraged the development of microwave energy devices in Western countries, and this development has been noted recently.
The excellent intraoperative blood loss control by MTC during liver parenchyma resection was reported more than 10 years ago. Our results were compatible with those reported in the past and the results of liver resection using HCN induced by radiofrequency energy[9,10]. Although most of our operations were minor liver resections, the median blood loss of 250 mL among more than one thousand cases was an outcome that was better than those in large series of hepatectomies for HCC using other resection techniques[2,10,15-19] (Table 5). Given that most of our patients had underlying liver dysfunction and 59% had histologically proven cirrhosis, our large-scale study clearly showed that MTC is effective in reducing intraoperative blood loss without using inflow control in hepatectomies for HCC. Moreover, a subgroup analysis showed that the intraoperative blood loss in cirrhotic patients was not significantly different from those without liver cirrhosis. The control of intraoperative blood loss without using inflow control, especially in patients with liver damage, is the biggest concern with techniques for liver parenchymal transection because it is closely related to postoperative short- and long-term prognoses of HCC. The effectiveness of MTC in these patients has been reported and our results confirmed its effectiveness[9,10].
| Transection method | n | Study period | Etiology | LC | OT (median/mean) | OBL (median/mean) | MTS | BL/AFC | 5-yr RFS/OS | Authors | Ref. |
| UD | 398 | 1983-1997 | HBV 22 | 52 | - | -/1848 | 5.0 cm | - | 23/34 | Hanazaki | [15] |
| HCV45 | - | ||||||||||
| - | 154 | 1991-1999 | HBV 24 | 65 | - | - | - | 3.2/5.8 | 37/- | Fong et al | [16] |
| HCV 6 | |||||||||||
| MTC | 214 | 1992-2001 | HBV 17 | 47 | 273/284 min | 1010/1628 mL | 3.9 cm | 13%/- | 28/58 | Satoi | [10] |
| HCV 72 | |||||||||||
| CC/UD | 532 | 1994-2002 | - | - | - | -/635 mL | - | 9.0/8.4 | -/- | Imamura et al | [17] |
| - | 168 | 1999-2003 | - | 66 | - | -/322 mL | 5.1 cm | < 1%/< 1% | 47/61 | Shi et al | [18] |
| RFAR | 55 | 2001-2007 | HBV 24 | 100 | -/165 | -/22 | - | 1.8/9.0 | -/- | Curro et al | [2] |
| HCV 38 | |||||||||||
| UD | 359 | 2001-2010 | HBV 34 | - | -/291 | -/1288 | - | 12.8/8.6 | -/- | Sadamori | [19] |
| HCV 45 | |||||||||||
| MTC | 1118 | 1990-2013 | HBV 25 | 59 | 165/178 min | 250/497 mL | 2.2 cm | 3.1/4.4 | 40/78 | Present study | |
| HCV 60 |
Despite the superior control of intraoperative blood loss during liver parenchyma resection, the use of MTC in liver resections has decreased gradually in Japan as its use near the hepatic hilum and/or major vasculature was regarded to be technically difficult because of the potential risk of heat injury to these structures. Moreover, MTC was considered to increase postoperative bile leakage and resection surface abscess due to infection of the remnants of heat-coagulated tissue. However, both concerns have not been fully investigated by examining large patient numbers in the past; this study therefore focused on those complications.
Our study dispelled the misconception about heat injury to major vasculature and the hepatic hilum. This series included 275 cases of AR that needed HCN near the major Glisson’s sheath and major hepatic vein, and there were 103 cases of hepatectomy that needed hepatic hilum dissection. None of them showed relevant clinical postoperative vascular and biliary tract problems due to heat injury. Recently, several studies from other institutes also reported the safety and effectiveness of hepatectomy using HCN in AR including cases that needed hepatic hilar dissection[20,21]. It is true that expert knowledge about intrahepatic anatomy and a certain amount of experience of liver resection are needed if MTC is used near the hepatic hilum, and surgeons have to pay close attention to avoid direct puncture of the first and second branch of Glisson’s sheath or the main branch of the hepatic vein. However, considering that experienced hepatologists can perform percutaneous tumor ablation treatment by using radiofrequency energy and microwave energy near the hepatic hilum or major hepatic vein, it would be easy to understand that experienced surgeons can perform antenna insertion under direct visual and intraoperative ultrasonography guidance without injury to the major Glisson’s sheath or major hepatic vein[22,23]. Moreover, the abundant blood flow of the major vasculature takes away heat energy, known as the “heat sink” effect, which might decrease heat injury to those structures unless they are punctured directly. Ng et al[24] reported that blood inflow occlusion during thermal ablation near the hepatic hilum caused bile duct injury and portal vein thrombosis. We therefore do not recommend the use of HCN near the hepatic hilum during blood inflow occlusion.
Intraoperative ultrasonography technique is critically important in determining the transection plane in AR using MTC. In cases of AR using MTC, we could not expose the hepatic vein every time because our intended resection plane is about 5 mm away from the major hepatic vein[25,26]. Therefore, we could not use the major hepatic vein as a landmark of the transection plane. Both in-depth simulation of the resection plane by ultrasonography and precise chasing of the burnt black needle tract line (Figure 1D) are tips for performing AR using MTC.
Regarding postoperative bile leakage and intra-abdominal fluid/abscess formation, our results did not show a remarkable difference compared with previous large-scale reports. The reported incidence of bile leakage after hepatectomy for liver malignancies without biliary reconstruction in recent large series ranged from less than 1% to 12.8%, although the definitions of postoperative bile leakage and tumor characteristics in each study were not consistent (Table 5). The overall incidence of bile leakage in our study, using our definition, was 3.0% overall and 4.4% in the group of patients who underwent AR. Considering the liver parenchyma transection area, the observed increase in the incidence of postoperative bile leakage in AR was not unexpected, and our results were compatible with those in previous reports (Table 5). Regarding postoperative intra-abdominal fluid/abscess formation, it is understandable that HCN tissue remnants of 5 mm thickness seemed to have caused postoperative infectious complications. Our results were not remarkably different from previous studies that focused on postoperative fluid/abscess formation (Table 5). Although this study did not compare other parenchyma transection devices, our findings examining a large number of patients were compatible with those of previous reports using non-HCN devices with respect to postoperative bile leakage and fluid/abscess formation. Possible reasons for the lower rate of bile leakage and fluid/abscess formation in our study include the higher proportion of non-anatomical limited resections compared with other studies and the retrospective study design. The shorter operative time and smaller operative blood loss might also decrease the risk of postoperative bile leakage because these operative results were reported as significant risk factors for postoperative bile leakage in a previous study[19]. Moreover, meticulous ligation of exposed Glisson’s sheath approximately 3-5 mm in diameter would result in a lower bile leakage rate. Subsequently, the low postoperative bile leakage rate would result in a decrease in the incidence of postoperative fluid/abscess formation because of their relation to bile leakage.
The significant deviation of operative procedure (more minor hepatectomy and less major hepatectomy) in the present study needs to be assessed. There were two clear reasons: one is the unique characteristic of our study population. Most of our patients had underlying liver disease with impaired liver function, and they were therefore closely followed up and received HCC screening examinations. As a result, many of the HCCs that were detected were small and solitary, and the indicated procedure tended to be non-anatomical limited resection. The other reason is our policy of hepatectomy for HCC in patients with multicentric carcinogenic potential. Although some surgeons believe that AR with strict exposure of the hepatic vein should be applied in every case if possible, we believe that minimizing the extent of resection and preserving remnant liver function would benefit overall survival in patients with multicentric carcinogenic potential. Consensus on the treatment strategy for HCC in patients with multicentric carcinogenesis potential has not been reached. The postoperative overall survival rates in the present study were compatible with those in previous large-scale reports, and our results justify our treatment strategy.
With respect to study limitations, the current study was retrospective in its study design with a wide period of recruitment and it analyzed patients in a single center. Moreover, the current study was not designed to compare the postoperative results of MTC with those of other devices, and therefore, the reduction in blood loss and complications was not verified statistically. Comparing the incidence of postoperative bile leakage and fluid/abscess formation in our study with those of other studies might be inaccurate because patient characteristics and definition were not consistent. However, we think that the small blood loss and the low complication rate among more than 1000 hepatectomies are worth reporting because our study is the biggest study on single liver parenchyma transection technique.
Differences between the use of radiofrequency energy and microwave energy to induce HCN in hepatectomy were not examined in this study. A direct comparison study of liver resection using radiofrequency energy and microwave energy does not exist. In contrast, there are reports comparing these two energies to induce coagulation in percutaneous tumor ablation[27,28]. However, whether radiofrequency energy is superior to microwave energy with respect to the size of the coagulated area, local tumor control and safety has not been fully explored in those studies. A direct comparison of the two thermal ablation energies for hepatectomy is needed to determine the superiority of one energy over the other in hepatectomy.
In conclusion, this study demonstrated an extensive experience of hepatectomy using HCN by MTC. Although the current study did not directly compare with other transection techniques, the prevalence of postoperative fluid/abscess formation and bile leakage seemed to be consistent with that seen in other large-scale studies reported in the past.
The authors thank Dr Daisuke Morioka and Dr William Ng, whose comments and suggestions were of inestimable value for our study.
Hepatectomy using heat coagulative necrosis has been widely adopted, hepatectomies using microwave tissue coagulator have increased in Western countries in recent years. In Japan, there have been several small-scale reports on clinical outcomes of hepatectomy using MTC that showed notable results in reducing intraoperative blood loss compared with traditional methods, however, liver resection for hepatocellular carcinoma (HCC) using MTC has not become a gold standard procedure.
During the past three decades, the authors performed hepatectomies using MTC in more than 1000 cases of HCC, which included major and minor hepatectomies, they present their extensive surgical experience, with an emphasis on intraoperative blood loss, adaptability to hepatectomies that needed hepatic hilar dissection, and postoperative complications of postoperative bile leakage and intra-abdominal fluid/abscess formation.
It is a very important study with a large patients population.
P- Reviewer: Rodriguez-Peralvarez M S- Editor: Yu J L- Editor: Logan S E- Editor: Ma S
| 1. | Curro G, Bartolotta M, Barbera A, Jiao L, Habib N, Navarra G. Ultrasound-guided radiofrequency-assisted segmental liver resection: a new technique. Ann Surg. 2009;250:229-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 2. | Curro G, Jiao L, Scisca C, Baccarani U, Mucciardi M, Habib N, Navarra G. Radiofrequency-assisted liver resection in cirrhotic patients with hepatocellular carcinoma. J Surg Oncol. 2008;98:407-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Ayav A, Navarra G, Habib NA, Jiao LR. New technique for liver resection using heat coagulative necrosis. Ann Surg. 2005;242:751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Li M, Zhang W, Li Y, Li P, Li J, Gong J, Chen Y. Radiofrequency-assisted versus clamp-crushing parenchyma transection in cirrhotic patients with hepatocellular carcinoma: a randomized clinical trial. Dig Dis Sci. 2013;58:835-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Pai M, Frampton AE, Mikhail S, Resende V, Kornasiewicz O, Spalding DR, Jiao LR, Habib NA. Radiofrequency assisted liver resection: analysis of 604 consecutive cases. Eur J Surg Oncol. 2012;38:274-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Daylami R, Kargozaran H, Khatri VP. Liver resection using bipolar InLine multichannel radiofrequency device: impact on intra- and peri-operative outcomes. Eur J Surg Oncol. 2012;38:531-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Tabuse K, Katsumi M. Application of a microwave tissue coagulator to hepatic surgery the hemostatic effects on spontaneous rupture of hepatoma and tumor necrosis. Nihon Geka Hokan. 1981;50:571-579. [PubMed] |
| 8. | Christian DJ, Khithani A, Jeyarajah DR. Making liver transection even safer: a novel use of microwave technology. Am Surg. 2011;77:417-421. [PubMed] |
| 9. | Ryu M, Watanabe K, Yamamoto H. Hepatectomy with microwave tissue coagulation for hepatocellular carcinoma. J Hepatobiliary Pancreat Surg. 1998;5:184-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Satoi S, Kamiyama Y, Matsui Y, Kitade H, Kaibori M, Yamamoto H, Yanagimoto H, Takai S, Kwon AH. Clinical outcome of 214 liver resections using microwave tissue coagulation. Hepatogastroenterology. 2005;52:1180-1185. [PubMed] |
| 11. | Kudo M, Izumi N, Kokudo N, Matsui O, Sakamoto M, Nakashima O, Kojiro M, Makuuchi M. Management of hepatocellular carcinoma in Japan: Consensus-Based Clinical Practice Guidelines proposed by the Japan Society of Hepatology (JSH) 2010 updated version. Dig Dis. 2011;29:339-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 663] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 12. | Strasberg SM. Nomenclature of hepatic anatomy and resections: a review of the Brisbane 2000 system. J Hepatobiliary Pancreat Surg. 2005;12:351-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 679] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 13. | Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibañes E, Pekolj J, Slankamenac K, Bassi C. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6210] [Cited by in RCA: 8570] [Article Influence: 535.6] [Reference Citation Analysis (0)] |
| 14. | Tabuse K. Basic knowledge of a microwave tissue coagulator and its clinical applications. J Hepatobiliary Pancreat Surg. 1998;5:165-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 45] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Hanazaki K, Kajikawa S, Shimozawa N, Mihara M, Shimada K, Hiraguri M, Koide N, Adachi W, Amano J. Survival and recurrence after hepatic resection of 386 consecutive patients with hepatocellular carcinoma. J Am Coll Surg. 2000;191:381-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 177] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 16. | Fong Y, Sun RL, Jarnagin W, Blumgart LH. An analysis of 412 cases of hepatocellular carcinoma at a Western center. Ann Surg. 1999;229:790-799; discussion 799-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 614] [Cited by in RCA: 590] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 17. | Imamura H, Seyama Y, Kokudo N, Maema A, Sugawara Y, Sano K, Takayama T, Makuuchi M. One thousand fifty-six hepatectomies without mortality in 8 years. Arch Surg. 2003;138:1198-206; discussion 1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 597] [Cited by in RCA: 614] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 18. | Shi M, Guo RP, Lin XJ, Zhang YQ, Chen MS, Zhang CQ, Lau WY, Li JQ. Partial hepatectomy with wide versus narrow resection margin for solitary hepatocellular carcinoma: a prospective randomized trial. Ann Surg. 2007;245:36-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 402] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 19. | Sadamori H, Yagi T, Shinoura S, Umeda Y, Yoshida R, Satoh D, Nobuoka D, Utsumi M, Fujiwara T. Risk factors for major morbidity after liver resection for hepatocellular carcinoma. Br J Surg. 2013;100:122-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 20. | Imura S, Shimada M, Utsunomiya T, Morine Y, Ikemoto T, Mori H, Hanaoka J, Iwahashi S, Saito Y, Miyake H. Ultrasound-guided microwave coagulation assists anatomical hepatic resection. Surg Today. 2012;42:35-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Tan K, DU X, Yin J, Dong R, Zang L, Yang T, Chen Y. Microwave tissue coagulation technique in anatomical liver resection. Biomed Rep. 2014;2:177-182. [PubMed] |
| 22. | Takayama T, Makuuchi M, Kubota K, Harihara Y, Hui AM, Sano K, Ijichi M, Hasegawa K. Randomized comparison of ultrasonic vs clamp transection of the liver. Arch Surg. 2001;136:922-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 209] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 23. | Torzilli G, Donadon M, Montorsi M, Makuuchi M. Concerns about ultrasound-guided radiofrequency-assisted segmental liver resection. Ann Surg. 2010;251:1191-1192; author reply 1192-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Ng KK, Lam CM, Poon RT, Shek TW, Fan ST, Wong J. Delayed portal vein thrombosis after experimental radiofrequency ablation near the main portal vein. Br J Surg. 2004;91:632-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | Thanos L, Mylona S, Galani P, Pomoni M, Pomoni A, Koskinas I. Overcoming the heat-sink phenomenon: successful radiofrequency thermal ablation of liver tumors in contact with blood vessels. Diagn Interv Radiol. 2008;14:51-56. [PubMed] |
| 26. | Huang S, Yu J, Liang P, Yu X, Cheng Z, Han Z, Li Q. Percutaneous microwave ablation for hepatocellular carcinoma adjacent to large vessels: a long-term follow-up. Eur J Radiol. 2014;83:552-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 27. | Ding J, Jing X, Liu J, Wang Y, Wang F, Wang Y, Du Z. Comparison of two different thermal techniques for the treatment of hepatocellular carcinoma. Eur J Radiol. 2013;82:1379-1384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 96] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 28. | Mertyna P, Goldberg W, Yang W, Goldberg SN. Thermal ablation a comparison of thermal dose required for radiofrequency-, microwave-, and laser-induced coagulation in an ex vivo bovine liver model. Acad Radiol. 2009;16:1539-1548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |