Published online Sep 28, 2015. doi: 10.3748/wjg.v21.i36.10327
Peer-review started: April 24, 2015
First decision: May 18, 2015
Revised: June 11, 2015
Accepted: August 30, 2015
Article in press: August 31, 2015
Published online: September 28, 2015
Processing time: 158 Days and 4.3 Hours
Hepatocellular carcinoma (HCC), the fifth most common cancer that predominantly occurs in liver cirrhosis patients, requires staging systems to design treatments. The barcelona clinic liver cancer staging system (BCLC) is the most commonly used HCC management guideline. For BCLC stage B (intermediate HCC), transarterial chemoembolization (TACE) is the standard treatment. Many studies support the use of TACE in early and advanced HCC patients. For BCLC stage 0 (very early HCC), TACE could be an alternative for patients unsuitable for radiofrequency ablation (RFA) or hepatic resection. In patients with BCLC stage A, TACE plus RFA provides better local tumor control than RFA alone. TACE can serve as bridge therapy for patients awaiting liver transplantation. For patients with BCLC B, TACE provides survival benefits compared with supportive care options. However, because of the substantial heterogeneity in the patient population with this stage, a better patient stratification system is needed to select the best candidates for TACE. Sorafenib represents the first line treatment in patients with BCLC C stage HCC. Sorafenib plus TACE has shown a demonstrable effect in delaying tumor progression. Additionally, TACE plus radiotherapy has yielded better survival in patients with HCC and portal venous thrombosis. Considering these observations together, TACE clearly has a critical role in the treatment of HCC as a stand-alone or combination therapy in each stage of HCC. Diverse treatment modalities should be used for patients with HCC and a better patient stratification system should be developed to select the best candidates for TACE.
Core tip: This article describes the role of transarterial chemoembolization (TACE) in the treatment of hepatocellular carcinoma (HCC) according to the barcelona clinic liver cancer (BCLC) staging system. Notably, TACE is the treatment of choice in the treatment of intermediate HCC (BCLC stage B). However, in clinical practice, TACE has been used as an alternative or combination therapy in patients with early or advanced HCC. Therefore, diverse treatment modalities, including TACE, should be considered for the best interests of patients with HCC.
- Citation: Han K, Kim JH. Transarterial chemoembolization in hepatocellular carcinoma treatment: Barcelona clinic liver cancer staging system. World J Gastroenterol 2015; 21(36): 10327-10335
- URL: https://www.wjgnet.com/1007-9327/full/v21/i36/10327.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i36.10327
Hepatocellular carcinoma (HCC) is the fifth most common type of cancer worldwide, predominantly occurs in patients with liver cirrhosis, and its rate of incidence is increasing[1,2]. HCC is a unique type of tumor because in addition to the extent of the tumor, the underlying liver function affects the prognosis[3]. The barcelona clinic liver cancer (BCLC) staging system staging system is the most widely accepted model worldwide as it integrates both tumor characteristics and general health status with hepatic function to provide a clinical algorithm to help guide treatment decision-making according to disease stages[4-6]. Notably, the BCLC staging system stipulates that transarterial chemoembolization (TACE) is the standard of care for patients with intermediate HCC. A growing body of evidence supports the use of TACE for patients with early and advanced HCC. This narrative review offers a critical appraisal of the available data regarding the role of TACE in the treatment of HCC based on the BCLC staging system.
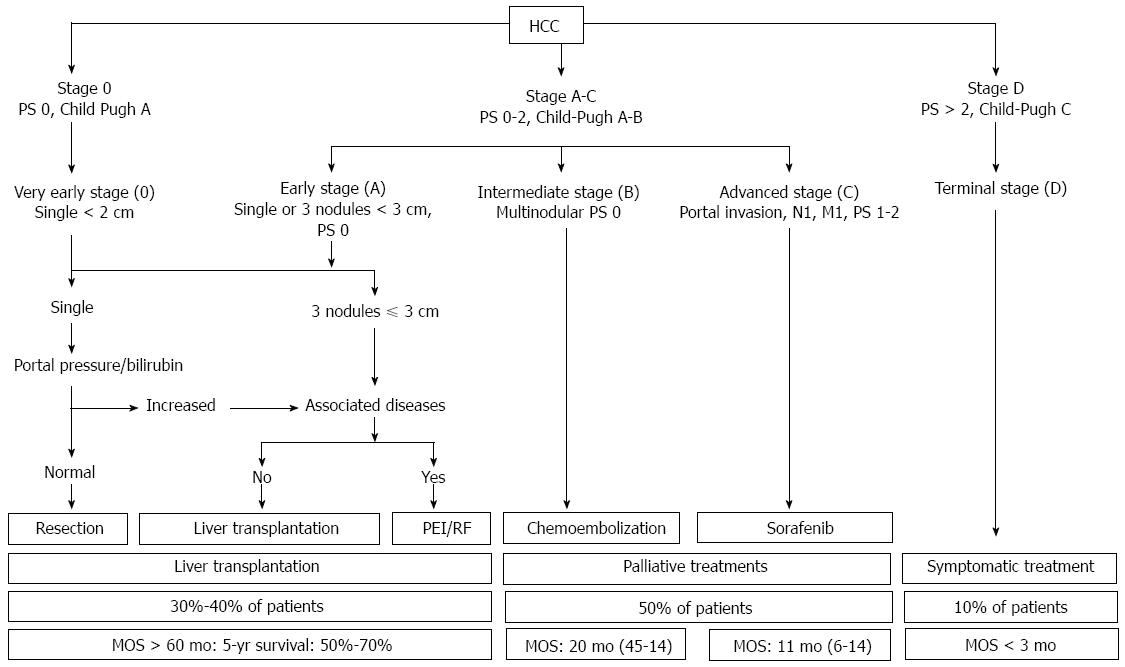
Recently, diverse HCC staging systems have been proposed, including TNM staging, the Cancer of the Liver Italian Program (CLIP), the Chinese University Prognostic Index (CUPI), the Japanese Integrated Staging (JIS) system, and the BCLC staging and treatment strategy[7-10]. Among these systems, only BCLC staging has been externally validated and allocates management choices to the following five different disease categories: very early, early, intermediate, advanced, and terminal. Importantly, liver expert groups (EASL and AASLD) generally agree that the BCLC system is preferred for HCC staging because it helps to predict survival outcomes and plan treatment options, and it is likely to be updated to reflect the latest molecular research to enhance prognostic and stage-specific management strategies[11,12]. We summarize the BCLC staging system and treatment strategies for each disease stage in Figure 1.
According to the BCLC system, TACE is the standard of care for both intermediate HCC. As described in the BCLC guidelines, this stratum of patients shows a survival benefit from TACE, which will be discussed later. However, in clinical practice, TACE has been widely used for different stages of HCC that extend beyond those recommended in the BCLC system (early or even advanced HCC). Irrespective of the heterogeneity in TACE techniques, chemotherapeutic agents, and treatment intervals, the term “conventional TACE” generally refers to the use of Lipiodol as an embolic material. For conventional TACE, various anticancer drugs are vigorously mixed with Lipiodol, which functions as a microvessel embolic agent, a chemotherapeutic agent carrier, and an augmenter of antitumor effects by promoting efflux into the portal vein[13]. As an alternative to conventional Lipiodol-based regimens, non-resorbable microspheres loaded with cytotoxic drugs can be administered intra-arterially to HCC patients. These particles are termed “drug-eluting beads” and were developed to sequester doxorubicin from solution and release it in a sustained manner. It has been reported that the amount of chemotherapeutic agents that reach systemic circulation compared with Lipiodol-based TACE can be substantially reduced, thus sharply increasing the local drug concentration[14].
The phase II PRECISION V trial compared doxorubicin-loaded DEBs with conventional TACE and demonstrated a significant reduction in liver toxicity and drug-related adverse events. However, to date, no prospective study has yet reported a significant difference in clinical efficacy between Lipiodol-based TACE and DEB TACE[15].
Herein, we review the clinical implications of conventional TACE in each BCLC category.
This stage refers to patients with a single tumor ≤ 2 cm or in situ. The American Association for the Study of Liver Diseases (AASLD) and European Association for the Study of the Liver (EASL) recommend that hepatic resection (HR) or liver transplantation (LT) should be the first option in BCLC 0 patients[11,12]. However, various risks, such as insufficient liver function, major blood loss, further injury to the normal parenchyma, and a shortage of liver donors, can prevent some patients from undergoing HR or LT[16,17]. In stage 0 patients who are not suitable for HR or LT, diverse logoregional ablation techniques have been employed. Among these patients, radiofrequency ablation (RFA) is recognized to be the modality of choice. Recently, RFA was shown to be as effective as HR for small HCCs in terms of overall survival, and some investigators suggest that RFA may be the first option for patients with a single HCC that is 2 cm or smaller, even when they can be surgically resected[18-20]. However, in patients with HCCs with a subcapsular or dome location or HCCs adjacent to the main bile duct or bowel loop, RFA may not be technically feasible because of the associated risks that include bowel perforation, major bleeding, and bile leakage[21].
Notably, TACE was previously only considered in this group of patients when HR, RFA, and LT were all not possible for various reasons. Kim et al[22] recently compared the effectiveness of TACE and RFA for stage 0 HCC, and reported no statistically significant difference in overall survival between the two groups, although RFA showed a better tumor response and delayed tumor progression. TACE may be considered a viable alternative treatment to RFA for treating single HCCs that are 2 cm or smaller when RFA is not feasible.
This stage includes patients with a single HCC or up to three nodules < 3 cm. Currently, if patients have well-preserved liver function without major vascular or lymphatic invasion, HR is considered to be the standard of care for early HCCs[12]. Unfortunately, in this stage, many patients do not satisfy the BCLC criteria for HR because HCC usually occurs in liver cirrhosis. As mentioned above, RFA has been found to be equally safe and effective as a first-line treatment for a single HCC up to 5 cm in diameter[23]. However, the local tumor progression rate, an important prognostic factor for RFA-treated HCC, was reported to sharply increase for tumors that exceeded 3 cm in size[24,25]. Notably, it is rarely possible to achieve complete ablation for tumors larger than 5 cm because of limitations for the ablation zone[26,27]. Kim et al[28] compared the effectiveness and safety of combined RFA and TACE to RFA alone in the treatment of mid-sized HCC (3-5 cm). In the combined therapy group, the long-term local tumor progression rates were lower than those of the RFA alone group (1-, 3-, 5-, and 7-year LTP rate: 9%, 40%, 55%, and 66% vs 45%, 76%, 86%, and 89%, respectively; all P < 0.001). The observed advantages appear to be attributed to reduced heat-sink effects by occluding the arterial flow and allowing for more microscopic satellite tumor control[29-31].
Because the BCLC staging system categorizes solitary HCC as an early stage disease irrespective of tumor size, large single HCCs (> 5 cm) without vascular invasion also belong to the BCLC A stage. Jin et al[32] compared the outcomes of HR and TACE for solitary large HCC. They reported that HR offered a significantly better 5-year survival rate in the surgical group than in the TACE group (65% vs 17%, P < 0.01) irrespective of tumor size. In the study of Zhu et al[33], the propensity score matched findings also demonstrated a better 5-year survival rate in the surgical group than in the TACE group with propensity score matching (41.3% vs 18.5%, P = 0.007). Recently, Lee et al[34] conducted the largest study (159 total patients: 91 patients for HR and 68 patients for TACE) to date that compared long-term survival after HR and TACE as the initial treatment for large solitary HCC (> 5 cm), which yielded contradicting results. The 5-year overall survival rates of HR and TACE were 66% and 50%, respectively, and TTP was longer in the HR group. After propensity score matching (58 pairs), the overall survival of TACE patients was comparable to that of HR patients, and TTP remained significantly longer in patients treated with HR. The difference in overall survival between the two groups might result from differences in baseline patient characteristics rather than the treatment modality. They concluded that TACE could be considered as an alternative initial treatment for large solitary HCCs if HR is not feasible, particularly in patients with clinically presumed portal hypertension. A large, randomized, controlled study is warranted to compare the long-term outcomes of HR and TACE in the treatment of large solitary HCCs.
The Milan criteria (one lesion ≤ 5 cm in diameter or up to 3 lesions ≤ 3 cm) can be applied as a basis for selecting patients with cirrhosis and HCC for LT. However, liver transplant candidates greatly outnumber liver donors. It has been suggested that TACE can be used to downstage a tumor within the Milan criteria before transplantation[35-39]. Additionally, TACE can be used as a bridge to LT in cirrhotic patients with HCC within the Milan criteria[40].
The intermediate stage constitutes asymptomatic, large, or multifocal HCCs without evidence of vascular invasion or extrahepatic metastasis. TACE is the recommended treatment modality for this stratum of patients[4]. This recommendation is based on a meta-analysis of seven trials, which demonstrated that TACE showed a significant improvement in 2-year survival compared with best supportive care (OR = 0.53; 95%CI: 0.32-0.89; P = 0.017)[41]. However, patients enrolled in these studies were not categorized according to the BCLC staging system and many patients with early HCCs were included. Furthermore, many patients had compensated liver function (Child-Pugh A) and, thus, the role of TACE in HCC patients with Child-Pugh class B is relatively insufficient. Similarly, one of the great problems of BCLC stage B is the enormous heterogeneity of the population in tumor load, age, liver function, and potential comorbidities. However, no subgroup stratification exists for this stage, making it difficult to provide optimal treatment strategies[42]. Therefore, in clinical practice, TACE is often used outside of the current treatment guidelines.
Recently, several groups have proposed patient stratification systems. Bolondi et al[43] proposed a subclassification system of intermediate HCC based on key parameters related to tumor burden and liver function. The key parameters in such four-subgroup systems included the Child-Pugh score, tumor load (within or beyond the up-to-seven criteria), the ECOG performance and portal venous thrombosis, and the first and alternative treatment options were assigned to each category (Table 1). Ha et al[44] evaluated the usefulness of such subclassifications. In their study, patients belonging to the B1 and B2 subclasses had a median overall survival of 41 or 22 months, respectively. They did not observe any survival difference between the B3 and B4 groups (14.1 mo vs 17.2 mo, P = 0.48) and proposed a modified subclassification system by merging the B3 and B4 patients to facilitate per-subclass-based treatment options (median OS: 16.6 mo).
| BCLC substage | B1 | B2 | B3 | B4 |
| Child-Pugh Score | 5-6-7 | 5-6 | 7 | 8-9 |
| Beyond Milan and Within Ut-7 | In | Out | Out | Any |
| ECOG-PS | 0 | 0 | 0 | 0-1 |
| PVT | No | No | No | No |
| 1st option | TACE | TACE or TARE | BSC | |
| Alternative | LT, TACE + ablation | SOR | Research trialsTACE, SOR | LT |
Despite the established survival benefit of TACE in patients with intermediate HCC, and as TACE is a palliative treatment that does not result in complete tumor necrosis, tumor recurrence after TACE is common. Additionally, repeat TACE might damage liver function and adversely affect patient survival. Nevertheless, RFA is known to provide better local control of disease than TACE and can achieve complete necrosis for small HCCs. However, the effectiveness of RFA in patients with intermediate or large HCC is unsatisfactory, with a relatively low complete necrosis rate that ranges from 29% to 70%, even if an overlapping technique or repeated procedures are used. However, Tanaka et al[45] investigated the long-term effects of combination therapy for intermediate HCC. A total of 58 patients with BCLC stage B (single nodule > 5 cm or measuring more than 30 mm in diameter or two to three nodules, each measuring more than 30 mm in diameter, or more than three nodules, no vascular invasion, and no extrahepatic metastasis) were included in that study. They reported that the 1-, 2-, 3- and 5-year overall survival rates of the combination therapy group (91%, 65%, 53%, and 27%, respectively) were significantly better than those of the supportive care group (42%, 8%, 8%, and 0%, respectively). The overall survival rates for the combination therapy group tended to be higher than those of patients treated with TACE alone in previous studies (1-, 2-, and 3-year survival rates of 75%, 50%, and 29%, respectively). Although a large-scale randomized controlled trial will be required to compare those results with TACE alone, TACE combined with RFA seems to be a safe and effective treatment strategy for patients with intermediate HCC.
Sorafenib, an oral multikinase tyrosine inhibitor, is the treatment of choice in BCLC C patients. However, a subanalysis of the SHARP trial revealed that sorafenib was safe and effective, irrespective of whether patients were either BCLC stage B or C (median OS: 14.5 mo in BCLC B stage vs 9.7 mo in BCLC C)[46]. Some investigators demonstrated that sorafenib may provide benefits for patients with BCLC B HCC who are ineligible for or have progressed after TACE[46,47]. Furthermore, it is suggested that patients who do not meet the allocated treatment criteria within a stage should be offered the next most suitable treatment within the same or next stage[11]. Patients with intermediate HCC who do not respond to TACE may benefit from sorafenib. Sorafenib will be discussed more thoroughly in the following section on the BCLC C stage.
This category applies to patients who have symptoms and/or vascular invasion or extrahepatic spread. No effective systemic chemotherapy exists for advanced HCC, and systemic chemotherapy might even adversely affect patient survival[46]. In this context, sorafenib, an oral multikinase inhibitor with antiproliferative and antiangiogenic effects, has emerged as a promising drug for advanced HCC interventions. The SHARP trial reported an improved median overall survival without significant drug toxicity in patients treated with sorafenib (10.7 mo in the sorafenib group vs 7.9 mo in the placebo group; HR = 0.69; 95%CI: 0.55-0.87; P < 0.001), and this improvement in survival was also identified in the Asian-Pacific population (6.5 mo in the sorafenib group vs 4.2 mo in the placebo group; HR = 0.68; 95%CI: 0.50-0.93; P = 0.014)[48,49]. Subsequently, sorafenib has been considered as the standard of care for BCLC stage C HCC.
As demonstrated by the aforementioned previous studies, the survival benefit observed after sorafenib treatment is limited to less than 3 mo, which highlights the need for better treatment strategies. Under these circumstances, several investigators have reported that TACE has the potential to benefit this group of patients[50-55]. Chung et al[56] compared the efficacy and safety of TACE in patients with HCC who initially presented with main portal vein invasion. They showed that repeated TACE showed significant survival benefits compared with supportive care in both Child-Pugh class A (median OS: 7.4 mo vs 2.6 mo) and B (median OS: 2.8 mo vs 1.9 mo). Furthermore, irrespective of the use of sorafenib, the use of TACE to control intrahepatic HCC has been found to offer survival benefits compared with conservative management in patients with HCC and extrahepatic spread[57].
TACE-induced hypoxia in surviving tumor cells results in the release of angiogenic growth factors, which contribute to tumor recurrence or metastases and a worse outcome[58,59]. Sorafenib inhibits tumor cell proliferation by blocking the Raf-MEK-ERK signaling pathway at the Raf kinase level, and exerts an antiangiogenic effect by blocking vascular endothelial growth factor receptor-2 and -3 and platelet-derived growth factor receptor tyrosine kinase[60]. Therefore, in theory a combination of TACE with sorafenib might provide a benefit for patients with HCC. Choi et al[61] studied the time to progression and overall survival in patients with advanced HCC who were treated with sorafenib plus TACE versus sorafenib alone. They reported that the median TTP and OS in the combined group were longer than those in the monotherapy group (TTP: 2.5 mo vs 2.1 mo, P = 0.008; OS: 8.9 mo vs 5.9 mo, P = 0.009). Therefore, the addition of TACE to established sorafenib therapy has a demonstrable effect in delaying tumor progression in patients with advanced HCC, although the survival benefit is uncertain.
TACE combined with radiotherapy has resulted in improved outcomes for patients with HCC and portal vein thrombosis[62-70]. The rationale for this combined treatment is that reducing PVT with RT can delay intravascular tumor growth and the deterioration of liver function by preserving adequate portal flow, as well as by facilitating subsequent treatment of the primary tumor[66,67]. Recently, Kim et al[71] compared the efficacy of TACE with or without RT vs sorafenib for advanced HCC with PVT. In this single center study, patients were divided into three different pairs (TACE vs TACE + RT, TACE vs sorafenib, and TACE + RT vs sorafenib). By propensity score matched analysis, the TACE + RT group had a longer median time to progression and overall survival than the TACE-alone [102 pairs; TTP 8.7 mo vs 3.6 mo (P < 0.01); OS, 11.4 mo vs 7.4 mo (P = 0.023)] and sorafenib groups [30 pairs; TTP, 3.4 mo vs 1.8 mo (P < 0.01); OS, 5.9 mo vs 4.4 mo (P = 0.03)]. Although these findings need to be verified with future randomized controlled trials, concurrent treatment with TACE and RT could represent an alternative option to the current standard sorafenib therapy for the treatment of HCC with PVT.
The BCLC staging system has served as the backbone of HCC treatment strategies as it stratifies patients according to outcomes and allocates treatments. Notably, despite the substantial heterogeneity of the HCC patient population with BCLC stage B, TACE has played a key role in the treatment of intermediate HCC. Additionally, as discussed in this review, TACE has been used as an alternative or combination therapy in patients with early or advanced HCC (Table 2). Therefore, diverse treatment modalities should be utilized for the best interest of patients with HCC. In future studies, we should also develop a better patient stratification system to select suitable candidates for TACE and identify the best alternative treatment for patients who are refractory to TACE.
| Stage | Potential role of TACE |
| BCLC 0 | TACE may be considered a viable alternative treatment to RFA for treating single HCCs measuring 2 cm or smaller when RFA is not feasible |
| BCLC A | 1: The combination of TACE and RFA is safe and provides better local tumor control than RFA alone in the treatment of medium sized HCC (3-5 cm) |
| 2: For a large solitary HCC (> 5 cm), HR provides better overall survival than HCC, but TACE could be considered as an alternative initial treatment when HR is not feasible | |
| 3: TACE can be used to downstage the tumor within the Milan criteria before LT or serve as a bridge to LT | |
| BCLC B | 1: TACE is the standard of care for this stratum of patients |
| 2: Combination with other therapies such as RFA and sorafenib may provide better patient survival or local tumor control | |
| BCLC C | 1: Repeated TACE showed significant survival benefits in patients with advanced HCC compared with supportive care |
| 2: Sorafenib plus TACE has a demonstrable effect in delaying tumor progression | |
| 3: Combination with radiotherapy has resulted in better survival in patients with HCC and PVT |
P- Reviewer: Gong JS, Sugimoto K, Yoshida H S- Editor: Ma YJ L- Editor: A E- Editor: Zhang DN
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25541] [Article Influence: 1824.4] [Reference Citation Analysis (7)] |
| 2. | Bosch FX, Ribes J, Díaz M, Cléries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127:S5-S16. [PubMed] |
| 3. | Lencioni R, Chen XP, Dagher L, Venook AP. Treatment of intermediate/advanced hepatocellular carcinoma in the clinic: how can outcomes be improved? Oncologist. 2010;15 Suppl 4:42-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 124] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 4. | Forner A, Reig ME, de Lope CR, Bruix J. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis. 2010;30:61-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 764] [Cited by in RCA: 866] [Article Influence: 57.7] [Reference Citation Analysis (0)] |
| 5. | Cillo U, Vitale A, Grigoletto F, Farinati F, Brolese A, Zanus G, Neri D, Boccagni P, Srsen N, D’Amico F. Prospective validation of the Barcelona Clinic Liver Cancer staging system. J Hepatol. 2006;44:723-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 332] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 6. | Marrero JA, Fontana RJ, Barrat A, Askari F, Conjeevaram HS, Su GL, Lok AS. Prognosis of hepatocellular carcinoma: comparison of 7 staging systems in an American cohort. Hepatology. 2005;41:707-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 474] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 7. | Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Handbook. 7th ed. New York: Springer 2010; . |
| 8. | A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology. 1998;28:751-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 977] [Cited by in RCA: 963] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 9. | Leung TW, Tang AM, Zee B, Lau WY, Lai PB, Leung KL, Lau JT, Yu SC, Johnson PJ. Construction of the Chinese University Prognostic Index for hepatocellular carcinoma and comparison with the TNM staging system, the Okuda staging system, and the Cancer of the Liver Italian Program staging system: a study based on 926 patients. Cancer. 2002;94:1760-1769. [PubMed] |
| 10. | Nanashima A, Sumida Y, Morino S, Yamaguchi H, Tanaka K, Shibasaki S, Ide N, Sawai T, Yasutake T, Nakagoe T. The Japanese integrated staging score using liver damage grade for hepatocellular carcinoma in patients after hepatectomy. Eur J Surg Oncol. 2004;30:765-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4059] [Cited by in RCA: 4520] [Article Influence: 347.7] [Reference Citation Analysis (2)] |
| 12. | Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6573] [Article Influence: 469.5] [Reference Citation Analysis (1)] |
| 13. | Ahrar K, Gupta S. Hepatic artery embolization for hepatocellular carcinoma: technique, patient selection, and outcomes. Surg Oncol Clin N Am. 2003;12:105-126. [PubMed] |
| 14. | Varela M, Real MI, Burrel M, Forner A, Sala M, Brunet M, Ayuso C, Castells L, Montañá X, Llovet JM. Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol. 2007;46:474-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 694] [Cited by in RCA: 719] [Article Influence: 39.9] [Reference Citation Analysis (1)] |
| 15. | Lammer J, Malagari K, Vogl T, Pilleul F, Denys A, Watkinson A, Pitton M, Sergent G, Pfammatter T, Terraz S. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010;33:41-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1063] [Cited by in RCA: 1208] [Article Influence: 75.5] [Reference Citation Analysis (0)] |
| 16. | Cucchetti A, Piscaglia F, Cescon M, Colecchia A, Ercolani G, Bolondi L, Pinna AD. Cost-effectiveness of hepatic resection versus percutaneous radiofrequency ablation for early hepatocellular carcinoma. J Hepatol. 2013;59:300-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 313] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 17. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5110] [Cited by in RCA: 5311] [Article Influence: 183.1] [Reference Citation Analysis (0)] |
| 18. | Choi D, Lim HK, Rhim H, Kim YS, Lee WJ, Paik SW, Koh KC, Lee JH, Choi MS, Yoo BC. Percutaneous radiofrequency ablation for early-stage hepatocellular carcinoma as a first-line treatment: long-term results and prognostic factors in a large single-institution series. Eur Radiol. 2007;17:684-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 245] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 19. | Chan AC, Poon RT, Ng KK, Lo CM, Fan ST, Wong J. Changing paradigm in the management of hepatocellular carcinoma improves the survival benefit of early detection by screening. Ann Surg. 2008;247:666-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 72] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 20. | Livraghi T, Meloni F, Di Stasi M, Rolle E, Solbiati L, Tinelli C, Rossi S. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice? Hepatology. 2008;47:82-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 850] [Cited by in RCA: 825] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 21. | Komorizono Y, Oketani M, Sako K, Yamasaki N, Shibatou T, Maeda M, Kohara K, Shigenobu S, Ishibashi K, Arima T. Risk factors for local recurrence of small hepatocellular carcinoma tumors after a single session, single application of percutaneous radiofrequency ablation. Cancer. 2003;97:1253-1262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 255] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 22. | Kim JW, Kim JH, Sung KB, Ko HK, Shin JH, Kim PN, Choi HK, Ko GY, Yoon HK, Chun SY. Transarterial chemoembolization vs. radiofrequency ablation for the treatment of single hepatocellular carcinoma 2 cm or smaller. Am J Gastroenterol. 2014;109:1234-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 23. | N’Kontchou G, Mahamoudi A, Aout M, Ganne-Carrié N, Grando V, Coderc E, Vicaut E, Trinchet JC, Sellier N, Beaugrand M. Radiofrequency ablation of hepatocellular carcinoma: long-term results and prognostic factors in 235 Western patients with cirrhosis. Hepatology. 2009;50:1475-1483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 356] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 24. | Xu HX, Lu MD, Xie XY, Yin XY, Kuang M, Chen JW, Xu ZF, Liu GJ. Prognostic factors for long-term outcome after percutaneous thermal ablation for hepatocellular carcinoma: a survival analysis of 137 consecutive patients. Clin Radiol. 2005;60:1018-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 80] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 25. | Llovet JM, Bruix J. Novel advancements in the management of hepatocellular carcinoma in 2008. J Hepatol. 2008;48 Suppl 1:S20-S37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 599] [Cited by in RCA: 639] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 26. | Yamasaki T, Kurokawa F, Shirahashi H, Kusano N, Hironaka K, Okita K. Percutaneous radiofrequency ablation therapy for patients with hepatocellular carcinoma during occlusion of hepatic blood flow. Comparison with standard percutaneous radiofrequency ablation therapy. Cancer. 2002;95:2353-2360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 98] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 27. | Rossi S, Garbagnati F, De Francesco I, Accocella F, Leonardi L, Quaretti P, Zangrandi A, Paties C, Lencioni R. Relationship between the shape and size of radiofrequency induced thermal lesions and hepatic vascularization. Tumori. 1999;85:128-132. [PubMed] |
| 28. | Kim JH, Won HJ, Shin YM, Kim SH, Yoon HK, Sung KB, Kim PN. Medium-sized (3.1-5.0 cm) hepatocellular carcinoma: transarterial chemoembolization plus radiofrequency ablation versus radiofrequency ablation alone. Ann Surg Oncol. 2011;18:1624-1629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 112] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 29. | Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Ierace T, Solbiati L, Gazelle GS. Hepatocellular carcinoma: radio-frequency ablation of medium and large lesions. Radiology. 2000;214:761-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 806] [Cited by in RCA: 741] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 30. | Takaki H, Yamakado K, Nakatsuka A, Fuke H, Murata K, Shiraki K, Takeda K. Radiofrequency ablation combined with chemoembolization for the treatment of hepatocellular carcinomas 5 cm or smaller: risk factors for local tumor progression. J Vasc Interv Radiol. 2007;18:856-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 31. | Shibata T, Isoda H, Hirokawa Y, Arizono S, Shimada K, Togashi K. Small hepatocellular carcinoma: is radiofrequency ablation combined with transcatheter arterial chemoembolization more effective than radiofrequency ablation alone for treatment? Radiology. 2009;252:905-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 185] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 32. | Jin YJ, Lee JW, Choi YJ, Chung HJ, Kim YS, Lee KY, Ahn SI, Shin WY, Cho SG, Jeon YS. Surgery versus transarterial chemoembolization for solitary large hepatocellular carcinoma of BCLC stage A. J Gastrointest Surg. 2014;18:555-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 33. | Zhu SL, Ke Y, Peng YC, Ma L, Li H, Li LQ, Zhong JH. Comparison of long-term survival of patients with solitary large hepatocellular carcinoma of BCLC stage A after liver resection or transarterial chemoembolization: a propensity score analysis. PLoS One. 2014;9:e115834. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 34. | Lee YB, Lee DH, Cho Y, Yu SJ, Lee JH, Yoon JH, Lee HS, Kim HC, Yi NJ, Lee KW. Comparison of transarterial chemoembolization and hepatic resection for large solitary hepatocellular carcinoma: a propensity score analysis. J Vasc Interv Radiol. 2015;26:651-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 35. | Kim do Y, Choi MS, Lee JH, Koh KC, Paik SW, Yoo BC, Shin SW, Choo SW, Do YS, Rhee JC. Milan criteria are useful predictors for favorable outcomes in hepatocellular carcinoma patients undergoing liver transplantation after transarterial chemoembolization. World J Gastroenterol. 2006;12:6992-6997. [PubMed] |
| 36. | Graziadei IW, Sandmueller H, Waldenberger P, Koenigsrainer A, Nachbaur K, Jaschke W, Margreiter R, Vogel W. Chemoembolization followed by liver transplantation for hepatocellular carcinoma impedes tumor progression while on the waiting list and leads to excellent outcome. Liver Transpl. 2003;9:557-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 336] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 37. | Yao FY, Kerlan RK, Hirose R, Davern TJ, Bass NM, Feng S, Peters M, Terrault N, Freise CE, Ascher NL. Excellent outcome following down-staging of hepatocellular carcinoma prior to liver transplantation: an intention-to-treat analysis. Hepatology. 2008;48:819-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 409] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 38. | Decaens T, Roudot-Thoraval F, Bresson-Hadni S, Meyer C, Gugenheim J, Durand F, Bernard PH, Boillot O, Boudjema K, Calmus Y. Impact of pretransplantation transarterial chemoembolization on survival and recurrence after liver transplantation for hepatocellular carcinoma. Liver Transpl. 2005;11:767-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 164] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 39. | Yoon HM, Kim JH, Kim EJ, Gwon DI, Ko GY, Ko HK. Modified cisplatin-based transcatheter arterial chemoembolization for large hepatocellular carcinoma: multivariate analysis of predictive factors for tumor response and survival in a 163-patient cohort. J Vasc Interv Radiol. 2013;24:1639-1646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 40. | Bouchard-Fortier A, Lapointe R, Perreault P, Bouchard L, Pomier-Layrargues G. Transcatheter arterial chemoembolization of hepatocellular carcinoma as a bridge to liver transplantation: a retrospective study. Int J Hepatol. 2011;2011:974514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 41. | Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2207] [Cited by in RCA: 2271] [Article Influence: 103.2] [Reference Citation Analysis (0)] |
| 42. | Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3249] [Cited by in RCA: 3596] [Article Influence: 276.6] [Reference Citation Analysis (4)] |
| 43. | Bolondi L, Burroughs A, Dufour JF, Galle PR, Mazzaferro V, Piscaglia F, Raoul JL, Sangro B. Heterogeneity of patients with intermediate (BCLC B) Hepatocellular Carcinoma: proposal for a subclassification to facilitate treatment decisions. Semin Liver Dis. 2012;32:348-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 303] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 44. | Ha Y, Shim JH, Kim SO, Kim KM, Lim YS, Lee HC. Clinical appraisal of the recently proposed Barcelona Clinic Liver Cancer stage B subclassification by survival analysis. J Gastroenterol Hepatol. 2014;29:787-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 45. | Tanaka M, Ando E, Simose S, Hori M, Kuraoka K, Ohno M, Yutani S, Harada K, Sata M. Radiofrequency ablation combined with transarterial chemoembolization for intermediate hepatocellular carcinoma. Hepatol Res. 2014;44:194-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 46. | Bruix J, Raoul JL, Sherman M, Mazzaferro V, Bolondi L, Craxi A, Galle PR, Santoro A, Beaugrand M, Sangiovanni A. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a phase III trial. J Hepatol. 2012;57:821-829. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 664] [Cited by in RCA: 654] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 47. | Raoul JL, Sangro B, Forner A, Mazzaferro V, Piscaglia F, Bolondi L, Lencioni R. Evolving strategies for the management of intermediate-stage hepatocellular carcinoma: available evidence and expert opinion on the use of transarterial chemoembolization. Cancer Treat Rev. 2011;37:212-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 409] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 48. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10268] [Article Influence: 604.0] [Reference Citation Analysis (2)] |
| 49. | Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3854] [Cited by in RCA: 4650] [Article Influence: 273.5] [Reference Citation Analysis (0)] |
| 50. | Kim KM, Kim JH, Park IS, Ko GY, Yoon HK, Sung KB, Lim YS, Lee HC, Chung YH, Lee YS. Reappraisal of repeated transarterial chemoembolization in the treatment of hepatocellular carcinoma with portal vein invasion. J Gastroenterol Hepatol. 2009;24:806-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 118] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 51. | Georgiades CS, Hong K, D’Angelo M, Geschwind JF. Safety and efficacy of transarterial chemoembolization in patients with unresectable hepatocellular carcinoma and portal vein thrombosis. J Vasc Interv Radiol. 2005;16:1653-1659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 178] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 52. | Lee HS, Kim JS, Choi IJ, Chung JW, Park JH, Kim CY. The safety and efficacy of transcatheter arterial chemoembolization in the treatment of patients with hepatocellular carcinoma and main portal vein obstruction. A prospective controlled study. Cancer. 1997;79:2087-2094. [PubMed] |
| 53. | Luo J, Guo RP, Lai EC, Zhang YJ, Lau WY, Chen MS, Shi M. Transarterial chemoembolization for unresectable hepatocellular carcinoma with portal vein tumor thrombosis: a prospective comparative study. Ann Surg Oncol. 2011;18:413-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 274] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 54. | Okusaka T, Okada S, Ishii H, Nose H, Nagahama H, Nakasuka H, Ikeda K, Yoshimori M. Prognosis of hepatocellular carcinoma patients with extrahepatic metastases. Hepatogastroenterology. 1997;44:251-257. [PubMed] |
| 55. | Uka K, Aikata H, Takaki S, Shirakawa H, Jeong SC, Yamashina K, Hiramatsu A, Kodama H, Takahashi S, Chayama K. Clinical features and prognosis of patients with extrahepatic metastases from hepatocellular carcinoma. World J Gastroenterol. 2007;13:414-420. [PubMed] |
| 56. | Chung GE, Lee JH, Kim HY, Hwang SY, Kim JS, Chung JW, Yoon JH, Lee HS, Kim YJ. Transarterial chemoembolization can be safely performed in patients with hepatocellular carcinoma invading the main portal vein and may improve the overall survival. Radiology. 2011;258:627-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 251] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 57. | Yoo DJ, Kim KM, Jin YJ, Shim JH, Ko GY, Yoon HK, Sung KB, Lee JL, Kang YK, Lim YS. Clinical outcome of 251 patients with extrahepatic metastasis at initial diagnosis of hepatocellular carcinoma: does transarterial chemoembolization improve survival in these patients? J Gastroenterol Hepatol. 2011;26:145-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 107] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 58. | Sergio A, Cristofori C, Cardin R, Pivetta G, Ragazzi R, Baldan A, Girardi L, Cillo U, Burra P, Giacomin A. Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): the role of angiogenesis and invasiveness. Am J Gastroenterol. 2008;103:914-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 397] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 59. | Shim JH, Park JW, Kim JH, An M, Kong SY, Nam BH, Choi JI, Kim HB, Lee WJ, Kim CM. Association between increment of serum VEGF level and prognosis after transcatheter arterial chemoembolization in hepatocellular carcinoma patients. Cancer Sci. 2008;99:2037-2044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 113] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 60. | Wilhelm SM, Adnane L, Newell P, Villanueva A, Llovet JM, Lynch M. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther. 2008;7:3129-3140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1149] [Cited by in RCA: 1109] [Article Influence: 65.2] [Reference Citation Analysis (0)] |
| 61. | Choi GH, Shim JH, Kim MJ, Ryu MH, Ryoo BY, Kang YK, Shin YM, Kim KM, Lim YS, Lee HC. Sorafenib alone versus sorafenib combined with transarterial chemoembolization for advanced-stage hepatocellular carcinoma: results of propensity score analyses. Radiology. 2013;269:603-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 116] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 62. | Han K, Kim JH, Yoon HM, Kim EJ, Gwon DI, Ko GY, Yoon HK, Ko HK. Transcatheter arterial chemoembolization for infiltrative hepatocellular carcinoma: clinical safety and efficacy and factors influencing patient survival. Korean J Radiol. 2014;15:464-471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 63. | Yoon SM, Lim YS, Won HJ, Kim JH, Kim KM, Lee HC, Chung YH, Lee YS, Lee SG, Park JH. Radiotherapy plus transarterial chemoembolization for hepatocellular carcinoma invading the portal vein: long-term patient outcomes. Int J Radiat Oncol Biol Phys. 2012;82:2004-2011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 180] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 64. | Kim DY, Park W, Lim DH, Lee JH, Yoo BC, Paik SW, Kho KC, Kim TH, Ahn YC, Huh SJ. Three-dimensional conformal radiotherapy for portal vein thrombosis of hepatocellular carcinoma. Cancer. 2005;103:2419-2426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 152] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 65. | Yamada K, Izaki K, Sugimoto K, Mayahara H, Morita Y, Yoden E, Matsumoto S, Soejima T, Sugimura K. Prospective trial of combined transcatheter arterial chemoembolization and three-dimensional conformal radiotherapy for portal vein tumor thrombus in patients with unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2003;57:113-119. [PubMed] |
| 66. | Zeng ZC, Fan J, Tang ZY, Zhou J, Qin LX, Wang JH, Sun HC, Wang BL, Zhang JY, Jiang GL. A comparison of treatment combinations with and without radiotherapy for hepatocellular carcinoma with portal vein and/or inferior vena cava tumor thrombus. Int J Radiat Oncol Biol Phys. 2005;61:432-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 142] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 67. | Ishikura S, Ogino T, Furuse J, Satake M, Baba S, Kawashima M, Nihei K, Ito Y, Maru Y, Ikeda H. Radiotherapy after transcatheter arterial chemoembolization for patients with hepatocellular carcinoma and portal vein tumor thrombus. Am J Clin Oncol. 2002;25:189-193. [PubMed] |
| 68. | Tazawa J, Maeda M, Sakai Y, Yamane M, Ohbayashi H, Kakinuma S, Miyasaka Y, Nagayama K, Enomoto N, Sato C. Radiation therapy in combination with transcatheter arterial chemoembolization for hepatocellular carcinoma with extensive portal vein involvement. J Gastroenterol Hepatol. 2001;16:660-665. [PubMed] |
| 69. | Nakazawa T, Adachi S, Kitano M, Isobe Y, Kokubu S, Hidaka H, Ono K, Okuwaki Y, Watanabe M, Shibuya A. Potential prognostic benefits of radiotherapy as an initial treatment for patients with unresectable advanced hepatocellular carcinoma with invasion to intrahepatic large vessels. Oncology. 2007;73:90-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 70. | Toya R, Murakami R, Baba Y, Nishimura R, Morishita S, Ikeda O, Kawanaka K, Beppu T, Sugiyama S, Sakamoto T. Conformal radiation therapy for portal vein tumor thrombosis of hepatocellular carcinoma. Radiother Oncol. 2007;84:266-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 89] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 71. | Kim GA, Shim JH, Yoon SM, Jung J, Kim JH, Ryu MH, Ryoo BY, Kang YK, Lee D, Kim KM. Comparison of chemoembolization with and without radiation therapy and sorafenib for advanced hepatocellular carcinoma with portal vein tumor thrombosis: a propensity score analysis. J Vasc Interv Radiol. 2015;26:320-9.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |