Published online Sep 28, 2015. doi: 10.3748/wjg.v21.i36.10375
Peer-review started: April 9, 2015
First decision: May 18, 2015
Revised: May 26, 2015
Accepted: July 3, 2015
Article in press: July 3, 2015
Published online: September 28, 2015
Processing time: 171 Days and 21.9 Hours
AIM: To investigate the effects of Recql5 deficiency on liver injury induced by lipopolysaccharide/D-galactosamine (LPS/D-Gal).
METHODS: Liver injury was induced in wild type (WT) or Recql5-deficient mice using LPS/D-Gal, and assessed by histological, serum transaminases, and mortality analyses. Hepatocellular apoptosis was quantified by transferase dUTP nick end labeling assay and Western blot analysis of cleaved caspase-3. Liver inflammatory chemokine and cytochrome P450 expression was analyzed by quantitative reverse transcription-PCR. Neutrophil infiltration was evaluated by myeloperoxidase activity. Expression and phosphorylation of ERK, JNK, p65, and H2A.X was determined by Western blot. Oxidative stress was evaluated by measuring malondialdehyde production and nitric oxide synthase, superoxide dismutase, glutathione peroxidase, catalase, and glutathione reductase activity.
RESULTS: Following LPS/D-Gal exposure, Recql5-deficient mice exhibited enhanced liver injury, as evidenced by more severe hepatic hemorrhage, higher serum aspartate transaminase and alanine transaminase levels, and lower survival rate. As compared to WT mice, Recql5-deficient mice showed an increased number of apoptotic hepatocytes and higher cleaved caspase-3 levels. Recql5-deficient mice exhibited increased DNA damage, as evidenced by increased γ-H2A.X levels. Inflammatory cytokine levels, neutrophil infiltration, and ERK phosphorylation were also significantly increased in the knockout mice. Additionally, Recql5-deficicent mice exhibited increased malondialdehyde production and elevated inducible nitric oxide synthase, superoxide dismutase, glutathione peroxidase, catalase, and glutathione reductase activity, indicative of enhanced oxidative stress. Moreover, CYP450 expression was significantly downregulated in Recql5-deficient mice after LPS/D-Gal treatment.
CONCLUSION: Recql5 protects the liver against LPS/D-Gal-induced injury through suppression of hepatocyte apoptosis and oxidative stress and modulation of CYP450 expression.
Core tip: Wild type and Recql5-deficient mice were intraperitoneally injected with lipopolysaccharide and D-galactosamine (LPS/D-Gal). The aim of the study was to explore the effects of Recql5 deficiency on LPS/D-Gal-induced liver injury. Our findings reveal that Recql5 protects against liver injury via inhibition of hepatocyte apoptosis and oxidative stress and regulation of hepatic CYP450 expression levels.
- Citation: Liao WQ, Qi YL, Wang L, Dong XM, Xu T, Ding CD, Liu R, Liang WC, Lu LT, Li H, Li WF, Luo GB, Lu XC. Recql5 protects against lipopolysaccharide/D-galactosamine-induced liver injury in mice. World J Gastroenterol 2015; 21(36): 10375-10384
- URL: https://www.wjgnet.com/1007-9327/full/v21/i36/10375.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i36.10375
The RecQ family is a highly conserved group of DNA helicases that play a critical role in DNA replication, recombination, transcription, and repair[1]. Mammals express five RecQ homologues: RECQL1, BLM, WRN, RECQL4, and RECQL5[2], which share a conserved helicase domain, but differ in their C- and/or N-terminal domains[3]. Mutations in BLM, WRN, and RECQL4 are linked to the human genetic disorders Bloom’s syndrome (BS), Werner’s syndrome (WS), and Rothmund-Thomson’s syndrome (RTS), respectively. These disorders are characterized by increased genomic instability and cancer susceptibility[2]. RECQL5 has not been directly linked to human genetic disease, but has been implicated in DNA double strand break (DSB) repair and DNA transcription[4-10].
Several lines of evidence suggest that RECQ helicases also play a role in hepatic cell proliferation and metabolism. For example, RECQL1 expression is significantly correlated with histological grade and MIB-1 indices of hepatocellular carcinoma (HCC) development. Silencing RECQL1 expression suppresses HCC cell proliferation both in vitro and in vivo[11]. BLM-deficient cells from patients with BS show slower growth and increased irradiation-mediated apoptosis. Deletion of BLM in mice leads to a reduced number of fetal liver cells and increased cell death[12]. In addition, a recent report has shown that Wrn-mutant mice exhibit accelerated typical age-related liver changes, including pseudocapillarization that directly affects hepatic metabolism[13]. Moreover, WRN regulates the transcription of hepatic cytochrome P450 2B (CYP2B) genes, which are involved in the metabolism of various active substances[14], suggesting that WRN may function in liver metabolism. Hepatocyte cell death and impaired hepatic metabolism are associated with many liver diseases, including chronic and acute liver injury and liver cancer[15-17]. Previously, we reported that Recql5 deficiency in mice resulted in increased susceptibility to cancers, including liver cancer[4,6]; however, it remains unknown whether Recql5 also has a role in liver injury.
Lipopolysaccharide/D-galactosamine (LPS/D-Gal) treated mice are a known model of acute liver injury. D-Gal is an amino sugar that blocks RNA synthesis and greatly increases the sensitivity of mice to LPS-induced hepatotoxicity[18,19]. Using this model, we examined the function of Recql5 in liver injury. We demonstrated that Recql5 protects against LPS/D-Gal-induced liver injury and found that the enhanced liver injury in Recql5-deficient mice occurs due to increased hepatic apoptosis, elevated oxidative stress, and downregulation of CYP450 expression.
Male, 6-8-wk-old Recql5-deficient and wild type (WT) C57BL/6 mice were used in this study. The Recql5-deficient mice used in this study have been characterized previously[4-7]. Mice were fed a commercial diet and maintained in a controlled environment at 20-25 °C and 50% ± 5% relative humidity with a 12:12 h dark-light cycle. All animal studies were approved by the Wenzhou Medical University Institutional Animal Care and Use Committee.
LPS (E. coli, 0111:B4) and D-Gal were purchased from Sigma (St Louis, MO, United States). Caspase-3 (rabbit polyclonal, 1:1000), ERK (rabbit polyclonal, 1:2000), phospho-ERK (rabbit polyclonal, 1:2000), JNK (rabbit polyclonal, 1:1000), phospho-JNK (mouse monoclonal, 1:2000), phospho-p65 (mouse monoclonal, 1:1000), phospho-H2A.X (γ-H2A.X, rabbit polyclonal, 1:1000), β-actin (rabbit polyclonal, 1:2000), and GAPDH (rabbit polyclonal, 1:3000) antibodies were obtained from Cell Signaling Technology (Waltham, MA, United States). Peroxidase-conjugated secondary antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA, United States).
Liver injury was induced in 6-8-wk-old male mice via an intraperitoneal injection of LPS/D-Gal. For mortality assay, mice were intraperitoneally injected with 20 μg/kg LPS and 400 mg/kg D-Gal and mortality was recorded for 72 h. To induce acute liver injury, mice were intraperitoneally injected with 10 μg/kg LPS and 300 mg/kg D-Gal. Mice were scarified at 1 and 6 h after LPS/D-Gal administration. Blood and liver samples were collected for further experiments.
The serum activity of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) was measured with a commercial assay kit (Nanjing Jiancheng Biological Technology, Inc., Nanjing, China). The enzyme activity was expressed as international units per liter (U/L). Serum tumor necrosis factor (TNF)-α and interleukin (IL)-6 levels were measured using commercial assay kits (Nanjing Jiancheng Biological Technology).
Formalin-fixed specimens were embedded in paraffin and stained with hematoxylin-eosin for conventional morphological evaluation under a light microscope.
Myeloperoxidase (MPO) activity was determined using an MPO detection kit according to the manufacturer’s instructions (Nanjing Jiancheng Biological Technology, Inc.).
Lipid peroxidation was determined by measuring malondialdehyde (MDA) levels using an assay kit (Beyotime Institute of Biotechnology, Inc., Shanghai, China). The activity of inducible nitric oxide synthase (iNOS), superoxide dismutase (SOD), glutathione peroxidase (GPX), catalase (CAT), and glutathione reductase (GR) was tested using commercial assay kits (Nanjing Jiancheng Biological Technology, Inc.).
Hepatocellular apoptosis was evaluated by transferase dUTP nick end labeling (TUNEL) assay using the DeadEndTM Colorimetric TUNEL System (Promega, Madison, WI, United States). The terminal transferase reactions produced a dark-brown precipitate. For each mouse liver section, the number of TUNEL-positive cells in five randomly selected fields was counted.
Total RNA was isolated from liver tissue using TRIZOL reagent (Invitrogen) and was treated with DNase to remove contaminating DNA before cDNA synthesis. RNA (2 μg) was reverse-transcribed to cDNA with murine leukemia virus (MLV)-reverse transcriptase (Invitrogen). Each cDNA sample was analyzed in triplicate on an ABI 7300 Real-Time Detection system (Applied Biosystems, Foster City, CA, United States) using SYBR Green (Tiangen, Beijing, China). An endogenous housekeeping gene (GAPDH) was used as an internal standard. The primer sequences are shown in Table 1. The primer concentration used in the PCR assay was 0.5 μmol/L. Cycle conditions were as follows: 95 °C for 2 min followed by 40 cycles of 95 °C for 15 s, 60 °C for 30 s, and 68 °C for 30 s. Relative mRNA quantification was calculated using the comparative threshold cycle (Ct) method. ∆∆Ct was converted to a fold change of expression using the formula 2-∆∆Ct.
| Gene | Forward primer (5'→3') | Reverse primer (5'→3') | Length of product |
| TNF-α | GAACTGGCAGAAGAGGCACT | AGGGTCTGGGCCATAGAACT | 203 bp |
| IL-6 | GAGGATACCACTCCCAACAGACC | AAGTGCATCATCGTTGTTCATAC | 141 bp |
| Cyp2A4 | CGGAAGACGAACGGTGCTTTC | GAGGCTTCCCAGCATCATTCTAAGA | 123 bp |
| Cyp2A5 | TCGGAAGACGAACGGTGCTTTT | GCTTCCCAGCATCATTCGAAGC | 124 bp |
| Cyp2B9 | TGAAGCTTTTCTGCCCTTCT | GTGTGAGCAGCTACCAATG | 147 bp |
| Cyp2B10 | GACTTTGGGATGGGAAAGAG | CCAAACACAATGGAGCAGAT | 68 bp |
| GAPDH | ACGGATTTGGTCGTATTGGGC | CTCGCTCCTGGAAGATGGTGAT | 216 bp |
Liver tissue was lysed in lysis buffer [50 mmol/L Tris-HCl pH 7.6, 150 mmol/L NaCl, 1% NP-40 and 0.1% (w/v) SDS] supplemented with a protease/phosphatase inhibitor cocktail (Cell Signaling Technology). After sonication, the lysate was centrifuged at 12000 rpm for 15 min at 4 °C, and the supernatant was collected. Proteins were separated using SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride (PVDF) membranes (Bio-Rad, Hercules, CA, United States). After blocking with 5% (w/v) milk in TTBS (TBS plus 0.1% Tween-20), the membranes were incubated with primary antibodies followed by horseradish peroxidase (HRP)-conjugated secondary antibodies. Protein bands were visualized with the Immun-Star HRP chemiluminescence kit (Bio-Rad). For densitometric analysis, Image J software was used.
Statistical comparisons were performed using Student’s t-test or analysis of variance (ANOVA) where appropriate. Data are expressed as the mean ± SD. Kaplan-Meier survival analysis was performed using the log-rank test. P-values less than 0.05 were considered significant.
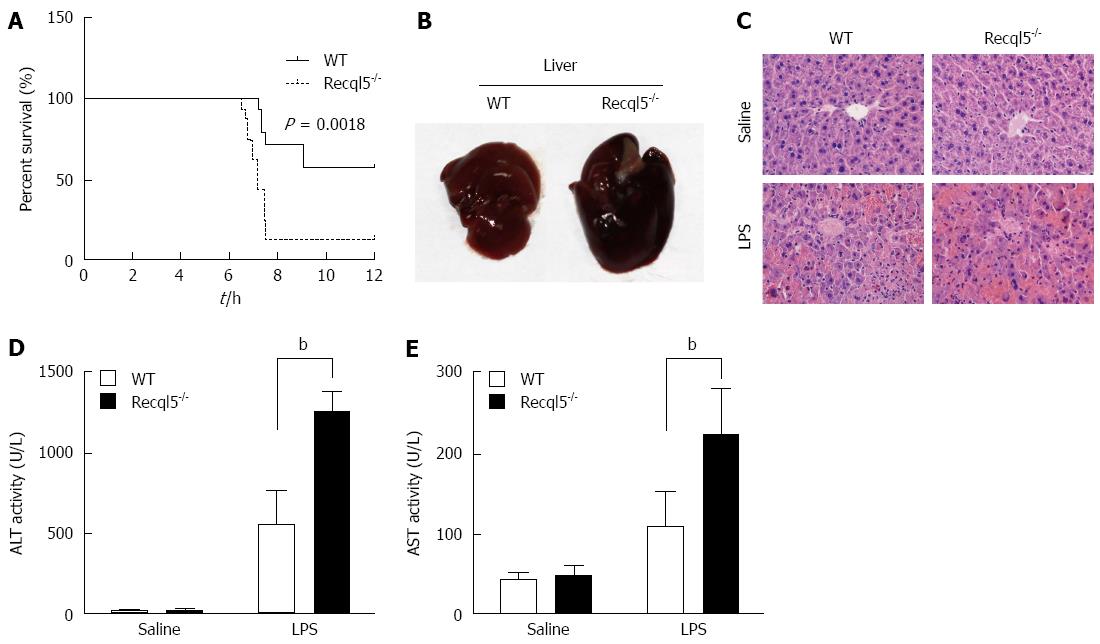
To investigate the role of Recql5 in liver injury, we first examined the effect of Recql5 deficiency on mouse mortality after LPS shock. Following injection of 20 μg/kg LPS and 400 mg/kg D-Gal, the mortality was significantly increased in Recql5-deficient mice as compared to WT mice (Figure 1A). Lethal shock in D-Gal/LPS-treated mice is characterized by acute liver injury[20]. To further elucidate the direct effects of Recql5 on liver injury, we used a low-dose LPS/D-Gal model (10 μg/kg LPS and 300 mg/kg D-Gal). Liver morphology analysis[21] showed that the liver of Recql5-deficient mice was swollen and exhibited more bleeding on the surface, as compared to the liver of WT mice, indicating that there was more severe liver hemorrhage in knockout mice after treatment (Figure 1B). These data were further confirmed by HE staining (Figure 1C). Moreover, the serum ALT and AST levels, two well-established biochemical markers of hepatocellular damage, were significantly increased in Recql5-deficient mice 6 h after injection (Figure 1D and E). Together, these results indicate that Recql5 has a protective role in liver injury induced by LPS/D-Gal.
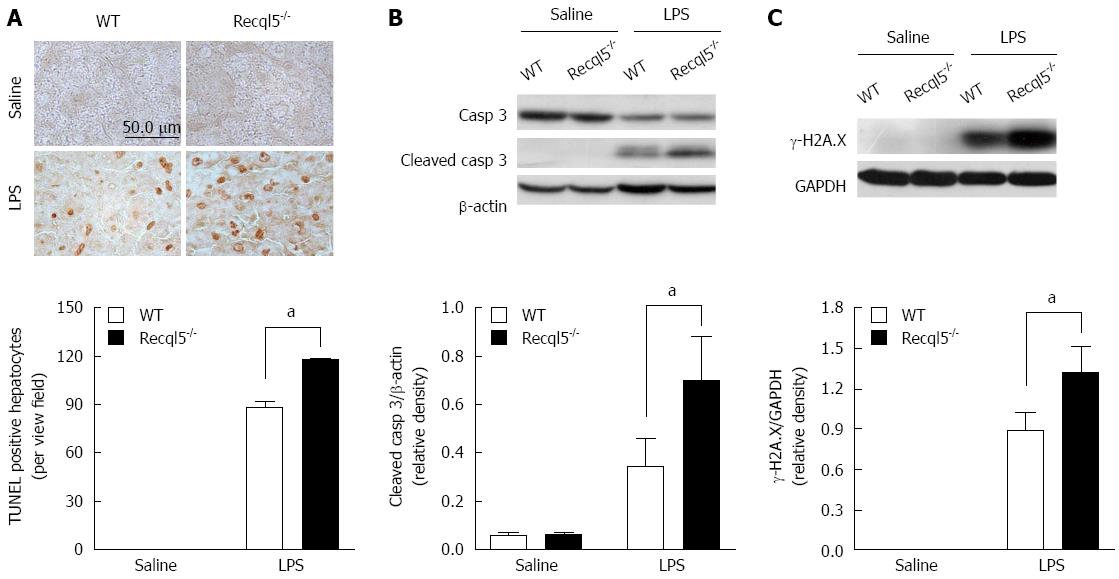
Hepatocyte apoptosis is considered a main cause of liver injury in the LPS/D-Gal model[22]. Thus we evaluated whether Recql5 deficiency affected hepatocyte apoptosis. The TUNEL assay showed that the number of apoptotic hepatocytes was significantly increased in Recql5-deicient mice, as compared to WT mice after LPS/D-Gal administration (Figure 2A). In agreement with this observation, Western blot analysis showed that the cleaved caspase-3 levels were significantly elevated in Recql5-deicient mice (Figure 2B). Given the important role of Recql5 in genomic stability, we assumed that LPS/D-Gal treatment would result in elevated DNA damage in Recql5-deficient mice, which, in turn, would trigger apoptosis. Indeed, Western blot showed that the level of γ-H2A.X, a biomarker of DNA damage, was significantly increased in Recql5-deficient mice (Figure 2C). Together, these results suggest that Recql5 deficiency results in increased LPS/D-Gal-induced DNA damage and hepatocyte apoptosis, thereby inducing liver injury.
The release of pro-inflammatory cytokines is involved in liver injury stimulated by LPS/D-Gal[18,22]. Among these, cytokines TNF-α and IL-6 are key mediators of hepatocyte apoptosis. To examine whether Recql5 deficiency could alter TNF-α and IL-6 expression, we measured their hepatic mRNA levels. As compared with WT mice, the mRNA levels of TNF-α and IL-6 were significantly elevated in Recql5-deficient mice at 1 and 6 h, respectively. Similar results were found for serum TNF-α and IL-6 levels (Figure 3C and D). Consistent with these data, the neutrophil infiltration, which can be triggered by TNF-α signaling[21], was significantly increased in knockout mice, as evaluated by MPO activity (Figure 3E). LPS/D-Gal-induced secretion of inflammatory cytokines is primarily dependent on the activation of the mitogen activated protein kinase (MAPK) and nuclear factor (NF)-κB pathways[18,23]. We then tested whether deletion of Recql5 affected these pathways. Our results showed that the phosphorylation of extracellular signal-related kinase (ERK) was significantly increased in Recql5-deficient mice, whereas there was no significant difference in c-Jun N-terminal kinase (JNK) and p65 phosphorylation between Recql5-deficient mice and control mice (Figure 3F), suggesting that Recql5 deficiency results in ERK activation.
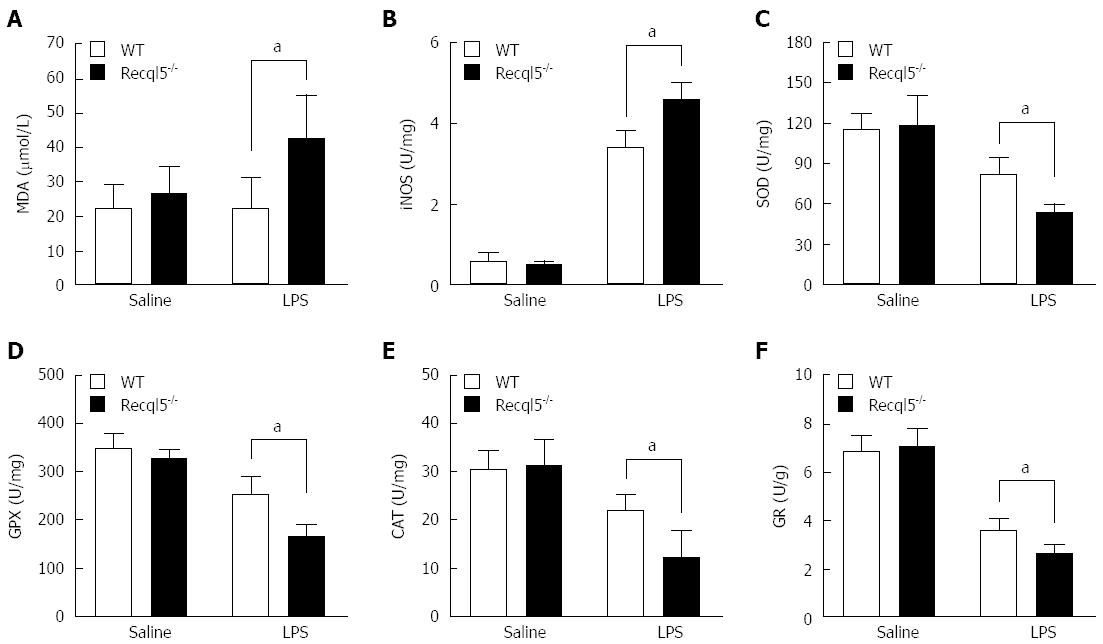
Oxidative stress is a known contributor to LPS/D-Gal-induced liver injury[24]. To investigate the effects of Recql5 deficiency on oxidative stress, we measured several parameters involved in this process, including MDA production and iNOS, SOD, GPX, CAT and GR activity. Our data showed that the levels of MDA, an end product of lipid peroxidation, were significantly increased in Recql5-deficient mice, as compared to WT mice (Figure 4A). Furthermore, there was a significant increase in iNOS activity in Recql5-deficient mice, indicative of enhanced NO production (Figure 4B). Additionally, SOD, GPX, CAT, and GR activity in Recql5-deficient mice was significantly reduced (Figure 4C-F). These results suggest that Recql5 deficiency leads to increased LPS/D-Gal-mediated oxidative stress.
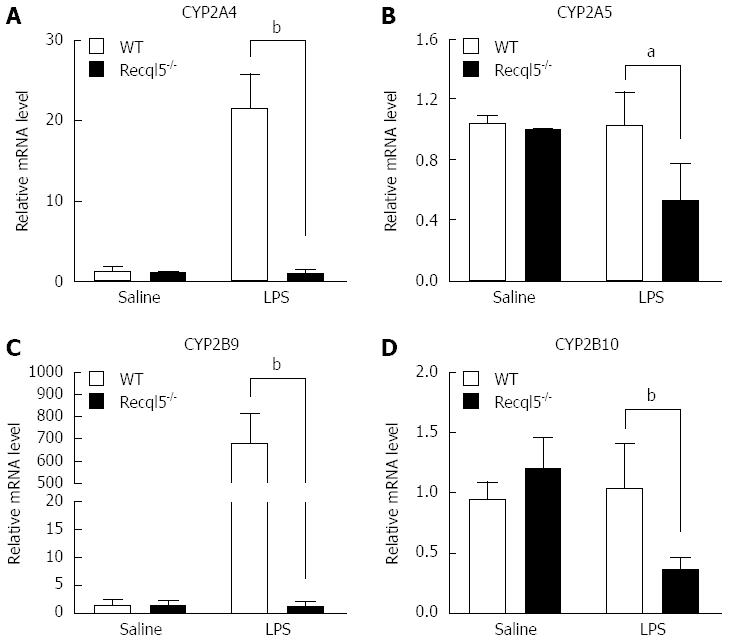
CYP450s are important for the metabolism of a variety of endogenous and exogenous substrates[25,26]. It has been reported that reduced CYP450 gene expression is associated with enhanced liver injury[27]. Therefore, we examined whether Recql5 deficiency could alter CYP450 expression. The mRNA levels of four CYP450 members were detected, including CYP2A4, CYP2A5, CYP2B9 and CYP2B10, which are regulated by LPS as reported previously[28-30]. Our results showed that, following LPS/D-Gal exposure, mRNA levels of CYP2A4, CYP2A5, CYP2B9, and CYP2B10 were significantly reduced in Recql5-deficient mice, as compared to WT mice (Figure 5). These data indicate that Recql5 deficiency results in the downregulation of CYP450 expression, which may impair LPS and/or D-Gal disposition.
In the present study, we demonstrated that Recql5 has a protective role against LPS/D-Gal-induced liver injury, as Recql5-deficient mice exhibited increased hepatic hemorrhage, elevated serum aminotransferase levels, and decreased survival rate. LPS/D-Gal-induced liver injury is a well-established experimental animal model of acute hepatic failure. The outcomes of this model are associated with increased hepatocyte apoptosis, inflammation, and oxidation[18,31,32]. First, we speculated that Recql5 deficiency might increase hepatocyte apoptosis, which could lead to enhanced liver damage. Indeed, TUNEL assays and Western blot confirmed an increase in hepatocyte apoptosis in Recql5-deficient mice. Moreover, consistent with the role of Recql5 in genomic stability, we found that Recql5-deficient liver exhibited increased DNA damage, which has been recognized as an inducer of apoptosis[33]. Our data suggest that Recql5 is a regulator of cell apoptosis. In agreement with these findings, a previous study suggested that BLM also regulates cell apoptosis, and that BLM deficiency results in increased cell death and apoptosis, which is associated with p53 dysfunction[12].
TNF-α and IL-6 are two proximal mediators of hepatotoxicity in several models of liver damage, including LPS/D-Gal[34,35]. TNF-α-induced hepatocyte apoptosis may be an early causal event during LPS/D-Gal-induced liver injury[32]. We found that LPS/D-Gal upregulated TNF-α and IL-6 in Recql5-deficient mice. Moreover, it has been reported that LPS activates various signaling pathways, including MAPK and NF-κB, thereby inducing the production of inflammatory cytokines[18]. In line with these data, we observed an upregulation in ERK phosphorylation in knockout mice following LPS/D-Gal challenge. Together, our data suggest that, in the LPS/D-Gal model, Recql5 deficiency activates ERK signaling, resulting in inflammatory cytokine production and subsequently, hepatocyte apoptosis and damage.
Oxidative stress is associated with damage to a wide range of molecules, including lipids, proteins, and nucleic acids, and may play a crucial role in LPS/D-Gal-stimulated liver injury. For example, treatment with antioxidants significantly reduces LPS/D-Gal-induced liver injury in mice, whereas inhibition of antioxidant enzyme activity enhances liver damage[24,36]. Oxidative stress can be triggered by increased free radical production and/or decreased antioxidant activity[37]. We found that MDA and NO production, indicative of free radicals, were significantly increased in Recql5-deficient mice. In contrast, the activity of the antioxidant enzymes SOD, GPX, CAT and GR was significantly reduced in mice deficient in Recql5. These data suggest that Recql5 deficiency results in an imbalance between free radical generation and antioxidant defenses, thereby enhancing oxidative stress-induced liver injury.
CYP450 oxidases are the predominant enzymes involved in Phase I detoxification. Downregulation of CYP450 increases the risk of liver damage after hepatoxin exposure[27,38]. We found that Recql5 deficiency resulted in reduced expression of CYP2A4, CYP2A5, CYP2B9, and CYP2B10, indicative of impaired LPS and/or D-Gal disposition, which might further aggravate liver injury. The mechanism by which Recql5 regulates CYP450 expression remains unknown. It has been shown that WRN regulates CYP2B transcription by forming complex with the Wrn binding site within the CYP2B promoter[14]. Further investigations are required to figure out whether Recql5 regulates CYP450 expression in the same manner as WRN.
In summary, the current study showed for the first time that Recql5 protects against liver injury induced by LPS/D-Gal. The protective effect of Recql5 is attributed to the inhibition of hepatocyte apoptosis and oxidative stress, as well as the regulation of CYP450 expression. Our findings indicate a hepatoprotective role for Recql5 in liver injury.
RecQ helicases are regulators of genomic integrity and play important roles in hepatic cell proliferation and metabolism. Deficiency in one of the RecQ family member, Recql5, results in elevated susceptibility to various types of cancers, including liver cancer; however, the possible function of Recql5 in liver injury remains unknown.
Recql5 is a member of the RecQ helicase family that plays critical roles in DNA replication, recombination, transcription, and repair. This study analyzes the possible functions of Recql5 in liver injury.
The study showed for the first time that Recql5 has a protective role against liver injury induced by LPS/D-Gal. The protection is mediated via the suppression of apoptosis, DNA damage, oxidative stress and CYP450 expression.
This study will help to improve the readers’ understanding of the mechanisms involved in acute liver injury and may represent a novel target for the acute liver failure therapy.
Oxidative stress is a term used to describe the condition of oxidative damage resulting when the critical balance between free radical generation and antioxidant defenses is unfavorable.
This is an interesting study investigating the role of Recql5, a member of the RecQ helicase family, in liver injury. The authors found that Recql5 protects against LPS/D-Gal-induced liver injury. They further showed that this effect is attributed to inhibition of hepatocyte apoptosis and oxidative stress, as well as to downregulation of CYP450.
P- Reviewer: Conti B, Pajares MA S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Wang CH
| 1. | Croteau DL, Popuri V, Opresko PL, Bohr VA. Human RecQ helicases in DNA repair, recombination, and replication. Annu Rev Biochem. 2014;83:519-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 428] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 2. | Chu WK, Hickson ID. RecQ helicases: multifunctional genome caretakers. Nat Rev Cancer. 2009;9:644-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 360] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 3. | Popuri V, Tadokoro T, Croteau DL, Bohr VA. Human RECQL5: guarding the crossroads of DNA replication and transcription and providing backup capability. Crit Rev Biochem Mol Biol. 2013;48:289-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Hu Y, Lu X, Luo G. Effect of Recql5 deficiency on the intestinal tumor susceptibility of Apc(min) mice. World J Gastroenterol. 2010;16:1482-1486. [PubMed] |
| 5. | Hu Y, Lu X, Zhou G, Barnes EL, Luo G. Recql5 plays an important role in DNA replication and cell survival after camptothecin treatment. Mol Biol Cell. 2009;20:114-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Hu Y, Raynard S, Sehorn MG, Lu X, Bussen W, Zheng L, Stark JM, Barnes EL, Chi P, Janscak P. RECQL5/Recql5 helicase regulates homologous recombination and suppresses tumor formation via disruption of Rad51 presynaptic filaments. Genes Dev. 2007;21:3073-3084. [PubMed] |
| 7. | Hu Y, Lu X, Barnes E, Yan M, Lou H, Luo G. Recql5 and Blm RecQ DNA helicases have nonredundant roles in suppressing crossovers. Mol Cell Biol. 2005;25:3431-3442. [PubMed] |
| 8. | Ramamoorthy M, May A, Tadokoro T, Popuri V, Seidman MM, Croteau DL, Bohr VA. The RecQ helicase RECQL5 participates in psoralen-induced interstrand cross-link repair. Carcinogenesis. 2013;34:2218-2230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Paliwal S, Kanagaraj R, Sturzenegger A, Burdova K, Janscak P. Human RECQ5 helicase promotes repair of DNA double-strand breaks by synthesis-dependent strand annealing. Nucleic Acids Res. 2014;42:2380-2390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 10. | Saponaro M, Kantidakis T, Mitter R, Kelly GP, Heron M, Williams H, Söding J, Stewart A, Svejstrup JQ. RECQL5 controls transcript elongation and suppresses genome instability associated with transcription stress. Cell. 2014;157:1037-1049. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 162] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 11. | Futami K, Ogasawara S, Goto H, Yano H, Furuichi Y. RecQL1 DNA repair helicase: A potential tumor marker and therapeutic target against hepatocellular carcinoma. Int J Mol Med. 2010;25:537-545. [PubMed] |
| 12. | Kaneko H, Fukao T, Kasahara K, Yamada T, Kondo N. Augmented cell death with Bloom syndrome helicase deficiency. Mol Med Rep. 2011;4:607-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Cogger VC, Svistounov D, Warren A, Zykova S, Melvin RG, Solon-Biet SM, O’Reilly JN, McMahon AC, Ballard JW, De Cabo R. Liver aging and pseudocapillarization in a Werner syndrome mouse model. J Gerontol A Biol Sci Med Sci. 2014;69:1076-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Lachaud AA, Auclair-Vincent S, Massip L, Audet-Walsh E, Lebel M, Anderson A. Werner’s syndrome helicase participates in transcription of phenobarbital-inducible CYP2B genes in rat and mouse liver. Biochem Pharmacol. 2010;79:463-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Lehmann K, Tschuor C, Rickenbacher A, Jang JH, Oberkofler CE, Tschopp O, Schultze SM, Raptis DA, Weber A, Graf R. Liver failure after extended hepatectomy in mice is mediated by a p21-dependent barrier to liver regeneration. Gastroenterology. 2012;143:1609-1619.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 100] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 16. | Waidmann O, Köberle V, Bettinger D, Trojan J, Zeuzem S, Schultheiß M, Kronenberger B, Piiper A. Diagnostic and prognostic significance of cell death and macrophage activation markers in patients with hepatocellular carcinoma. J Hepatol. 2013;59:769-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 17. | Degli Esposti D, Hamelin J, Bosselut N, Saffroy R, Sebagh M, Pommier A, Martel C, Lemoine A. Mitochondrial roles and cytoprotection in chronic liver injury. Biochem Res Int. 2012;2012:387626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 116] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 18. | Wang X, Zhang L, Wei Z, Zhang X, Gao Q, Ma Y, Liu X, Jiang Y, Liu X, Guo C. The inhibitory action of PDCD4 in lipopolysaccharide/D-galactosamine-induced acute liver injury. Lab Invest. 2013;93:291-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Shang Y, Liu Y, Du L, Wang Y, Cheng X, Xiao W, Wang X, Jin H, Yang X, Liu S. Targeted expression of uncoupling protein 2 to mouse liver increases the susceptibility to lipopolysaccharide/galactosamine-induced acute liver injury. Hepatology. 2009;50:1204-1216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Lehmann V, Freudenberg MA, Galanos C. Lethal toxicity of lipopolysaccharide and tumor necrosis factor in normal and D-galactosamine-treated mice. J Exp Med. 1987;165:657-663. [PubMed] |
| 21. | González-Terán B, Cortés JR, Manieri E, Matesanz N, Verdugo Á, Rodríguez ME, González-Rodríguez Á, Valverde ÁM, Martín P, Davis RJ. Eukaryotic elongation factor 2 controls TNF-α translation in LPS-induced hepatitis. J Clin Invest. 2013;123:164-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 22. | Jing Y, Ai Q, Lin L, Dai J, Jia M, Zhou D, Che Q, Wan J, Jiang R, Zhang L. Protective effects of garcinol in mice with lipopolysaccharide/D-galactosamine-induced apoptotic liver injury. Int Immunopharmacol. 2014;19:373-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Chen L, Ren F, Zhang H, Wen T, Piao Z, Zhou L, Zheng S, Zhang J, Chen Y, Han Y. Inhibition of glycogen synthase kinase 3β ameliorates D-GalN/LPS-induced liver injury by reducing endoplasmic reticulum stress-triggered apoptosis. PLoS One. 2012;7:e45202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 24. | Lu J, Chen YP, Wan R, Guo CY, Wang XP. Protective effects of ulinastatin on acute liver failure induced by lipopolysaccharide/D-galactosamine. Dig Dis Sci. 2012;57:399-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Ingelman-Sundberg M. Human drug metabolising cytochrome P450 enzymes: properties and polymorphisms. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:89-104. [PubMed] |
| 26. | Korzekwa K. Enzyme kinetics of oxidative metabolism: cytochromes P450. Methods Mol Biol. 2014;1113:149-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Xie Y, Hao H, Wang H, Guo C, Kang A, Wang G. Reversing effects of lignans on CCl4-induced hepatic CYP450 down regulation by attenuating oxidative stress. J Ethnopharmacol. 2014;155:213-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 28. | Shah P, Guo T, Moore DD, Ghose R. Role of constitutive androstane receptor in Toll-like receptor-mediated regulation of gene expression of hepatic drug-metabolizing enzymes and transporters. Drug Metab Dispos. 2014;42:172-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 29. | Li-Masters T, Morgan ET. Effects of bacterial lipopolysaccharide on phenobarbital-induced CYP2B expression in mice. Drug Metab Dispos. 2001;29:252-257. [PubMed] |
| 30. | De-Oliveira AC, Poça KS, Totino PR, Paumgartten FJ. Modulation of cytochrome P450 2A5 activity by lipopolysaccharide: low-dose effects and non-monotonic dose-response relationship. PLoS One. 2015;10:e0117842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 31. | Weber MA, Schnyder-Candrian S, Schnyder B, Quesniaux V, Poli V, Stewart CL, Ryffel B. Endogenous leukemia inhibitory factor attenuates endotoxin response. Lab Invest. 2005;85:276-284. [PubMed] |
| 32. | Kuhla A, Eipel C, Abshagen K, Siebert N, Menger MD, Vollmar B. Role of the perforin/granzyme cell death pathway in D-Gal/LPS-induced inflammatory liver injury. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1069-G1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 33. | Roos WP, Kaina B. DNA damage-induced cell death by apoptosis. Trends Mol Med. 2006;12:440-450. [PubMed] |
| 34. | Liu J, Wu KC, Lu YF, Ekuase E, Klaassen CD. Nrf2 protection against liver injury produced by various hepatotoxicants. Oxid Med Cell Longev. 2013;2013:305861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 112] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 35. | Vandendriessche B, Goethals A, Simats A, Van Hamme E, Brouckaert P, Cauwels A. MAPK-activated protein kinase 2-deficiency causes hyperacute tumor necrosis factor-induced inflammatory shock. BMC Physiol. 2014;14:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 36. | Jia M, Jing Y, Ai Q, Jiang R, Wan J, Lin L, Zhou D, Che Q, Li L, Tang L. Potential role of catalase in mice with lipopolysaccharide/D-galactosamine-induced fulminant liver injury. Hepatol Res. 2014;44:1151-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 37. | Aksu B, Ayvaz S, Aksu F, Karaca T, Cemek M, Ayaz A, Demirtaş S. Effects of sphingosylphosphorylcholine against oxidative stress and acute lung ınjury ınduced by pulmonary contusion in rats. J Pediatr Surg. 2015;50:591-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 38. | Liu YF, Zha BS, Zhang HL, Zhu XJ, Li YH, Zhu J, Guan XH, Feng ZQ, Zhang JP. Characteristic gene expression profiles in the progression from liver cirrhosis to carcinoma induced by diethylnitrosamine in a rat model. J Exp Clin Cancer Res. 2009;28:107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |