Published online Sep 21, 2015. doi: 10.3748/wjg.v21.i35.10104
Peer-review started: January 10, 2015
First decision: March 13, 2015
Revised: April 25, 2015
Accepted: June 15, 2015
Article in press: June 15, 2015
Published online: September 21, 2015
Processing time: 250 Days and 11.8 Hours
AIM: To investigate the effects of salvianolic acid B (Sal B) on the morphological characteristics and functions of liver mitochondria of rats with nonalcoholic steatohepatitis (NASH).
METHODS: A total of 60 male Sprague-Dawley rats were randomly divided into three groups: (1) a normal group fed a normal diet; (2) an NASH model group; and (3) a Sal B-treated group fed a high-fat diet. Two rats from each group were executed at the end of the 12th week to detect pathological changes. The rats in the Sal B-treated group were gavaged with 20 mL/kg Sal B (1 mg/mL) daily. The model group received an equal volume of distilled water as a control. At the end of the 24th weekend, the remaining rats were executed. Serum biochemical parameters and liver histological characteristics were observed. Malondialdehyde (MDA) and superoxide dismutase (SOD) in the liver were determined. Protein expression of CytC and caspase-3 was determined by immunohistochemistry. The mRNA transcripts of mitofusin-2 (Mfn2) and NF-κB in the liver tissue were detected by real-time PCR. Mitochondrial membrane potential was detected using a fluorescence spectrophotometer. Mitochondrial respiratory function was detected using a Clark oxygen electrode.
RESULTS: The model group showed significantly higher ALT, AST, TG, TC and MDA but significantly lower SOD than the normal group. In the model group, the histological characteristics of inflammation and steatosis were also evident; mitochondrial swelling and crest were shortened or even disappeared. CytC (18.46 ± 1.21 vs 60.01 ± 3.43, P < 0.01) and caspase-3 protein expression (30.26 ± 2.56 vs 83.31 ± 5.12, P < 0.01) increased significantly. The mRNA expression of NF-κB increased (0.81 ± 0.02 vs 0.91 ± 0.03, P < 0.05), whereas the mRNA expression of Mfn2 decreased (1.65 ± 0.31 vs 0.83 ± 0.16, P < 0.05). Mitochondrial membrane potential also decreased and breathing of rats was weakened. Steatosis and inflammation degrees in the treatment group were significantly alleviated compared with those of the model group. In the treatment group, mitochondrial swelling was alleviated. CytC (60.01 ± 3.43 vs 30.52 ± 2.01, P < 0.01) and caspase-3 protein expression (83.31 ± 5.12 vs 40.15 ± 3.26, P < 0.01) significantly decreased. The mRNA expression of NF-κB also decreased (0.91 ± 0.03 vs 0.74 ± 0.02, P < 0.01), whereas the mRNA expression of Mfn2 increased (0.83 ± 0.16 vs 1.35 ± 0.23, P < 0.01). Mitochondrial membrane potential increased and respiratory function was enhanced.
CONCLUSION: Sal B can treat NASH by protecting the morphological characteristics and functions of liver mitochondria, regulating lipid metabolism, controlling oxidative stress and lipid peroxidation and inhibiting apoptosis.
Core tip: This study applied salvianolic acid B (Sal B) as intervention for rats with nonalcoholic steatohepatitis (NASH). We also observed the changes in biochemical indexes, mitochondrial morphological characteristics and functions and apoptosis index before and after Sal B treatment was administered to explore the pathogenesis of NASH and therapeutic effects of Sal B. Sal B can treat NASH by protecting the morphological characteristics and functions of liver mitochondria, regulating lipid metabolism, controlling oxidative stress and lipid peroxidation and inhibiting apoptosis.
- Citation: Wang YC, Kong WZ, Jin QM, Chen J, Dong L. Effects of salvianolic acid B on liver mitochondria of rats with nonalcoholic steatohepatitis. World J Gastroenterol 2015; 21(35): 10104-10112
- URL: https://www.wjgnet.com/1007-9327/full/v21/i35/10104.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i35.10104
Non-alcoholic steatohepatitis (NASH) is a pathological type of nonalcoholic fatty liver diseases (NAFLD); however, the pathogenesis of NASH remains unclear and is possibly because of heredity, environment, metabolism and stress. This disease can also progress to liver cirrhosis and liver cancer, and thus, NASH is a chronic liver disease extensively investigated in China[1-3]. Sanyal et al[4] indicated that NASH is associated with an increased risk of diabetes, hypertension and cardiovascular diseases; NASH can also increase the mortalities of patients with cardiovascular diseases and malignant tumours. Liver mitochondria play an important role in the pathogenesis of NAFLD[5]. Free fatty acids (FFAs) can be oxidised in liver mitochondria; as a result, excessive reactive oxygen species (ROS) are produced, the body’s anti-oxidation and auxo-oxidation become unstable and oxidative stress and lipid peroxidation occur. FFAs are also the theoretical foundation of the “secondary attack” of NAFLD[6-8]. In this study, salvianolic acid B (Sal B) was applied as intervention for rats with NASH. We also observed the changes in biochemical indexes, mitochondrial morphological characteristics and functions and apoptosis index before and after Sal B treatment was administered to investigate the pathogenesis of NASH and the therapeutic effects of Sal B.
A total of 60 clean-grade male Sprague-Dawley rats, weighing 150 ± 10 g, were purchased from the Laboratory Animal Centre of Dalian Medical University. After the adaptive feed was administered for one week, the rats were randomly divided into three groups: (1) a normal group fed a normal diet; (2) an NASH model group; and (3) a Sal B-treated group fed a high-fat diet (88% ordinary feed + 10% lard + 2% cholesterol produced by Beijing HuaFuKang Biotechnology Co., Ltd., China). The experimental animals were fed individually and provided free access to water and diet. Room temperature was kept at 20 °C ± 2 °C; illumination was adjusted according to normal circadian rhythm. Two rats from each group were sacrificed at the end of 12th week to confirm the success of NASH modelling. The treatment group and the model group were intragastrically treated with Sal B solution daily (concentration = 1 mg/mL; 20 mL/kg body weight; Sichuan Plantpharm Chemical Co., Ltd., China) and with distilled water (20 mL/kg body weight), respectively; each group was then fed the same diet provided before treatment was administered. At the end of the 24th week, the rats were subjected to fasting overnight and anesthetized by intraperitoneally injecting 10% chloral hydrate solution (2.0 mL/kg body weight) on the next day. After inferior venous blood samples were obtained, the rats were sacrificed. The liver was quickly removed, and the colour, texture and weight of the liver were observed macroscopically to calculate liver index [liver index = liver weight/body weight (100%)].
A Vitros5.1 FS automatic biochemical analyser (J&J, United States) was used to determine ALT, AST, TG and TC. Liver tissues were homogenized, and Coomassie brilliant blue method was used to quantify proteins. Thiobarbital acid method was used to determine MDA content, and colorimetry was performed to detect SOD. Kits were bought from Nanjing Keygen Biotech Co., Ltd., China.
Liver tissues were obtained from the same site on the right lobe, fixed in neutral formaldehyde, embedded in paraffin, serially sectioned and stained with HE. The degrees of hepatic steatosis, inflammatory reactions and balloon-like changes of the samples were evaluated according to a previously described method[9].
Approximately 0.5 mm3 to 1 mm3 of fresh liver tissue was obtained and rapidly placed in a clean small bottle containing 2 to 3 mL of precooled fixation fluid (2.5% glutaraldehyde) for 2 h of internal fixation at 4 °C. The samples were then washed with 0.1 mol/L phosphate buffer several times, fixed with 1% OsO4 for 2 h, rinsed thoroughly with 0.1 mol/L phosphate buffer thrice (15 min each), and serially dehydrated with 50%, 70%, 90% and 100% ethanol. The samples were soaked in pure acetone and embedding agent mixture (2:1, v/v) at room temperature for 2 h and then soaked in pure acetone and embedding agent mixture (1:2, v/v) at room temperature for 3 h. The samples were embedded in configured 618 epoxy resin overnight at 37 °C and solidified at 60 °C for 48 h. The samples were sliced using Leica LKB-1 ultra-thin slicing machine (Sweden) and stained with lead citrate and uranyl acetate. Afterwards, the samples were observed under a Tecnai Spirit 120 kV transmission electron microscope (FEI, United States) and photographs were obtained. The rats of each group were randomly selected for three samples, which were randomly selected for five photographing visions under the same magnification; each vision was randomly selected for 10 mitochondria for further analysis. Scoring was performed in accordance with Flameng Grading method[10]: level 0 (0 points), mitochondrial structure is normal and full of particles; level I (1 point), mitochondrial structure is normal but loses some matrix particles (there is mild swelling, matrix density decreases and ridge is separated); level II (2 points), the mitochondria swell (matrix density is severely reduced and ridge is separated), the matrix is transparent and the ridge is not broken; level III (3 points), the mitochondrial ridge is fractured, and the matrix solidifies (severely swells); and level IV (4 points), outer and inner mitochondrial membranes completely disappear and are vacuolated (severe swelling, accompanied with ridge fracture, outer and inner membrane rupture). The mean of each group was used as the final score of each group.
Liver tissues were fixed, embedded in paraffin and sectioned in accordance with the manufacturer’s instructions provided with the CytC and caspase-3 immunohistochemical kit (Nanjing KeyGen). Phosphate buffer was used to replace primary antibody as a negative control. Image-Pro Plus 6.0 Professional Image analysis software was used for the semi-quantitative analysis of the immunohistochemical images of each group.
Approximately 40 mg of liver tissues were obtained, and total RNA was extracted using a pillar animal tissue total RNA extraction-purification kit and quantified by spectrophotometry. Gel electrophoresis (1.0%) was performed to identify the integrity of total RNA. According to the instructions of M-MuLV first-strand cDNA synthesis kit, total RNA was reversely transcribed to cDNA; cDNA was then used as a template to detect gene transcripts of NF-κB and mitofusin-2 (Mfn2) according to the instructions of fluorescence quantitative PCR kit (SYBR staining method). GAPDH was used as an internal reference. All of the PCR reagents and primers were purchased from Shanghai Sangon Biological Engineering Co., Ltd., China. Forward and reverse primer sequences of each gene are as follows: NF-κB: 5’-TCT GTT TCC CCTS CAT CTT TCC C-3’, 5’-GTC TTA GTG GTA TCT GTG CTT CTC-3’; Mfn2: 5’-AAT TTC GAG AGG CGA TTT GA-3’, 5’-TGG GTG AAA GTC CAT CTG GT-3’; GAPDH: 5’-ACC ACA GTC CAT GAC ATC AC-3’, 5’-TCC ACC ACC CTG TTG CTG TA-3’. Three samples were selected from each group and re-detected thrice. The 2-∆∆CT method[11] was used to calculate the relative expression of target genes.
Mitochondrial separation medium (100 mL; Nanjing Keygen) was precooled at 4 °C; fresh liver tissues (1 g) were placed in precooled mitochondrial separation medium (5 mL) and cut into pieces. Afterwards, the liver tissues were subjected to intermittent homogenization in an ice bath glass homogenator for 5-7 times. The tissues were then transferred into a 50 mL centrifuge tube, mixed evenly with 35 mL of mitochondrial separation medium and centrifuged in a low temperature high-speed centrifuge machine at 600 g for 7 min. The precipitate was discarded; the supernatant was poured into a precooled clean centrifuge tube and centrifuged at 8000 g for 10 min. The supernatant was discarded; the precipitate was washed with the separation medium and centrifuged at 8000 g for another 10 min. Afterwards, the supernatant was again discarded, and 2 mL of bovine serum-free albumin preservation medium was added to prepare the mitochondrial suspension. A part of this suspension was obtained and subjected to protein quantification; the remaining part of the suspension was added to an ice bath of bovine serum albumin for subsequent determination.
The excitation wavelength of fluorescence spectrophotometer was set at 500 nm, and the emission wavelength was set at 525 nm. Approximately 1 μL of rhodamin-123 and 2 mL of reaction medium of the mitochondrial membrane potential were added to the test tube and mixed evenly to detect basic fluorescence value F1 (Nanjing KeyGen). The liquid was sucked into the original tube, and 100 μL of mitochondrial suspension was added; the resulting mixture was incubated at 25 °C for 15 min and centrifuged at 5000 g for 10 min. The fluorescence value F2 of the supernatant was then detected. The changed value of the fluorescence caused by 1 mg of mitochondria was then calculated as follows: ∆F = (F1-F2)/(0.1 mL × 5 mg/mL), where the amount of rhodamin-123 combined with the mitochondrial matrix reflected the value of average mitochondrial membrane potential.
Oxygen electrode method was used to detect mitochondrial oxygen consumption. Mitochondria (1 mg) were added to the reaction pool at 37 °C; the reaction medium was preheated at 37 °C and the total volume was adjusted to 2 mL. Malic acid (20 μL), sodium glutamate (0.5 mmol/L) and ADP (4 μL; 0.1 mmol/L) were added to determine ADP-free oxygen consumption rate (ST4) and oxygen consumption rate after ADP (ST3) was added. The respiratory control rate (RCR) was the rate of ST3 to ST4. The ratio of the added amount of ADP to ST3 oxygen consumption was P/O ratio (P/O).
SPSS13.0 was used to statistically analyse data; measurement data are expressed as mean ± SD. One-way ANOVA and pairwise comparison were performed. P < 0.05 was considered statistically significant.
The statistical methods of this study were reviewed by Hong Li from Dalian University.
Compared with the normal group, the NASH model group exhibited slightly increased liver volume, whereas the liver edge became dull, the color became yellow, the tangent plane became greasy and the texture became brittle. Compared with model group, the hepatic color and texture of the Sal B treatment group were improved. The model group exhibited the highest liver index, which exhibited a statistically significant difference from the normal group and the treatment group (F = 37.97, P < 0.01; Table 1).
| Group | n | Liver index (%) | TG (mmol/L) | TC (mmol/L) | ALT (U/L) | AST (U/L) |
| Normal group | 18 | 2.35 ± 0.18 | 0.35 ± 0.12 | 0.91 ± 0.05 | 45.23 ± 2.23 | 83.52 ± 6.67 |
| Model group | 18 | 3.80 ± 0.30 | 0.73 ± 0.15 | 1.50 ± 0.07 | 85.12 ± 3.38 | 130.23 ± 3.23 |
| Treatment group | 18 | 3.20 ± 0.18 | 0.40 ± 0.07 | 1.10 ± 0.03 | 50.10 ± 1.67 | 85.30 ± 6.63 |
| F | 37.97 | 8.77 | 87.18 | 92.16 | 72.98 | |
| P value | 0.000 | 0.017 | 0.000 | 0.000 | 0.000 |
The levels of TG, TC, ALT and AST of the model group were higher than those of the normal group, whereas the above indicators of the treatment group were reduced to some extent when compared with the model group, and the differences were statistically significant (P < 0.05; Table 1). Compared with the normal group (0.63 ± 0.07 nmol/mg prot), the MDA of the model group (1.62 ± 0.05 nmol/mg prot) was increased, whereas MDA of the treatment group (0.70 ± 0.62 nmol/mg prot) was reduced than the model group, and the difference was statistically significant (F = 89.69, P < 0.05). The SOD of the model group (340 ± 23.79 U/mg prot) was reduced than that of the normal group (390 ± 38.91 U/mg prot), and the SOD of the treatment group (410 ± 30.62 U/mg prot) was increased compared with that of the model group, and the difference was statistically significant (F = 110.87, P < 0.05).
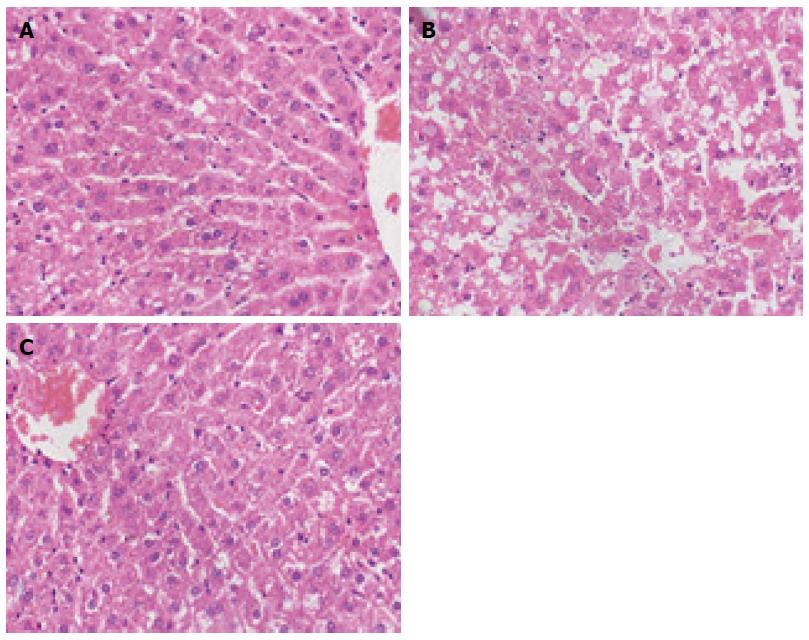
After HE staining was performed, the hepatic lobule structures of normal rats were clear, without steatosis or inflammatory infiltration. The model group exhibited disordered hepatic lobule structures, with various sizes of fat vacuoles inside the liver cells, which also had the balloon-like changes, as well as different levels of inflammatory infiltration and liver cell debris-like necrosis. A little bridging necrosis occurred, and partial portal areas exhibited mild to moderate inflammatory reactions. The degrees of liver cell fatty degeneration, necrosis and inflammatory reactions of the Sal B treatment group were obviously lower than those of the model group (Figure 1). The comparison of NAFLD activity score among the groups showed that the treatment group was significantly lower than the model group (F = 86.57, P < 0.01; Table 2).
| Group | n | Fatty degeneration of hepatocytes (0-3) | Liver lobular inflammatory reactions (0-3) | Balloon-like changes (0-2) | NAS (0-8) | |||||
| 0-1 | 2-3 | 0-1 | 2-3 | 0 | 1-2 | 0-2 | 3-4 | 5-8 | ||
| Normal group | 18 | 18 | 0 | 18 | 0 | 18 | 0 | 18 | 0 | 0 |
| Model group | 18 | 0 | 18 | 8 | 10 | 2 | 16 | 0 | 1 | 17 |
| Treatment group | 18 | 2 | 16 | 12 | 6 | 3 | 15 | 3 | 5 | 10 |
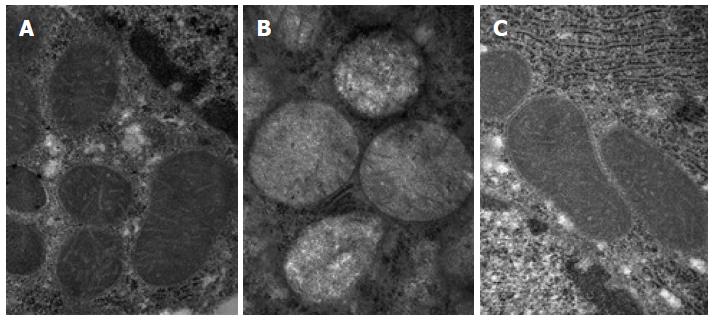
The electron microscopy revealed that the normal group exhibited much more numerous ovoid-shaped mitochondria, and the amounts of layer ridge were moderate. The mitochondria of the model group generally swelled to the ball-like shape, and the matrix was shallow. The mitochondrial swelling of the treatment group was alleviated, and the mitochondrial ridge was obvious (Figure 2). The Flameng semi-quantitative analysis of hepatic mitochondria showed that the differences among the three groups were statistically significant (F = 26.57, P < 0.01), the score of the model group (3.021 ± 0.302) was higher than that of the normal group (0.157 ± 0.033), and the difference was statistically significant (P < 0.01). The score of the treatment group (1.152 ± 0.223) was reduced than that of the model group, and the difference was statistically significant (P < 0.01).
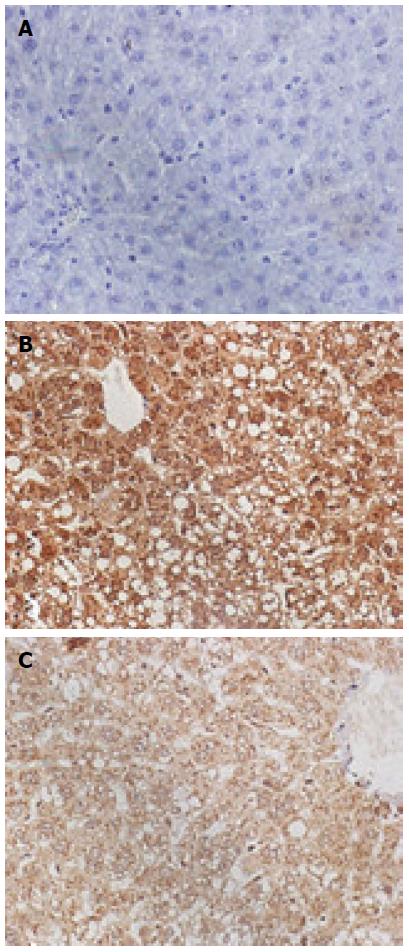
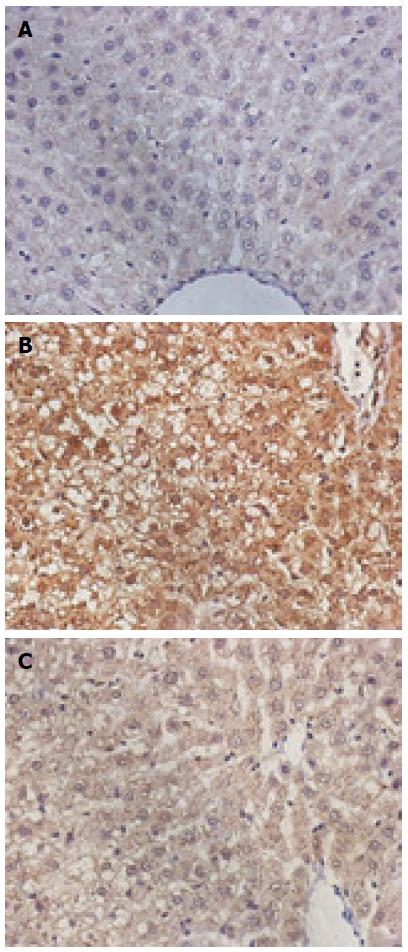
The CytC and caspase-3-positive cells exhibited the brown-stained cytoplasm. The expression of these two proteins in the model group was increased, the positive particles inside the liver cells were increased, and staining was deepened. The normal group exhibited small amounts of positive particles, and the positive particles of the treatment group were reduced. The average absorbance semi-quantitative analysis revealed that the expression of CytC protein exhibited statistically significant differences among the three groups (F = 38.67, P < 0.05). The expression of CytC in the normal group (18.46 ± 1.21) exhibited a statistically significant difference when compared with the model group (60.01 ± 3.43; P < 0.01), and the expression of CytC in the treatment group (30.52 ± 2.01) exhibited a statistically significant difference when compared with the model group (60.01 ± 3.43, P < 0.01). The expression of caspase-3 exhibited statistically significant differences among the three groups (F = 20.97, P < 0.05); the expression of caspase-3 in the normal (30.26 ± 2.56) and treatment groups (40.15 ± 3.26) exhibited a statistically significant difference when compared with the model group (83.31 ± 5.12; P < 0.01 for both) (Figures 3 and 4).
The mRNA expression levels of NF-κB in rat liver tissues were statistically significant among the three groups (F = 21.69, P < 0.05). NF-κB level in the model group (0.91 ± 0.03) was significantly increased compared with that of the normal group (0.81 ± 0.02, P < 0.05). NF-κB level in the treatment group (0.74 ± 0.02) was significantly reduced compared with that of the model group (P < 0.01). The mRNA expression levels of Mfn2 were also statistically significant among the three groups (F = 16.87, P < 0.05); Mfn2 level in the model group (0.83 ± 0.16) was decreased compared with that of the normal group (1.65 ± 0.31), and the difference was statistically significant (P < 0.01); Mfn2 level in the treatment group (1.35 ± 0.23) was significantly increased compared with that of the model group (P < 0.01).
The mitochondrial membrane potential exhibited statistically significant differences among the three groups (F = 85.73, P < 0.05). The mitochondrial membrane potential in the model group (573.51 ± 62.90) was significantly reduced when compared with that of the normal group (963.10 ± 59.81; P < 0.01); the mitochondrial membrane potential in the treatment group (788.31 ± 60.50) was significantly increased compared with that of the model group (P < 0.05).
The differences in mitochondrial RCR among the three groups were statistically significant (F = 15.62, P < 0.05). The RCR in the model group (3.31 ± 0.23) was significantly reduced compared with that of the normal group (4.86 ± 0.53), and RCR in the treatment group (3.79 ± 0.41) was significantly higher than that of the model group (P < 0.05). The P/O ratios among the three groups were also statistically significant (F = 13.92, P < 0.05); P/O ratio in the model group (3.41 ± 0.15) was significantly reduced compared with that of the normal group (4.15 ± 0.36, P < 0.01); P/O ratio in the treatment group (3.58 ± 0.30) was significantly increased compared with that of the model group (P < 0.05).
Although changing the lifestyle might be effective towards NASH treatment, this action would often be difficult to realise. Therefore, the development of new drugs and new treatment plans according to the pathogenesis of NASH would be very necessary[12]. Salvia miltiorrhiza was the dry root and rhizome of salvia plants, Labiatae, and its water-soluble active ingredient, Sal B, is condensed by three molecules of tanshinol and one molecule of caffeic acid, and has strong activities. Previous studies were more about the therapeutic effects of Sal B in treating cardiovascular diseases and liver fibrosis, which were mainly caused by Sal B activities, such as anti-oxidation, anti-inflammation and free-radical scavenging[13,14].
The mitochondrion is an important organelle inside the eukaryotic cells, and the cellular energy supply centre, the Krebs cycle, β-oxidation of fatty acid, oxidative phosphorylation and the urea cycle were all conducted inside the mitochondria[15]. The mitochondria also participated in processes such as regulating the intracellular Ca2+ concentration, information transmission and cell death. A large number of studies had shown that the mitochondrial structures and functions of NASH patients were abnormal[16-19]. The deacetylase gene Sirt3 of mitochondrion-knockout mice might make the lipid metabolism disordered, thus the fat toxicity was increased and NASH was formed[20-22]. Furthermore, the Mfn2 gene could promote the fusion of mitochondria, which was closely related to the mitochondrial morphology, structures and functions[23,24]. This study found that in the NASH rats, the liver mitochondria swelled, the matrix became shallower and the Mfn2 expression became weaker, which were consistent with the large amount of previous research. After Sal B treatment, the mitochondrial morphology basically returned to normal and the Mfn2 expression was enhanced, indicating that Sal B might protect the mitochondrial structures by Mfn2.
The energy needed for the lipid metabolism of liver cells is mainly created by mitochondria through the respiratory chain; the RCR and P/O are the main indexes that could reflect the phosphorylation activities and mitochondrial respiratory functions[25-27]. Once RCR is decreased, the energy metabolism disorders might occur, and the abilities of liver cells in processing and transporting fats are also reduced, thus leading to the hepatic fat deposition[28,29]. Moreover, domestic and foreign studies found that the mitochondrial energy metabolism disorders could cause the reduction of mitochondrial membrane potential, which is an important index to reflect the mitochondrial functions[30,31]. The reduction of mitochondrial membrane potential could cause the cytochrome C release and the activation of caspase-9 and caspase-3, hence promoting cell apoptosis[15]. This study showed that in the NASH model group, the mitochondrial membrane potential was decreased, the ratios of RCR and P/O were reduced, whereas the protein expression of CytC and caspase-3 was increased, hence confirming that the mitochondrial energy metabolism disorders occurred in the NASH rats, the functions were thus impaired, and apoptosis occurred. After Sal B treatment was administered, mitochondrial membrane potential was increased, ratios of RCR and P/O were increased and protein expression of CytC and caspase-3 was decreased; these results indicated that Sal B could stabilise mitochondrial membrane potential. Thus, energy metabolism was improved, mitochondrial functions were protected, protein expression of CytC and caspase-3 was reduced and apoptosis was inhibited.
The results of this study showed that serum biochemical indexes, such as TG, TC, ALT and AST of the NASH group were increased, and the mRNA expression of NF-κB was increased. The liver tissue inflammation was evident, and the expression of MDA was also increased, whereas SOD was decreased, hence conforming with the results of a number of related research that the increased fatty acids inside the NASH liver strengthened the oxidative phosphorylation of mitochondria; accumulated ROS, which would cause lipid peroxidation, increase MDA and damage mitochondrial structures and functions. Excessive ROS could activate NF-κB and promote the Kupffer cells to secrete a large amount of TNF-α and induce chemotaxis of neutrophils; as a result, hepatic inflammatory reactions and fibrosis occur[32,33]. Hence, mitochondrial structure and function disorders are closely related to fat metabolism, oxidative stress and lipid peroxidation and liver tissue inflammation. After Sal B treatment was administered, the expression of TG, TC, ALT and AST was reduced, the expression of NF-κB was weakened, the inflammation of liver tissues was relieved and the MDA expression was reduced, whereas the SOD expression was increased; these results indicated that Sal B could regulate fat metabolism, control oxidative stress and lipid peroxidation and inhibit mRNA expression of NF-κB via the mitochondria. Thus, NASH can be treated.
The results of this study showed that Sal B could protect mitochondrial structures and functions, regulate fat metabolism, control oxidative stress and lipid peroxidation and inhibit apoptosis; thus, Sal B elicits therapeutic effects on NASH. However, whether Sal B induces therapeutic effects via other mechanisms should be further investigated.
Non-alcoholic steatohepatitis (NASH) has complex pathogenesis, which is widely accepted as “two-hit” hypothesis. Mitochondria are the major source of reactive oxygen species (ROS), and the main target of oxidative attack. Long-term exposure to free fatty acids can increase the ROS formation, thus oxidative stress is induced and excessive oxygen free radicals are produced. These conditions can cause lipid peroxidation in cells and mitochondrial membrane; as a result, mitochondrial structure and function are disrupted. Salvianolic acid B (Sal B) performs anti-inflammatory, antioxidant and free radical scavenging functions; Sal B also elicits therapeutic effects on NASH.
The factors initiating the “second hit” include oxidative stress, lipid peroxidation, inflammatory factors, and mitochondrial dysfunction. The excessive storage of free fatty acids in the liver can cause a compensatory increase in mitochondria β-oxidation; thus, ROS accumulate and exceed the scavenging capacity of the antioxidant system. ROS can attack adjacent tissues and cells and destroy liver cell function and structural integrity, thereby causing liver cell rupture and apoptosis. Moreover, ROS can activate neutrophils; thus, inflammation, necrosis and fibrosis of the liver lobule occur.
Sal B can affect the structure and function of mitochondria to ameliorate oxidative stress and elicit therapeutic effect on NASH. This study provided a theoretical basis for the clinical application of Sal B to treat NASH.
Mitochondrial dysfunction is involved in the development of NASH. Sal B can affect the structure and function of the mitochondria, regulate energy metabolism, alleviate oxidative stress and lipid peroxidation and inhibit apoptosis. Sal B also elicits therapeutic effects on NASH.
This study investigated the effects of Sal B on morphological characteristics and functions of liver mitochondria of rats with NASH. The experimental design is reasonable and practical; the results are satisfactory. This study provided a theoretical basis for the clinical application of Sal B to treat NASH.
P- Reviewer: Vollmers HP S- Editor: Yu J L- Editor: Wang TQ E- Editor: Ma S
| 1. | Jian-gao F. Guidelines for management of nonalcoholic fatty liver disease: an updated and revised edition. Zhonghua Ganzangbing Zazhi. 2010;18:163-166. [PubMed] |
| 2. | Fan JG, Farrell GC. Epidemiology of non-alcoholic fatty liver disease in China. J Hepatol. 2009;50:204-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 437] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 3. | Greenfield V, Cheung O, Sanyal AJ. Recent advances in nonalcholic fatty liver disease. Curr Opin Gastroenterol. 2008;24:320-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 72] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 4. | Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675-1685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2642] [Cited by in RCA: 2472] [Article Influence: 164.8] [Reference Citation Analysis (2)] |
| 5. | Paradies G, Paradies V, Ruggiero FM, Petrosillo G. Oxidative stress, cardiolipin and mitochondrial dysfunction in nonalcoholic fatty liver disease. World J Gastroenterol. 2014;20:14205-14218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 362] [Cited by in RCA: 373] [Article Influence: 33.9] [Reference Citation Analysis (2)] |
| 6. | Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114:842-845. [PubMed] |
| 7. | van Hoek B. Non-alcoholic fatty liver disease: a brief review. Scand J Gastroenterol Suppl. 2004;56-59. [PubMed] |
| 8. | Lee O, Bruce WR, Dong Q, Bruce J, Mehta R, O’Brien PJ. Fructose and carbonyl metabolites as endogenous toxins. Chem Biol Interact. 2009;178:332-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313-1321. [PubMed] |
| 10. | Flameng W, Andres J, Ferdinande P, Mattheussen M, Van Belle H. Mitochondrial function in myocardial stunning. J Mol Cell Cardiol. 1991;23:1-11. [PubMed] |
| 11. | Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402-408. [PubMed] |
| 12. | Mao YM. [Clinical diagnostic methods and evaluation of drug-induced liver injury]. Zhonghua Ganzangbing Zazhi. 2012;20:167-169. [PubMed] |
| 13. | Joe Y, Zheng M, Kim HJ, Kim S, Uddin MJ, Park C, Ryu do G, Kang SS, Ryoo S, Ryter SW. Salvianolic acid B exerts vasoprotective effects through the modulation of heme oxygenase-1 and arginase activities. J Pharmacol Exp Ther. 2012;341:850-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 14. | Ho JH, Hong CY. Salvianolic acids: small compounds with multiple mechanisms for cardiovascular protection. J Biomed Sci. 2011;18:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 199] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 15. | Zhao CY, Liu XY, Shen C. [Molecular mechanisms of mitochondrial damage in the development of liver failure]. Zhonghua Ganzangbing Zazhi. 2011;19:955-957. [PubMed] |
| 16. | Takaki A, Kawai D, Yamamoto K. Multiple hits, including oxidative stress, as pathogenesis and treatment target in non-alcoholic steatohepatitis (NASH). Int J Mol Sci. 2013;14:20704-20728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 230] [Cited by in RCA: 335] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 17. | Basaranoglu M, Basaranoglu G, Sentürk H. From fatty liver to fibrosis: a tale of “second hit”. World J Gastroenterol. 2013;19:1158-1165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 114] [Cited by in RCA: 134] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 18. | Sunny NE, Parks EJ, Browning JD, Burgess SC. Excessive hepatic mitochondrial TCA cycle and gluconeogenesis in humans with nonalcoholic fatty liver disease. Cell Metab. 2011;14:804-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 510] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 19. | Wei Y, Rector RS, Thyfault JP, Ibdah JA. Nonalcoholic fatty liver disease and mitochondrial dysfunction. World J Gastroenterol. 2008;14:193-199. [PubMed] |
| 20. | He J, Hu B, Shi X, Weidert ER, Lu P, Xu M, Huang M, Kelley EE, Xie W. Activation of the aryl hydrocarbon receptor sensitizes mice to nonalcoholic steatohepatitis by deactivating mitochondrial sirtuin deacetylase Sirt3. Mol Cell Biol. 2013;33:2047-2055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 90] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 21. | Hallows WC, Yu W, Smith BC, Devries MK, Ellinger JJ, Someya S, Shortreed MR, Prolla T, Markley JL, Smith LM. Sirt3 promotes the urea cycle and fatty acid oxidation during dietary restriction. Mol Cell. 2011;41:139-149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 318] [Cited by in RCA: 313] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 22. | Bao J, Scott I, Lu Z, Pang L, Dimond CC, Gius D, Sack MN. SIRT3 is regulated by nutrient excess and modulates hepatic susceptibility to lipotoxicity. Free Radic Biol Med. 2010;49:1230-1237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 128] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 23. | Gan KX, Wang C, Chen JH, Zhu CJ, Song GY. Mitofusin-2 ameliorates high-fat diet-induced insulin resistance in liver of rats. World J Gastroenterol. 2013;19:1572-1581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 58] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 24. | Chen XL, Lei YR. [Effects of hydrodynamics-mediated RNAi on Mfn2 expression, blood sugar and fat levels in mice]. Zhonghua Gan Zang Bing Za Zhi. 2010;18:778-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 25. | Lan CH, Fang DC, Fan LL, He ZG, Chen DF, Liu CY, Shi HT. [The role of mitochondrial damages in Helicobacter pylori-induced apoptosis of gastric cancer cells]. Zhonghua Neike Zazhi. 2005;44:748-750. [PubMed] |
| 26. | Ngo JK, Pomatto LC, Davies KJ. Upregulation of the mitochondrial Lon Protease allows adaptation to acute oxidative stress but dysregulation is associated with chronic stress, disease, and aging. Redox Biol. 2013;1:258-264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 120] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 27. | Zhu FS, Liu S, Chen XM, Huang ZG, Zhang DW. Effects of n-3 polyunsaturated fatty acids from seal oils on nonalcoholic fatty liver disease associated with hyperlipidemia. World J Gastroenterol. 2008;14:6395-6400. [PubMed] |
| 28. | Martel C, Esposti DD, Bouchet A, Brenner C, Lemoine A. Non-alcoholic steatohepatitis: new insights from OMICS studies. Curr Pharm Biotechnol. 2012;13:726-735. [PubMed] |
| 29. | Pessayre D, Fromenty B, Berson A, Robin MA, Lettéron P, Moreau R, Mansouri A. Central role of mitochondria in drug-induced liver injury. Drug Metab Rev. 2012;44:34-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 195] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 30. | Apraiz A, Boyano MD, Asumendi A. Cell-centric view of apoptosis and apoptotic cell death-inducing antitumoral strategies. Cancers (Basel). 2011;3:1042-1080. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 31. | Akar FG, O’Rourke B. Mitochondria are sources of metabolic sink and arrhythmias. Pharmacol Ther. 2011;131:287-294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 32. | Rolo AP, Teodoro JS, Palmeira CM. Role of oxidative stress in the pathogenesis of nonalcoholic steatohepatitis. Free Radic Biol Med. 2012;52:59-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 636] [Cited by in RCA: 719] [Article Influence: 55.3] [Reference Citation Analysis (1)] |
| 33. | Balmer ML, Dufour JF. [Non-alcoholic steatohepatitis - from NAFLD to MAFLD]. Ther Umsch. 2011;68:183-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |