Published online Sep 21, 2015. doi: 10.3748/wjg.v21.i35.10166
Peer-review started: March 25, 2015
First decision: April 23, 2015
Revised: May 13, 2015
Accepted: July 18, 2015
Article in press: July 18, 2015
Published online: September 21, 2015
Processing time: 181 Days and 18.9 Hours
AIM: To compare cases of xanthogranulomatous cholecystitis (XGC) and advanced gallbladder cancer and discuss the differential diagnoses and surgical options.
METHODS: From April 2000 to December 2013, 6 XGC patients received extended surgical resections. During the same period, 16 patients were proven to have gallbladder (GB) cancer, according to extended surgical resection. Subjects chosen for analysis in this study were restricted to cases of XGC with indistinct borders with the liver as it is often difficult to distinguish these patients from those with advanced GB cancer. We compared the clinical features and computed tomography findings between XGC and advanced GB cancer. The following clinical features were retrospectively assessed: age, gender, symptoms, and tumor markers. As albumin and the neutrophil/lymphocyte ratio (NLR) are prognostic in several cancers, we compared serum albumin levels and the NLR between the two groups. The computerized tomography findings were used to compare the two diseases, determine the coexistence of gallstones, the pattern of GB thickening (focal or diffuse), the presence of a hypoattenuated intramural nodule, and continuity of the mucosal line.
RESULTS: Based on the preoperative image findings, we suspected GB carcinoma in all cases including XGC in this series. In addition, by pathological examination, we found that the group of patients with XGC developed inflammatory disease after surgery. Patients with XGC tended to have abdominal pain (4/6, 67%). However, there was no significant difference in clinical symptoms, including fever, between the two groups. Serum albumin and NLR were also similar in the two groups. Serum tumor markers, such as carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9), tended to increase in patients with GB cancer. However, no significant differences in tumor markers were identified. On the other hand, gallstones were more frequently observed in patients with XGC (5/6, 83%) than in patients with GB cancer (4/16, 33%) (P = 0.0116). A hypoattenuated intramural nodule was found in 3 patients with XGC (3/6, 50%), but in only 1 patient with GB cancer (1/16, 6%) (P = 0.0024). The GB thickness, continuous mucosal line, and bile duct dilatation showed no significant differences between XGC and GB cancer.
CONCLUSION: Although XGC is often difficult to differentiate from GB carcinoma, it is possible to obtain an accurate diagnosis by careful intraoperative gross observation, and several intraoperative frozen sections.
Core tip: Xanthogranulomatous cholecystitis (XGC) is a rare inflammatory disease of the gallbladder. Differentiating between XGC and malignant gallbladder lesions is often difficult, especially in patients with severe proliferative fibrosis involving the gallbladder and surrounding organs. We compared the clinical features and computed tomography findings between patients with XGC and patients with advanced gallbladder cancer. There were almost no significant differences between the two groups. Although XGC is often difficult to differentiate from gallbladder carcinoma, it is possible to obtain an accurate diagnosis by careful intraoperative gross observation and several intraoperative frozen sections which could prevent extended resections.
- Citation: Suzuki H, Wada S, Araki K, Kubo N, Watanabe A, Tsukagoshi M, Kuwano H. Xanthogranulomatous cholecystitis: Difficulty in differentiating from gallbladder cancer. World J Gastroenterol 2015; 21(35): 10166-10173
- URL: https://www.wjgnet.com/1007-9327/full/v21/i35/10166.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i35.10166
Xanthogranulomatous cholecystitis (XGC) is a rare inflammatory disease of the gallbladder (GB). The characteristic macroscopic findings of XGC include abnormal thickening of the wall and severe proliferative fibrosis with the formation of multiple yellow-brown intramural nodules[1,2].
Although the mechanism that leads to this condition remains unclear, XGC is thought to start as a biliary obstruction with acute or chronic cholelithiasis and increasing intra-gallbladder pressure. It is believed that this pressure provokes a rupture of the Rokitansky-Aschoff sinuses or mucosal ulcer with extravasation of bile in the interstitial tissues and a consequent xanthogranulomatous inflammatory reaction[3,4]. This inflammatory process is often extensive and may extend to adjacent organs, forming dense adhesions with a large mass of inflammatory tissue surrounding the GB.
The clinical manifestations of XGC are usually acute or chronic cholecystitis. The main symptoms include right hypochondriac pain, radiating pain in the shoulder, fever, and nausea[5]. However, some patients with XGC do not have these symptoms. On computed tomography (CT), an enhanced continuous mucosal line in the CT image can aid the diagnosis of XGC. Moreover, Uchiyama et al[6] reported that an enhanced continuous mucosal line with gallstones was highly suggestive of XGC. However, despite the use of these imaging techniques, the differential diagnosis between XGC and malignant GB lesions is often difficult, especially in patients with severe proliferative fibrosis involving the GB and surrounding organs.
In this study, we compared cases of XGC who had indistinct borders with the liver suggestive of GB cancer and who required extended surgical resections with cases of advanced GB cancer that had invaded the liver. We discuss the differential diagnosis and surgical options in cases of XGC with extensive involvement of extra-gallbladder organs.
From April 2000 to December 2013, 6 XGC patients received extended surgical resections. During the same period, 16 patients were proven to have GB cancer, according to extended surgical resections at Gunma University Hospital, Department of Surgery 1. Subjects chosen for analysis in this study were restricted to cases of XGC with indistinct borders with the liver as it is often difficult to distinguish these patients from those with advanced GB cancer. Preoperative evaluation was carried out with ultrasonography (US), CT, magnetic resonance imaging, and FDG-PET. In addition, some patients underwent endoscopic retrograde cholangiopancreatography and/or percutaneous transhepatic biliary drainage for diagnosis and/or biliary decompression. Based on these image findings, surgical treatment was performed following the guidelines for the management of biliary tract and ampullary carcinomas[7]. The following clinical features were retrospectively assessed: age, gender, and symptoms. As albumin and the neutrophil/lymphocyte ratio (NLR) are prognostic in several cancers[8], we compared serum albumin levels and the NLR between the two groups. The NLR was calculated from a complete blood count in laboratory testing before the operation. Tumor markers CEA and CA19-9 were also serologically analyzed. CT findings were used to compare the two diseases, determine the coexistence of gallstones, the pattern of GB thickening (focal or diffuse), the presence of a hypoattenuated intramural nodule, and continuity of the mucosal line. Two radiologists evaluated these images independently and by consensus for the diagnosis.
Statistical computations were performed with JMP (SAS Institute, Cary, North Carolina, United States). Continuous variables were expressed as medians and were compared using the Wilcoxon test, whereas categorical variables were compared using Fisher’s exact test or the χ2 test. A P value of less than 0.05 indicated statistical significance.
The clinical symptoms, laboratory findings, and CT findings are summarized in Table 1. Based on the preoperative image findings, we suspected GB carcinoma in all cases including XGC in this series. In addition, by pathological examination we found that the group of patients with XGC developed inflammatory disease after surgery.
| XGC(n = 6) | GB carcinoma (n = 16) | P value | |
| Age (mean ± SD, yr) | 64.3 ± 9.7 | 67.9 ± 12.0 | 0.5148 |
| Gender | 0.8558 | ||
| Male | 4 | 8 | |
| Female | 2 | 8 | |
| Abdominal pain | 0.4806 | ||
| Presence | 4 | 8 | |
| Absence | 2 | 8 | |
| Fever | 0.4726 | ||
| Yes | 1 | 1 | |
| No | 5 | 15 | |
| Jaundice | 0.6707 | ||
| Yes | 1 | 4 | |
| No | 5 | 12 | |
| Albumin (mean ± SD, mg/dL) | 3.8 ± 0.4 | 3.8 ± 0.3 | 0.8090 |
| Neutrophil/lymphocyte ratio (mean ± SD) | 2.1 ± 0.9 | 2.4 ± 1.0 | 0.6280 |
| CEA (mean ± SD) | 1.9 ± 0.6 | 3.1 ± 3.5 | 0.2205 |
| CA19-9 (mean ± SD) | 232.7 ± 488.5 | 354.0 ± 737.1 | 0.6806 |
| Cholecystolithiasis | 0.0116 | ||
| Yes | 5 | 4 | |
| No | 1 | 12 | |
| Diffuse gallbladder wall thickening (CT findings) | 0.1551 | ||
| Yes | 3 | 3 | |
| No | 3 | 13 | |
| Continuous mucosal line (CT findings) | 0.0616 | ||
| Yes | 3 | 2 | |
| No | 3 | 14 | |
| Intramural hypoattenuated nodule | 0.0024 | ||
| Yes | 3 | 1 | |
| No | 3 | 15 | |
| Bile duct dilatation | 0.9035 | ||
| Yes | 1 | 3 | |
| No | 5 | 13 | |
Patients with XGC tended to have abdominal pain (4/6, 67%). However, there was no significant difference in clinical symptoms, including fever, between the two groups. Serum albumin and NLR were also similar in the two groups. Serum tumor markers, such as CEA and CA19-9, were increased in patients with GB cancer. However, no significant differences in tumor markers were identified.
On the other hand, gallstones were more frequent in patients with XGC (5/6, 83%) than in patients with GB cancer (4/16, 33%) (P = 0.0116). A hypoattenuated intramural nodule was found in 3 patients with XGC (3/6, 50%), but in only 1 patient with GB cancer (1/16, 6%) (P = 0.0024). The GB thickness, continuous mucosal line, and bile duct dilatation were not significantly different between XGC and GB cancer.
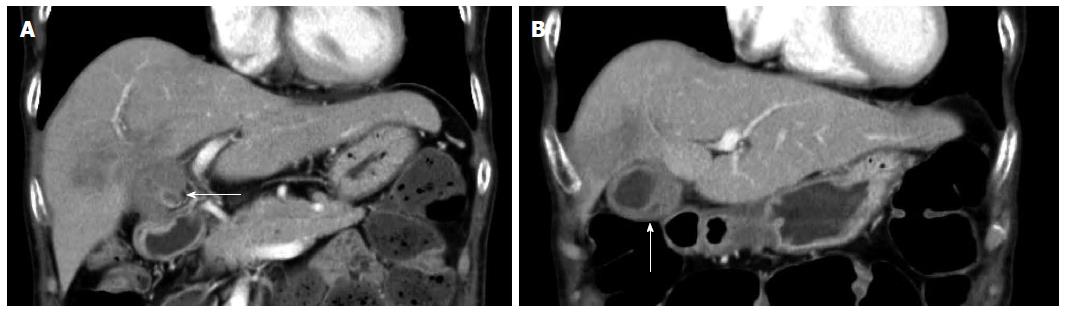
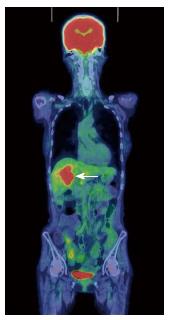
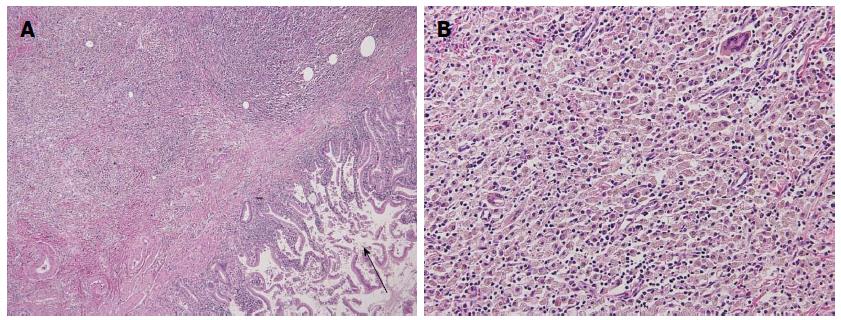
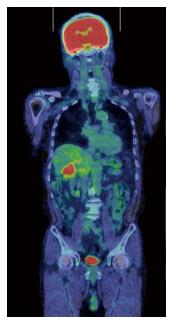
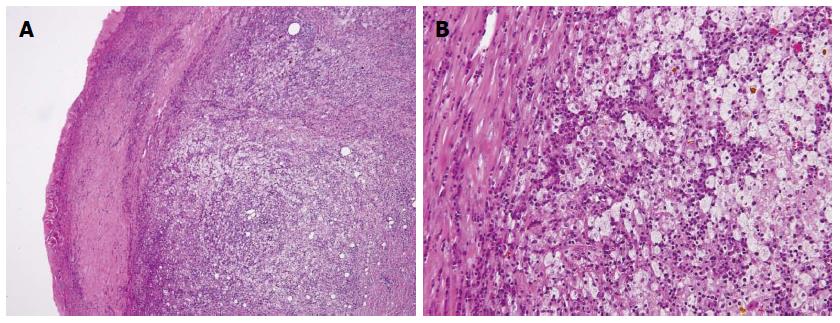
A 70-year-old woman was admitted to our hospital with abnormal findings on abdominal CT during follow-up of rectal cancer after a low anterior resection. She had neither fever nor abdominal pain. On admission, laboratory data, including tumor markers, were nearly normal. A CT scan showed a large mass and stone (arrow) with suspected hepatic invasion (Figure 1A). Moreover, CT findings detected an asymmetrically thickened GB wall with homogenous enhancement that was continuous along the mucosal line and a submucosal hypoattenuated nodule (arrow) (Figure 1B). Positron emission tomography with fluorine-18-labeled fluoro-deoxyglucose (FDG-PET) showed increased uptake at the tumor site, and the maximum standardized uptake value (SUV) was 10.2 (arrow) (Figure 2). Assuming an advanced GB carcinoma, we performed an extended right hepatectomy after portal vein embolization. Histologically, the GB mucosa showed hyperplasia, however, no atypical cells or malignant cells were observed (arrow) (Figure 3A). The adjacent liver showed diffuse inflammatory infiltrates consisting of giant histiocytes and foamy histiocytes with clear lipid-containing cytoplasm (xanthoma cells), lymphocytes, and polymorphonuclear cells (Figure 3B).
The postoperative course was uneventful, and the patient was discharged on the 21st postoperative day.
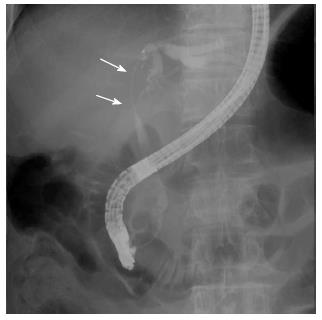
A 72-year-old man was found to be jaundiced during a medical examination. He was admitted to the local hospital for evaluation. Endoscopic retrograde cholangiography demonstrated a filiform stenosis of the proximal common bile duct and bifurcation with left intrahepatic bile duct dilatation. The right hepatic branch was not visualized on cholangiography (Figure 4). A complete sphincterotomy was performed with insertion of an internal stent for drainage.
CT confirmed the hypodense mass at the hilum (Figure 5A) and intrahepatic bile duct dilatation caused by this tumor (Figure 5B). The patient was then transferred to our hospital with the diagnosis of advanced GB cancer.
Tumor markers CA19-9 and CEA were not elevated, with values of 14 U/mL and 2.4 U/mL, respectively. However, FDG-PET demonstrated intense FDG activity at the hepatic hilum, which suggested GB carcinoma (SUVmax 5.2) (Figure 6). Therefore, the patient was diagnosed with advanced GB cancer and an extended right lobectomy with extirpation of the extrahepatic bile duct was planned. During the operation, a hard and thickened GB wall was identified at the hepatic hilum. A frozen section of the GB was negative for carcinoma. However, as there were many intrahepatic stones and severe bile duct stenosis at the right bile duct bifurcation, we performed a posterior hepatic resection with extirpation of the extrahepatic bile duct. Reconstruction was performed with a Roux-en-Y hepaticojejunostomy. Histologically, the GB wall was markedly thickened with severe inflammation and fibrosis (Figure 7A). Large xanthoma cells with clear-to-foamy lipid-containing cytoplasm and interspersed lymphocytes invaded the liver (Figure 7B). These findings are characteristic of xanthogranulomatous inflammation of the GB. The patient developed cholangitis after the operation. However, he was cured by conservative therapy and discharged approximately 2 mo after surgery.
Xanthogranulomatous cholecystitis (XGC) is a rare form of chronic cholecystitis and is seen in 1.3% to 5.2% of resected GB specimens[4,9]. The characteristic macroscopic findings of XGC include abnormal thickening of the GB wall with poorly demarcated soft-to-firm, yellow-brown intramural nodules of various sizes with cholecystitis[2,5]. Complications include GB perforation, abscess formation, fistulous tracts to the duodenum, and extension of the inflammatory process to adjacent abdominal organs, such as the liver and transverse colon[10]. These features that involve adjacent organs indicate that XGC develops aggressively, as does advanced GB cancer. Therefore, it is important to differentiate XGC from advanced GB cancer preoperatively to avoid unnecessary surgical treatment.
The clinical manifestations of XGC usually involve acute or chronic cholecystitis. The primary symptoms include right hypochondriac pain (93.9%), radiating shoulder and back pain (42.4%), fever (24.2%), nausea (33.3%), and vomiting (24.2%)[5]. Abdominal pain, jaundice, and fever are more frequently observed in patients with XGC as compared to patients with GB cancer[11]. However, these symptoms and signs are usually not helpful in differentiating these two conditions, except in advanced cases of malignancy presenting with weight loss or features of ascites or metastases. Despite the tendency for XGC patients in our study to have abdominal pain, it is difficult to differentiate XGC from GB cancer based only on symptoms.
The formation of XGC is thought to start as biliary obstruction with acute or chronic cholecystitis and increasing intra-gallbladder pressure, followed by a granulomatous reaction. Although the pathogenesis of this granulation is not well understood, it has been postulated that obstruction of the GB outflow leads to extravasation of bile into the GB wall, with involvement of the Rokitansky-Aschoff sinuses, or extravasation through a small ulceration in the mucosa. This causes a granulation reaction that leads to the formation of intramural nodules[4,12]. This inflammatory process is often extensive and may extend to adjacent organs, such as the liver, duodenum, and transverse colon. Dense adhesions with a large mass of inflammatory tissue surrounding the GB are then formed. Our six cases of XGC had inflammatory reactions that extended to the liver and adhesions of the GB to adjacent organs, such as the transverse colon and/or the duodenum; thus, we misdiagnosed them as advanced GB cancer and performed extended radical surgery, including liver resection in all six cases.
Extravasated bile causes histiocytes to accumulate in an effort to phagocytose insoluble cholesterol. A fibrous reaction and scarring result due to healing of the inflammatory reaction. Microscopically, the early stage of XGC is characterized by a large number of foamy histiocytes with clear lipid-containing cytoplasm and acute inflammatory cells, including lymphocytes, neutrophils, and plasma cells. In the later stage, a fibrous reaction occurs and extends to adjacent structures, such as the liver, omentum, duodenum, or colon[13]. The low-attenuation appearance of XGC nodules on CT is due to the histiocytes that have phagocytosed the extravasated bile and bile lipids and then accumulated in the GB wall. Kim et al[14] reported that intramural nodules were seen histologically in all patients with XGC, but radiologically in only 53% (10/19).
Imaging modalities are able to detect abnormalities in the GB, but are not always able to differentiate advanced GB cancer from XGC. The imaging characteristics of XGC closely resemble those of GB carcinoma in terms of thickening of the GB wall and the tendency to involve neighboring organs. However, Uchiyama et al[6] reported that an enhanced continuous mucosal line helped in the diagnosis of XGC. Moreover, the presence of gallstones with these findings indicates a high likelihood of XGC. On the other hand, there is some debate as to the coexistence of stones with GB cancer. In our study, the patients with XGC were associated with a higher incidence of gallstones than the patients with GB carcinoma (P = 0.0116). However, due to the limited number of cases, we could not conclude whether the existence of a gallstone is helpful in the differential diagnosis between XGC and GB carcinoma. In patients with GB carcinoma, the malignant process greatly disrupts the mucosal layer and the underlying muscle layer. Ultrasonographic characteristics of XGC include moderate-to-marked thickening of the GB wall with oval hypoechoic nodules[15,16]. Kim et al[17] reported that the combined ultrasonographic findings of diffuse wall thickening and intramural nodule formation are highly suggestive of XGC. FDG-PET may identify characterizing lesions of the GB[18]. However, XGC shows a positive image due to FDG uptake by active inflammatory cells[19]. In two of our patients showing XGC, the SUV of the tumor was also high, so we could not differentiate GB cancer from XGC by the SUV value. Therefore, FDG-PET would be expected to give a false positive result with cholecystitis, including XGC. A recent case report demonstrated that XGC showed FDG uptake on positron emission tomography which mimicked that of GB carcinoma[20]. FDG-PET may not be very useful in differentiating XGC from carcinoma, as inflammatory lesions also show increased FDG uptake.
XGC can be more easily mistaken for GB cancer macroscopically than radiologically, especially in patients with XGC and severe proliferative fibrosis involving the GB and surrounding organs. The combination of a gross check of the mucosa with frozen section examination, particularly in areas highly indicative of cancer, is more accurate for differentiating XGC from GB cancer and for excluding the simultaneous presence of XGC and GB cancer[21]. On the other hand, in cases showing extensive invasion of extra-gallbladder organs, the surgical strategy should not be determined only by frozen section examination, since it can give false negative results[2,22,23]. Moreover, it is estimated that XGC and GB cancer coexist in up to 12% of cases[16]. Therefore, even if a preoperative diagnosis is made with fine-needle aspiration cytology[24], it is important to be aware of the possible coexistence of XGC and cancer in the same GB. Zhuang et al[25] demonstrated that XGC is precancerous in nature, mainly depending on oncogenes such as BCL-2 and c-Myc, but not via the pathway associated with anti-oncogenes. Therefore, in addition to several frozen section examinations, careful gross observation during surgery is needed even if the pre-operative diagnosis is XGC.
With regard to the treatment of XGC, we must show skepticism with advanced GB cancer. If patients demonstrate features of XGC during preoperative examination, we need to perform fine-needle aspiration cytology of the GB preoperatively[24]. However, radiological differentiation from cancer can be extremely difficult in some cases in the presence of severe inflammation. In addition, although XGC is not believed to be a premalignant lesion, the frequency of coexisting XGC and GB cancer is nearly 10%[4]. Moreover, most of the reported cases with XGC and GB cancer were discovered by histologic examination of the cholecystectomy specimen[3]. Careful gross observation during surgery and several frozen section examinations are necessary to treat XGC which can extend to surrounding organs.
In conclusion, pseudotumoral XGC has puzzled surgeons in terms of surgical treatment. Despite the use of modern imaging techniques, a differential diagnosis between XGC and malignant GB lesions is often difficult. Even intraoperative differential diagnosis of XGC from GB carcinoma remains a challenge when XGC is associated with tumor formation and adhesions to adjacent organs. As GB carcinoma and XGC may coexist, radical resection, such as liver resection, is justified when malignancy cannot be completely excluded. However, in view of the small number of patients in this study, additional studies on a larger scale are warranted.
Xanthogranulomatous cholecystitis (XGC) is a rare inflammatory disease of the gallbladder. XGC is thought to start as a biliary obstruction with acute or chronic cholelithiasis and increasing intra-gallbladder pressure. This pressure provokes a rupture of the Rokitansky-Aschoff sinuses or mucosal ulcer with extravasation of bile in the interstitial tissues and a consequent xanthogranulomatous inflammatory reaction. This inflammatory process is often extensive and may extend to adjacent organs, forming dense adhesions with a large mass of inflammatory tissue surrounding the gallbladder. Differentiating XGC and malignant gallbladder lesions is often difficult, especially in patients with severe proliferative fibrosis involving the gallbladder and surrounding organs.
XGC often mimics gallbladder carcinoma, and may coexist with carcinoma, leading to a diagnostic dilemma. Characteristic pathological, radiological and clinical features are sometimes similar to those of gallbladder carcinoma and contribute to considerable treatment inaccuracy.
Although XGC is often difficult to differentiate from gallbladder carcinoma, it is possible to obtain an accurate diagnosis by careful intraoperative gross observation and several intraoperative frozen sections and could prevent extended resections.
XGC with severe proliferative fibrosis involving the gallbladder and surrounding organs requires careful intraoperative gross observation and several intraoperative frozen sections.
The xanthogranulomatous process is a form of acute and chronic inflammation characterized by a large number of foamy histiocytes with clear lipid-containing cytoplasm and acute inflammatory cells. In the later stage, a fibrous reaction occurs and extends to adjacent structures, such as the liver, duodenum or colon.
XGC is a rare benign disease of the gallbladder. It is difficult to identify with GB cancer before operation. The author tries to make a summary. This article has a better clinical value and is designed reasonably.
P- Reviewer: Cariati A, Lee KG, Ma YH, Wang W, Xu Z S- Editor: Ma YJ L- Editor: Webster JR E- Editor: Liu XM
| 2. | Spinelli A, Schumacher G, Pascher A, Lopez-Hanninen E, Al-Abadi H, Benckert C, Sauer IM, Pratschke J, Neumann UP, Jonas S. Extended surgical resection for xanthogranulomatous cholecystitis mimicking advanced gallbladder carcinoma: A case report and review of literature. World J Gastroenterol. 2006;12:2293-2296. [PubMed] |
| 3. | Benbow EW. Xanthogranulomatous cholecystitis associated with carcinoma of the gallbladder. Postgrad Med J. 1989;65:528-531. [PubMed] |
| 4. | Roberts KM, Parsons MA. Xanthogranulomatous cholecystitis: clinicopathological study of 13 cases. J Clin Pathol. 1987;40:412-417. [PubMed] |
| 5. | Yang T, Zhang BH, Zhang J, Zhang YJ, Jiang XQ, Wu MC. Surgical treatment of xanthogranulomatous cholecystitis: experience in 33 cases. Hepatobiliary Pancreat Dis Int. 2007;6:504-508. [PubMed] |
| 6. | Uchiyama K, Ozawa S, Ueno M, Hayami S, Hirono S, Ina S, Kawai M, Tani M, Yamaue H. Xanthogranulomatous cholecystitis: the use of preoperative CT findings to differentiate it from gallbladder carcinoma. J Hepatobiliary Pancreat Surg. 2009;16:333-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Kondo S, Takada T, Miyazaki M, Miyakawa S, Tsukada K, Nagino M, Furuse J, Saito H, Tsuyuguchi T, Yamamoto M. Guidelines for the management of biliary tract and ampullary carcinomas: surgical treatment. J Hepatobiliary Pancreat Surg. 2008;15:41-54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 157] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 8. | McNamara MG, Templeton AJ, Maganti M, Walter T, Horgan AM, McKeever L, Min T, Amir E, Knox JJ. Neutrophil/lymphocyte ratio as a prognostic factor in biliary tract cancer. Eur J Cancer. 2014;50:1581-1589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 118] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 9. | Yoshida J, Chijiiwa K, Shimura H, Yamaguchi K, Kinukawa N, Honda H, Tanaka M. Xanthogranulomatous cholecystitis versus gallbladder cancer: clinical differentiating factors. Am Surg. 1997;63:367-371. [PubMed] |
| 10. | Lee KC, Yamazaki O, Horii K, Hamba H, Higaki I, Hirata S, Inoue T. Mirizzi syndrome caused by xanthogranulomatous cholecystitis: report of a case. Surg Today. 1997;27:757-761. [PubMed] |
| 11. | Chang BJ, Kim SH, Park HY, Lim SW, Kim J, Lee KH, Lee KT, Rhee JC, Lim JH, Lee JK. Distinguishing xanthogranulomatous cholecystitis from the wall-thickening type of early-stage gallbladder cancer. Gut Liver. 2010;4:518-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Goodman ZD, Ishak KG. Xanthogranulomatous cholecystitis. Am J Surg Pathol. 1981;5:653-659. [PubMed] |
| 13. | Pinocy J, Lange A, König C, Kaiserling E, Becker HD, Kröber SM. Xanthogranulomatous cholecystitis resembling carcinoma with extensive tumorous infiltration of the liver and colon. Langenbecks Arch Surg. 2003;388:48-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Kim PN, Lee SH, Gong GY, Kim JG, Ha HK, Lee YJ, Lee MG, Auh YH. Xanthogranulomatous cholecystitis: radiologic findings with histologic correlation that focuses on intramural nodules. AJR Am J Roentgenol. 1999;172:949-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 39] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Lichtman JB, Varma VA. Ultrasound demonstration of xanthogranulomatous cholecystitis. J Clin Ultrasound. 1987;15:342-345. [PubMed] |
| 16. | Parra JA, Acinas O, Bueno J, Güezmes A, Fernández MA, Fariñas MC. Xanthogranulomatous cholecystitis: clinical, sonographic, and CT findings in 26 patients. AJR Am J Roentgenol. 2000;174:979-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 84] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | Kim PN, Ha HK, Kim YH, Lee MG, Kim MH, Auh YH. US findings of xanthogranulomatous cholecystitis. Clin Radiol. 1998;53:290-292. [PubMed] |
| 18. | Koh T, Taniguchi H, Yamaguchi A, Kunishima S, Yamagishi H. Differential diagnosis of gallbladder cancer using positron emission tomography with fluorine-18-labeled fluoro-deoxyglucose (FDG-PET). J Surg Oncol. 2003;84:74-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 75] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 19. | Anderson CD, Rice MH, Pinson CW, Chapman WC, Chari RS, Delbeke D. Fluorodeoxyglucose PET imaging in the evaluation of gallbladder carcinoma and cholangiocarcinoma. J Gastrointest Surg. 2004;8:90-97. [PubMed] |
| 20. | Makino I, Yamaguchi T, Sato N, Yasui T, Kita I. Xanthogranulomatous cholecystitis mimicking gallbladder carcinoma with a false-positive result on fluorodeoxyglucose PET. World J Gastroenterol. 2009;15:3691-3693. [PubMed] |
| 21. | Zhang LF, Hou CS, Liu JY, Xiu DR, Xu Z, Wang LX, Ling XF. Strategies for diagnosis of xanthogranulomatous cholecystitis masquerading as gallbladder cancer. Chin Med J (Engl). 2012;125:109-113. [PubMed] |
| 22. | Aoki T, Tsuchida A, Kasuya K, Inoue K, Saito H, Koyanagi Y. Is frozen section effective for diagnosis of unsuspected gallbladder cancer during laparoscopic cholecystectomy? Surg Endosc. 2002;16:197-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Rastogi A, Singh DK, Sakhuja P, Gondal R. Florid xanthogranulomatous cholecystitis masquerading as invasive gallbladder cancer leading to extensive surgical resection. Indian J Pathol Microbiol. 2010;53:144-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Krishnani N, Shukla S, Jain M, Pandey R, Gupta RK. Fine needle aspiration cytology in xanthogranulomatous cholecystitis, gallbladder adenocarcinoma and coexistent lesions. Acta Cytol. 2000;44:508-514. [PubMed] |
| 25. | Zhuang PY, Zhu MJ, Wang JD, Zhou XP, Quan ZW, Shen J. Xanthogranulomatous cholecystitis: a clinicopathological study of its association with gallbladder carcinoma. J Dig Dis. 2013;14:45-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |