Published online Sep 14, 2015. doi: 10.3748/wjg.v21.i34.9974
Peer-review started: March 10, 2015
First decision: April 13, 2015
Revised: May 12, 2015
Accepted: July 15, 2015
Article in press: July 15, 2015
Published online: September 14, 2015
Processing time: 191 Days and 23.7 Hours
AIM: To evaluate the clinical value of the newly modified Simple Endoscopic Score for Crohn’s disease (mSES-CD).
METHODS: Seventy-six Crohn’s disease (CD) patients who underwent transanal double balloon endoscopy (DBE) in our hospital between 2003 and 2012 were retrospectively reviewed. DBE is defined as small intestinal endoscopy using two attached balloons. We included patients with stenosis which hampered passage of the scope and those who underwent DBE with observation for at least 80 cm from the ileocecal valve. Our new mSES-CD assesses the endoscopic activity of two consecutive small intestinal segments located 0-40 cm and 40-80 cm from the ileocecal valve by DBE, in addition to the activity of four colorectal segments. To compare the usefulness of mSES-CD with SES-CD, we similarly divided the patients into two groups according to total mSES-CD score (low disease activity group, < 4; high disease activity group, ≥ 4). The clinical value of mSES-CD in predicting clinical outcome in patients with CD was evaluated using the occurrence of surgery after DBE as an endpoint.
RESULTS: Median age of the 76 CD patients was 36 years (range, 16-71). Thirty-nine patients had stenosis which hampered passage of the DBE to 80 cm on the proximal side from the ileocecal valve. Median evaluable length of small intestine by DBE was 80 cm (range, 3-200). A total of 74 patients had one or more small intestinal lesions detected by DBE, of which 62 (83.8%) were within 80 cm of the ileocecal valve on the proximal side. Only two patients (2.7%) with proximal-side lesions more than 80 cm from the ileocecal valve did not have lesions within 80 cm. Patients with high mSES-CD scores showed significantly shorter surgery-free survival than those with low scores (P < 0.05). In contrast, surgery-free survival did not significantly differ between the low and high SES-CD groups (P > 0.05). Multivariate analysis by a Cox proportional hazards model identified mSES-CD as an independent factor for surgery-free survival.
CONCLUSION: mSES-CD is useful in evaluating the risk of surgery-free survival in patients with CD.
Core tip: Modified Simple Endoscopic Score for Crohn’s disease (mSES-CD) is a new scoring method which includes assessment of the endoscopic activity of small intestinal as well as colorectal lesions by double balloon endoscopy. mSES-CD is useful in evaluating the risk of salvage surgery-free survival in patients with Crohn’s disease.
- Citation: Morise K, Ando T, Watanabe O, Nakamura M, Miyahara R, Maeda O, Ishiguro K, Hirooka Y, Goto H. Clinical utility of a new endoscopic scoring system for Crohn’s disease. World J Gastroenterol 2015; 21(34): 9974-9981
- URL: https://www.wjgnet.com/1007-9327/full/v21/i34/9974.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i34.9974
Crohn’s disease (CD) is an inflammatory bowel disease of unknown etiology. In Japan, the Research Committee of Inflammatory Bowel Disease, established by the Japanese Ministry of Health and Welfare, estimates that the number of patients with CD is increasing by 1500 every year. CD is characterized as a chronic process with repeating recurrence and recrudescence, and can affect any part of the gastrointestinal tract. Many CD patients require surgery, with Solberg et al[1] reporting a cumulative probability of surgery over 10 years of 37.9%.
The traditional goal of CD therapy has been the induction and maintenance of clinical remission. With the availability of monoclonal antibodies, however, the goal of treatment has switched to mucosal healing (MH). Previous reports showed that, because of its association with the prognosis of CD, MH is more important than clinical remission[2-4]. For example, Schnitzler et al[5] reported that MH was significantly associated with a lower need for major abdominal surgery during long-term follow-up in patients treated with infliximab (IFX). To evaluate MH, several conventional endoscopic scoring methods for CD have been proposed, including the Crohn’s Disease Endoscopic Index of Severity (CDEIS) and Simple Endoscopic Score for Crohn’s Disease (SES-CD)[6,7]. The SES-CD scores four variables - ulcer size, extent of ulcerated surface, extent of affected surface, and stenosis - from 0 to 3 in the five segments of the ileum, right colon, transverse colon, left colon, and rectum. Daperno et al[8] reported that the SES-CD is a simple, reproducible, and easily used endoscopic scoring system for CD, and showed a strong correlation with CDEIS (r = 0.920).
The recent availability of double balloon endoscopy (DBE) allows evaluation of the activity of small intestinal lesions as well as colorectal lesions by an endoscopic procedure. Although a number of incidents have been reported, serious complications with DBE are rare, particularly with diagnostic DBE[9-11]. To date, however, a method of evaluating MH in CD which includes the small intestine has not been established. To date, capsule endoscopy (CE) and magnetic resonance enterography (MRE) have both been shown to be capable of assessing mucosal healing[12-16]. However, a method of evaluating MH which includes small intestinal lesions in patients with CD using DBE has not been established.
Here, we assessed the importance of evaluating the endoscopic activity of small intestinal lesions by DBE. We also propose a newly modified Simple Endoscopic Score for Crohn’s disease (mSES-CD), which includes assessment of the endoscopic activity of small intestinal lesions, and evaluate its usefulness in predicting the prognosis of CD.
The study was approved by the ethical review committee of Nagoya University Hospital. CD was diagnosed on the basis of standard clinical, endoscopic, and histological criteria. Between 2003 and 2012, the medical records of 76 patients with CD who underwent transanal DBE in our hospital were retrospectively reviewed. We included patients with stenosis which hampered passage of the scope and those who underwent DBE with observation for at least 80 cm from the ileocecal valve. Median age was 36 years (range, 16-71 years) and 60 of 76 were male. Patient characteristics are summarized in Table 1.
DBE was first performed in humans by Yamamoto et al[17] in 2000, and has been available for the clinical care of CD patients in Japan since 2003. In brief, DBE is performed using two balloons, one attached to the tip of the endoscope and the second at the distal end of the overtube. The role of these balloons is to grip the intestinal wall, thereby allowing easy further insertion of the endoscope without the formation of redundant loops in the small intestine.
Demographic and clinical data were collected from the medical records. SES-CD was assessed according to previous reports. Briefly, five segments were evaluated, namely the right colon, transverse colon, left colon, rectum, and ileum. Four variables were assessed in each segment: ulcer size, extent of ulcerated surface, extent of affected surface, and stenosis. Each variable was scored from 0 to 3 giving a total score range of 0-56[8]. Patients were divided into two groups according to total SES-CD score, with < 4 classified in the low disease activity group and ≥ 4 in the high disease activity group[18].
In this study, we devised a newly modified SES-CD which incorporates evaluation of endoscopic activity in the small intestine by DBE. Scoring for this newly mSES-CD is done using endoscopic activity in the two consecutive small intestinal segments extending 0-40 cm and 40-80 cm from the ileocecal valve, and in the four colorectal segments. The right colon segment included the ileocecal valve. The scores of the two intestinal segments were evaluated using the same scoring method as SES-CD[8], giving a total score range for the mSES-CD of 0-67. To compare clinical values of the mSES-CD with SES-CD, we similarly divided the patients into two groups according to total mSES-CD score (low disease activity group, < 4; high disease activity group, ≥ 4)[18].
The clinical value of mSES-CD in predicting clinical outcome in patients with CD was evaluated using the occurrence of surgery after DBE as an endpoint. Surgery-free survival was defined as the time from the date of DBE to the date of surgery or date of last follow-up, whichever occurred first. Surgeries included laparotomies for intestinal resection, strictureplasty and abscess drainage. These surgeries were performed as a result of failure of medical therapy.
Categorical variables were compared by the χ2 test or Fisher’s exact test, and continuous variables were compared by the independent Student’s t test. The association of endoscopic activity (mSES-CD and SES-CD) with CRP was assessed by Spearman’s rank correlation coefficient. Surgery-free survival was analyzed by the Kaplan-Meier method and statistical differences were calculated by the log-rank test. Independent factors associated with surgery-free survival were identified by univariate and multivariate analyses using the Cox proportional hazards model. Statistical analyses were carried out with a statistical software package (IBM SPSS version 20), with P < 0.05 considered statistically significant.
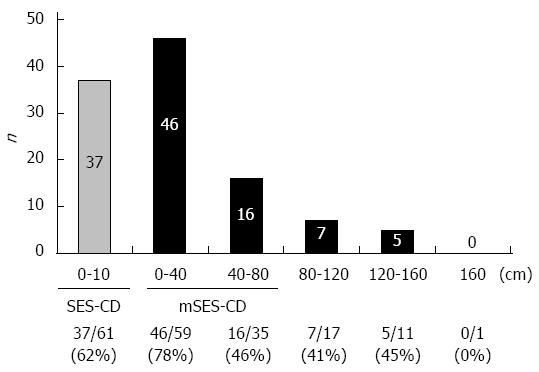
Of 76 patients, 39 had stenosis which hampered passage of the scope to 80 cm on the proximal side from the ileocecal valve. Median total DBE-evaluable length of small intestine was 80 cm (range, 3-200). A total of 74 patients with one or more small intestinal lesions were detected by DBE (Figure 1), of which 62 (83.8%) were within 80 cm of the ileocecal valve on the proximal side. In addition, only two patients (2.7%) with proximal-side lesions more than 80 cm from the ileocecal valve did not have lesions within 80 cm. These results support the validity of our new mSES-CD, which includes two small intestinal segments extending 0-40 cm and 40-80 cm from the ileocecal valve. In consequence, 62 (83.8%) of 74 patients with one or more small intestinal lesions were assessed by mSES-CD, whereas only 37 (50.0%) patients with small intestinal lesions were assessed by SES-CD. With regard to safety, no adverse events were observed, including major adverse events such as perforation of the small intestine, bleeding, or mucosal injury.
Among several laboratory markers of systemic inflammation in patients with CD, CRP is one of the most useful, and values show a good correlation with mucosal inflammation in the intestine[19-24]. We investigated the association of our new mSES-CD with CRP compared to that of SES-CD. mSES-CD showed a stronger correlation with CRP (r = 0.576, P < 0.001) than SES-CD (r = 0.446, P < 0.001). On subgroup analysis in the 32 patients with endoscopic activity in the small intestine only, mSES-CD endoscopic activity again showed a positive correlation with CRP (r = 0.536, P = 0.002), whereas SES-CD did not correlate with CRP (r = 0.259, P = 0.152). These results indicate that CRP value is influenced by not only colorectal inflammation but also small intestinal activity.
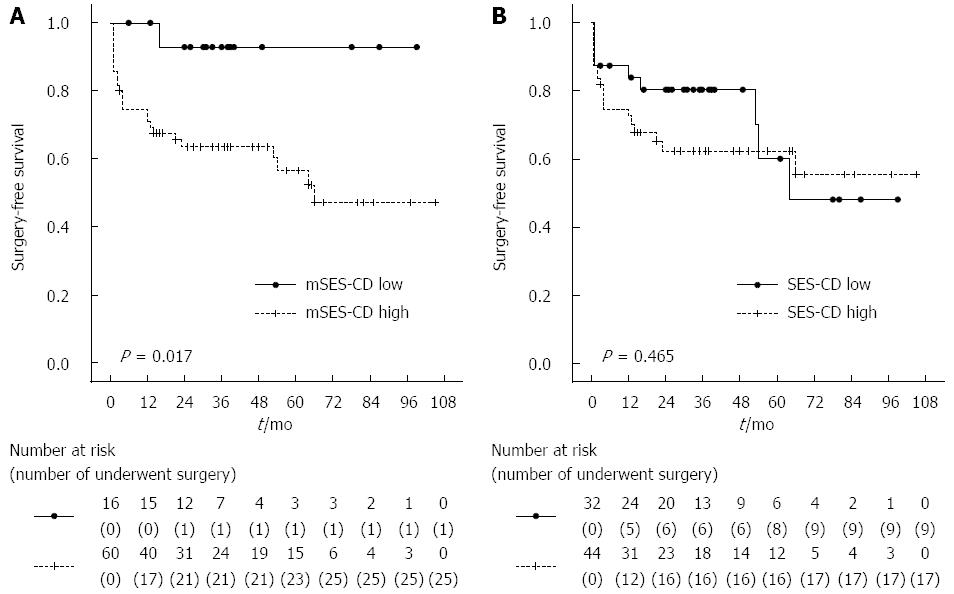
MH is a useful predictor of a good clinical outcome in patients with CD[3,5,25,26]. We evaluated the predictive value of mSES-CD with regard to the occurrence of surgery due to the worsening of CD inflammation. Table 2 compares patient characteristics between the low disease activity (mSES-CD < 4) and high disease activity (mSES-CD ≥ 4) groups. Although most clinical variables did not significantly differ between the low and high mSES-CD groups, CRP was significantly higher and albumin was significantly lower in the high mSES-CD group. The total number of patients who underwent surgery was 26 of 76 patients in this study. Of these, 3 (12%), 13 (50%) and 10 (38%) had a disease location in the colorectum, both colorectum and small intestine, and small intestine, respectively. We next examined the correlation between surgery-free survival and demographic and clinical data. On univariate analysis, mSES-CD, IFX treatment and disease duration were significantly associated with surgery-free survival, whereas SES-CD and CRP were not (Table 3). On multivariate analysis using a Cox proportional hazards model, mSES-CD was an independent factor associated with surgery-free survival, in addition to disease duration and treatment with IFX. Finally, we examined the correlation between surgery-free survival and the mSES-CD and SES-CD groups. The low mSES-CD group had significantly better survival than the high mSES-CD group (P < 0.05, log-rank test). In contrast, surgery-free survival did not significantly differ between the low and high SES-CD groups (P > 0.05, log-rank test). Kaplan-Meier analysis by mSES-CD and SES-CD groups is shown in Figure 2. In addition, we performed additional subgroup analysis in patients without stenosis. This analysis showed a tendency to poorer surgery-free survival in patients with a high mSES-CD score (≥ 4) compared to low mSES-CD score (P = 0.332 log-rank test), albeit without statistical significance.
| mSES-CD | Low (< 4) | High (≥4) | P value |
| Number | 16 | 60 | |
| Sex (Male:Female) | 15:1 | 45:15 | 0.092 |
| Mean age at diagnosis (yr) | 27.2 ± 8.9 | 25.8 ± 11.0 | 0.648 |
| Mean disease duration (yr) | 13.1 ± 8.0 | 9.0 ± 8.8 | 0.097 |
| Prior surgery (yes/no) | 10:6 | 30:30 | 0.374 |
| Infliximab (yes/no) | 8:8 | 21:39 | 0.272 |
| Azathioprine (yes/no) | 2:14 | 16:44 | 0.200 |
| Elemental diet (yes/no) | 14:2 | 47:13 | 0.335 |
| 5-ASA (yes/no) | 15:1 | 48:12 | 0.180 |
| CDAI | 90.0 ± 55.4 | 111.0 ± 54.1 | 0.186 |
| CRP (mg/dL) | 0.16 ± 0.21 | 1.13 ± 1.57 | < 0.001 |
| Albumin (g/dL) | 4.01 ± 0.82 | 3.47 ± 0.72 | 0.010 |
| Univariate analyses | Multivariate analyses | ||||||
| HR | 95%CI | P value | HR | 95%CI | P value | ||
| mSES-CD | ≥ 4 | 7.59 | 1.03-56.0 | 0.047 | 9.38 | 1.20-73.5 | 0.033 |
| SES-CD | ≥ 4 | 1.34 | 0.60-3.02 | 0.477 | - | ||
| Sex | Male | 0.97 | 0.39-2.42 | 0.942 | - | ||
| Age at diagnosis | < 20 | 1.07 | 0.48-2.35 | 0.876 | - | ||
| Disease duration | ≥ 10 | 2.21 | 0.98-4.81 | 0.056 | 2.75 | 1.18-6.45 | 0.020 |
| Prior surgery | Yes | 1.52 | 0.69-3.34 | 0.303 | - | ||
| Albumin | < 4 | 1.53 | 0.64-3.63 | 0.340 | - | ||
| Platelet | ≥ 410 × 104 | 1.91 | 0.76-4.77 | 0.166 | - | ||
| CRP | ≥ 0.3 | 1.56 | 0.72-3.38 | 0.259 | - | ||
| WBC | ≥ 8500 | 1.42 | 0.42-4.72 | 0.572 | - | ||
| CDAI | ≥ 150 | 1.85 | 0.80-4.30 | 0.151 | - | ||
| Infliximab | Yes | 0.34 | 0.13-0.90 | 0.029 | 0.31 | 0.12-0.83 | 0.019 |
| 5-ASA | Yes | 1.12 | 0.42-2.97 | 0.822 | - | ||
| Elemental diet | Yes | 1.41 | 0.48-4.10 | 0.530 | - | ||
| Azathioprine | Yes | 0.66 | 0.25-1.76 | 0.408 | - | ||
| Disease location | Colorectum | 0.38 | 0.11-1.27 | 0.114 | - | ||
| Colorectum + Small intestine | 2.17 | 1.00-4.69 | 0.050 | Excluded | |||
| Small intestine | 0.91 | 0.41-2.02 | 0.824 | - | |||
In this study, we found that small intestinal lesions detected by DBE influence the systemic inflammation of CD. In addition, mSES-CD, our new scoring method for the evaluation of mucosal activity using DBE, showed predictive value in the clinical outcome in patients with CD. These findings suggest that mSES-CD is useful in evaluating the risk of surgery-free survival in patients with CD.
CD patients in clinical remission do not always achieve endoscopic remission, and a DBE finding of active lesions in patients in clinical remission is not rare. As the disease progresses without symptoms, these patients are at risk of developing stenosis or fistula. Decision making for treatment adjustment therefore requires the accurate evaluation of mucosa. Recent advances in endoscopy have substantially benefited the diagnosis of lesions of the small intestine in patients with CD. With regard to DBE, this was first performed in humans by Yamamoto et al[17] and Sunada et al[27] in 2000, and has been available for the clinical care of CD patients in Japan since 2003.
In the present study, we proposed a newly modified SES-CD which incorporates evaluation of two 40-cm segments of the small intestine extending 0-40 cm and 40-80 cm from the ileocecal valve. Although it is technically possible to investigate the full length of the small intestine, determining a more appropriate evaluation range should be based on both the sensitivity of detection of small intestinal lesions and invasiveness. In our study, 83.8% of all patients with one or more lesions of the small intestine were localized within 80 cm of the ileocecal valve, and only two patients (2.7%) showed proximal-side lesions more than 80 cm from the ileocecal valve but no lesions within 80 cm. These findings support the validity of our scoring method of small intestinal lesions by DBE that includes evaluation of the small intestine for 80 cm from the ileocecal valve.
The utility of endoscopy for predicting intestinal surgery has not been fully established. Allez et al[28] reported that severe endoscopic lesions (SELs) have a more aggressive clinical course with an increased rate of surgery, while Jauregui-Amezaga et al[29] reported that the presence of SELs is not predictor of surgery in patients with CD. They emphasized the importance of stenosis and/or intra-abdominal fistulae on MRI for predicting an increased risk of surgery. We agree with their discussion of the clinical importance of evaluating stenosis, which is consistent with the present study. To determine the benefit of DBE more clearly, we performed additional subgroup analysis in patients without stenosis. The analysis showed a tendency to poorer surgery-free survival in patients with a high mSES-CD score (≥ 4) compared to low mSES-CD score, albeit without statistical significance, probably due to the small sample size. These results and previously reported evidence might suggest that the evaluation of lesions other than stenosis is also potentially useful in predicting the event of surgery. On this basis, evaluation of lesions other than stenosis using DBE would provide more accurate risk estimation for the event of surgery, although further validation studies are warranted. In addition, infliximab therapy and disease duration were significantly associated with the event of surgery. Jauregui-Amezaga et al[29] reported that immunosuppressants and anti-TNF therapy reduce the risk of the event of surgery, whereas prolonged disease duration significantly increases the risk of this event. Similar to these previous results, both infliximab and duration of disease were independent factors associated with surgery-free survival in this study.
Vermeire et al[19] reported that CRP is an objective marker of inflammation and showed good correlation with disease activity in CD compared to ESR, leucocytes, platelet count, and albumin. In our study, mSES-CD showed a better correlation with CRP than SES-CD in both the total study cohort and in a subgroup limited to those with small intestinal lesions only. Of note, the traditional SES-CD showed no correlation with CRP in this subgroup with small intestinal lesions only. These findings suggest that small intestinal lesions influence the systematic inflammation of CD. Although CRP value was not directly associated with clinical outcome (surgery-free survival in our study), we consider that CRP is useful as a non-invasive biomarker for decision making on whether to perform follow-up DBE in patients with CD. More specifically, elevated CRP may indicate the need for DBE evaluation of the inflammatory activity of not only colorectal but also small intestinal lesions.
When patients were divided into two groups by total mSES-CD score, surgery-free survival was significantly better in the low score group than in the high group. In contrast, the low and high SES-CD score groups did not significantly differ. Moreover, mSES-CD score was an independent factor for surgery-free survival in multivariate analysis. Frøslie et al[25] reported that MH was significantly associated with a low risk of future colectomy in UC patients (P = 0.02), and MH was significantly associated with less inflammation after 5 years in CD patients (P = 0.02) in a Norwegian population-based inflammatory bowel disease cohort. Our observation of inflammatory activity in both the colorectum and small intestine in CD suggests that our mSES-CD scoring, which includes evaluation of the small intestine, might be more useful in predicting the clinical outcome of CD than the conventional scoring system.
Recent reports have described the new modality of capsule endoscopy (CE) for the evaluation of small intestinal activity of CD. CE appears relatively non-invasive compared to DBE, but is contraindicated in the presence of small intestinal stenosis because of the risk of retention of the capsule endoscope. The major adverse events of CE involve capsule retention. Cheifetz et al[30] reported a retention rate of 13% in patients with known CD, but only 1.6% in those with suspected CD. The retained capsule has to be removed by endoscopy or surgical intervention. By contrast, there were no complications in the 76 DBEs in our study, while Oshitani et al[31] reported a single case of ileal perforation because of overtube balloon pressure in 53 examinations in patients with CD (1.9%). Further, Bourreille et al[32] reported that although CE had lower sensitivity than ileocolonoscopy in detecting recurrence in the neoterminal ileum, CE detected lesions outside the scope of ileocolonoscopy in more than two-thirds of patients. We consider that the combination of these two methods might provide more accurate evaluation of MH in patients with CD than either modality alone. Further prospective studies to compare the efficacy and safety of these procedures are needed.
Several limitations of this study warrant mention. First, it was conducted under a retrospective design and with a small sample size. Confirmation of the findings awaits larger prospective studies. Second, we identified the location of small intestinal lesions by the insertion length of DBE and radiography. However, precise determination of insertion length is hindered by expansion and contraction of the small intestine. Third, we assessed endoscopic activity in two small intestinal segments of 40 cm each, at 0-40 cm and 40-80 cm from the ileocecal valve, in addition to the four traditional colorectal segments of right colon, transverse colon, left colon, and rectum. However, whether assessment of these two segments represents the inflammatory status of the whole small intestine has not been confirmed. Fourth, a definition of mucosal healing has still not been established. In patients with a low endoscopic score, CD activity might be lower than in those with a high score. However, we were unable to diagnose whether mucosal healing was obtained, and therefore defined “endoscopic score < 4” as the “low disease activity group”, and “endoscopic score ≥ 4” as the “high disease activity group”. Fifth, physicians who recommended surgery were not blinded to information on endoscopic assessment. Surgery-free survival is a surrogate endpoint for evaluating the clinical course of patients with CD. Because of its surrogate nature, selection bias from the assessment of endoscopy influences the clinical decision for surgical treatment. Finally, we included all patients with CD who underwent transanal DBE in our hospital, which happened to include only a small number of L1 patients compared to L2 and L3. If more L1 patients had been included, SES-CD might have been more predictive.
In conclusion, this retrospective study showed that mSES-CD, our new scoring method for mucosal activity evaluated using DBE, has predictive value for clinical outcome in patients with CD. Further validation studies of mSES-CD are warranted.
Several conventional endoscopic scoring methods for Crohn’s disease (CD) have been proposed, including ‘Simple Endoscopic Score for Crohn’s Disease (SES-CD)’. The recent availability of double balloon endoscopy (DBE) allows evaluation of the activity of small intestinal lesions as well as colorectal lesions by an endoscopic procedure. The importance of evaluating the endoscopic activity of small intestinal lesions by DBE was assessed.
CD patients with high scores by modified Simple Endoscopic Score for Crohn’s disease (mSES-CD), this new scoring method for mucosal activity evaluated using DBE, showed significantly shorter surgery-free survival than those with low scores, while surgery-free survival did not significantly differ between the low and high SES-CD groups.
mSES-CD could offer predictive value for clinical outcome in patients with CD. The authors recommend the use of mSES-CD in evaluating the risk of salvage surgery-free survival in patients with CD
The newly mSES-CD is a new scoring method which includes assessment of the endoscopic activity of small intestinal as well as colorectal lesions by DBE.
This study’s efforts to validate an endoscopic scoring system using newer technology is important. It is somewhat limited by its retrospective nature that only includes those patients who have been pre-selected to undergo DBE.
P- Reviewer: Boirivant M, Hall BJ, Zimmerman LA S- Editor: Yu J L- Editor: A E- Editor: Ma S
| 1. | Solberg IC, Vatn MH, Høie O, Stray N, Sauar J, Jahnsen J, Moum B, Lygren I. Clinical course in Crohn’s disease: results of a Norwegian population-based ten-year follow-up study. Clin Gastroenterol Hepatol. 2007;5:1430-1438. [PubMed] |
| 2. | Castiglione F, Testa A, Rea M, De Palma GD, Diaferia M, Musto D, Sasso F, Caporaso N, Rispo A. Transmural healing evaluated by bowel sonography in patients with Crohn’s disease on maintenance treatment with biologics. Inflamm Bowel Dis. 2013;19:1928-1934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 3. | af Björkesten CG, Nieminen U, Sipponen T, Turunen U, Arkkila P, Färkkilä M. Mucosal healing at 3 months predicts long-term endoscopic remission in anti-TNF-treated luminal Crohn’s disease. Scand J Gastroenterol. 2013;48:543-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 4. | Paul S, Del Tedesco E, Marotte H, Rinaudo-Gaujous M, Moreau A, Phelip JM, Genin C, Peyrin-Biroulet L, Roblin X. Therapeutic drug monitoring of infliximab and mucosal healing in inflammatory bowel disease: a prospective study. Inflamm Bowel Dis. 2013;19:2568-2576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 189] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 5. | Schnitzler F, Fidder H, Ferrante M, Noman M, Arijs I, Van Assche G, Hoffman I, Van Steen K, Vermeire S, Rutgeerts P. Mucosal healing predicts long-term outcome of maintenance therapy with infliximab in Crohn’s disease. Inflamm Bowel Dis. 2009;15:1295-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 533] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 6. | Mary JY, Modigliani R. Development and validation of an endoscopic index of the severity for Crohn’s disease: a prospective multicentre study. Groupe d’Etudes Thérapeutiques des Affections Inflammatoires du Tube Digestif (GETAID). Gut. 1989;30:983-989. [PubMed] |
| 7. | De Cruz P, Kamm MA, Prideaux L, Allen PB, Moore G. Mucosal healing in Crohn’s disease: a systematic review. Inflamm Bowel Dis. 2013;19:429-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 120] [Article Influence: 10.0] [Reference Citation Analysis (1)] |
| 8. | Daperno M, D’Haens G, Van Assche G, Baert F, Bulois P, Maunoury V, Sostegni R, Rocca R, Pera A, Gevers A. Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: the SES-CD. Gastrointest Endosc. 2004;60:505-512. [PubMed] |
| 9. | Mensink PB, Haringsma J, Kucharzik T, Cellier C, Pérez-Cuadrado E, Mönkemüller K, Gasbarrini A, Kaffes AJ, Nakamura K, Yen HH. Complications of double balloon enteroscopy: a multicenter survey. Endoscopy. 2007;39:613-615. [PubMed] |
| 10. | Gerson LB, Tokar J, Chiorean M, Lo S, Decker GA, Cave D, Bouhaidar D, Mishkin D, Dye C, Haluszka O. Complications associated with double balloon enteroscopy at nine US centers. Clin Gastroenterol Hepatol. 2009;7:1177-1182, 1182.e1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 112] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 11. | Möschler O, May A, Müller MK, Ell C. Complications in and performance of double-balloon enteroscopy (DBE): results from a large prospective DBE database in Germany. Endoscopy. 2011;43:484-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 116] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 12. | Ordás I, Rimola J, Rodríguez S, Paredes JM, Martínez-Pérez MJ, Blanc E, Arévalo JA, Aduna M, Andreu M, Radosevic A. Accuracy of magnetic resonance enterography in assessing response to therapy and mucosal healing in patients with Crohn’s disease. Gastroenterology. 2014;146:374-82.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 294] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 13. | Papadia C, Maffei E, Del Rio P, Taylor S, Caini S, Montana C, Coruzzi A, Franzè A, Cademartiri F, Forbes A. Sensitivity and specificity of magnetic resonance enterography in the clinical management of fistulizing Crohn’s disease. Inflamm Bowel Dis. 2013;19:1896-1903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Hall B, Holleran G, Chin JL, Smith S, Ryan B, Mahmud N, McNamara D. A prospective 52 week mucosal healing assessment of small bowel Crohn’s disease as detected by capsule endoscopy. J Crohns Colitis. 2014;8:1601-1609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (37)] |
| 15. | Niv E, Fishman S, Kachman H, Arnon R, Dotan I. Sequential capsule endoscopy of the small bowel for follow-up of patients with known Crohn’s disease. J Crohns Colitis. 2014;8:1616-1623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 16. | Tillack C, Seiderer J, Brand S, Göke B, Reiser MF, Schaefer C, Diepolder H, Ochsenkühn T, Herrmann KA. Correlation of magnetic resonance enteroclysis (MRE) and wireless capsule endoscopy (CE) in the diagnosis of small bowel lesions in Crohn’s disease. Inflamm Bowel Dis. 2008;14:1219-1228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 91] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 17. | Yamamoto H, Sekine Y, Sato Y, Higashizawa T, Miyata T, Iino S, Ido K, Sugano K. Total enteroscopy with a nonsurgical steerable double-balloon method. Gastrointest Endosc. 2001;53:216-220. [PubMed] |
| 18. | Schoepfer AM, Beglinger C, Straumann A, Trummler M, Vavricka SR, Bruegger LE, Seibold F. Fecal calprotectin correlates more closely with the Simple Endoscopic Score for Crohn’s disease (SES-CD) than CRP, blood leukocytes, and the CDAI. Am J Gastroenterol. 2010;105:162-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 421] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 19. | Vermeire S, Van Assche G, Rutgeerts P. Laboratory markers in IBD: useful, magic, or unnecessary toys? Gut. 2006;55:426-431. [PubMed] |
| 20. | Filik L, Dagli U, Ulker A. C-reactive protein and monitoring the activity of Crohn’s disease. Adv Ther. 2006;23:655-662. [PubMed] |
| 21. | Denis MA, Reenaers C, Fontaine F, Belaïche J, Louis E. Assessment of endoscopic activity index and biological inflammatory markers in clinically active Crohn’s disease with normal C-reactive protein serum level. Inflamm Bowel Dis. 2007;13:1100-1105. [PubMed] |
| 22. | Kiss LS, Szamosi T, Molnar T, Miheller P, Lakatos L, Vincze A, Palatka K, Barta Z, Gasztonyi B, Salamon A. Early clinical remission and normalisation of CRP are the strongest predictors of efficacy, mucosal healing and dose escalation during the first year of adalimumab therapy in Crohn’s disease. Aliment Pharmacol Ther. 2011;34:911-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 23. | Jürgens M, Mahachie John JM, Cleynen I, Schnitzler F, Fidder H, van Moerkercke W, Ballet V, Noman M, Hoffman I, van Assche G. Levels of C-reactive protein are associated with response to infliximab therapy in patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2011;9:421-7.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 147] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 24. | Jones J, Loftus EV, Panaccione R, Chen LS, Peterson S, McConnell J, Baudhuin L, Hanson K, Feagan BG, Harmsen SW. Relationships between disease activity and serum and fecal biomarkers in patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2008;6:1218-1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 315] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 25. | Frøslie KF, Jahnsen J, Moum BA, Vatn MH. Mucosal healing in inflammatory bowel disease: results from a Norwegian population-based cohort. Gastroenterology. 2007;133:412-422. [PubMed] |
| 26. | Baert F, Moortgat L, Van Assche G, Caenepeel P, Vergauwe P, De Vos M, Stokkers P, Hommes D, Rutgeerts P, Vermeire S, D’Haens G; Belgian Inflammatory Bowel Disease Research Group; North-Holland Gut Club. Mucosal healing predicts sustained clinical remission in patients with early-stage Crohn’s disease. Gastroenterology. 2010;138:463-48; quiz e10-1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 603] [Cited by in RCA: 646] [Article Influence: 43.1] [Reference Citation Analysis (35)] |
| 27. | Sunada K, Yamamoto H. Double-balloon endoscopy: past, present, and future. J Gastroenterol. 2009;44:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Allez M, Lemann M, Bonnet J, Cattan P, Jian R, Modigliani R. Long term outcome of patients with active Crohn’s disease exhibiting extensive and deep ulcerations at colonoscopy. Am J Gastroenterol. 2002;97:947-953. [PubMed] |
| 29. | Jauregui-Amezaga A, Rimola J, Ordás I, Rodríguez S, Ramírez-Morros A, Gallego M, Masamunt MC, Llach J, González-Suárez B, Ricart E. Value of endoscopy and MRI for predicting intestinal surgery in patients with Crohn’s disease in the era of biologics. Gut. 2015;64:1397-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 30. | Cheifetz AS, Kornbluth AA, Legnani P, Schmelkin I, Brown A, Lichtiger S, Lewis BS. The risk of retention of the capsule endoscope in patients with known or suspected Crohn’s disease. Am J Gastroenterol. 2006;101:2218-2222. [PubMed] |
| 31. | Oshitani N, Yukawa T, Yamagami H, Inagawa M, Kamata N, Watanabe K, Jinno Y, Fujiwara Y, Higuchi K, Arakawa T. Evaluation of deep small bowel involvement by double-balloon enteroscopy in Crohn’s disease. Am J Gastroenterol. 2006;101:1484-1489. [PubMed] |