Published online Sep 14, 2015. doi: 10.3748/wjg.v21.i34.9966
Peer-review started: February 23, 2015
First decision: March 10, 2015
Revised: April 9, 2015
Accepted: May 7, 2015
Article in press: May 7, 2015
Published online: September 14, 2015
Processing time: 205 Days and 23.6 Hours
AIM: To evaluate the prognostic significance of the lymphocyte to monocyte ratio (LMR) in patients with unresectable metastatic colorectal cancer who received palliative chemotherapy.
METHODS: A total of 104 patients with unresectable metastatic colorectal cancer who underwent palliative chemotherapy were enrolled. The LMR was calculated from blood samples by dividing the absolute lymphocyte count by the absolute monocyte count. Pre-treatment LMR values were measured within one week before the initiation of chemotherapy, while post-treatment LMR values were measured eight weeks after the initiation of chemotherapy.
RESULTS: The median pre-treatment LMR was 4.16 (range: 0.58-14.06). We set 3.38 as the cut-off level based on the receiver operating characteristic curve. Based on the cut-off level of 3.38, 66 patients were classified into the high pre-treatment LMR group and 38 patients were classified into the low pre-treatment LMR group. The low pre-treatment LMR group had a significantly worse overall survival rate (P = 0.0011). Moreover, patients who demonstrated low pre-treatment LMR and normalization after treatment exhibited a better overall survival rate than the patients with low pre-treatment and post-treatment LMR values.
CONCLUSION: The lymphocyte to monocyte ratio is a useful prognostic marker in patients with unresectable metastatic colorectal cancer who receive palliative chemotherapy.
Core tip: We retrospectively analyzed 104 patients who had unresectable metastatic colorectal cancer. This study indicated that patients with a low pre-treatment lymphocyte to monocyte ratio (LMR) had a significantly worse overall survival rate. Moreover, patients who demonstrated low pre-treatment LMR and normalization after chemotherapy exhibited a better overall survival rate than patients with low pre-treatment and post-treatment LMR values.
- Citation: Shibutani M, Maeda K, Nagahara H, Ohtani H, Sakurai K, Yamazoe S, Kimura K, Toyokawa T, Amano R, Tanaka H, Muguruma K, Hirakawa K. Prognostic significance of the lymphocyte-to-monocyte ratio in patients with metastatic colorectal cancer. World J Gastroenterol 2015; 21(34): 9966-9973
- URL: https://www.wjgnet.com/1007-9327/full/v21/i34/9966.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i34.9966
Colorectal cancer (CRC) is the third leading cause of cancer-related death worldwide[1]. Patients with unresectable metastatic CRC have a particularly poor prognosis. Despite the recent major advances in new cytotoxic and molecular targeted therapies for unresectable CRC that have been developed within the last 10 years[2-5], the median survival time of patients with unresectable metastatic CRC is approximately only 30 mo[6,7]. According to the guidelines of the European Society for Medical Oncology (ESMO), it is recommended to individualize the treatment of patients with metastatic CRC based on their tumor- and disease-related characteristics[8]. Therefore, it is necessary to detect biomarkers for predicting survival.
It is well known that the systemic inflammatory response plays an important role in cancer progression[9]. Markers based on systemic inflammation, such as the neutrophil to lymphocyte ratio (NLR) and Glasgow prognostic score, have been reported to be useful for predicting the prognosis in patients with various types of cancer[10-14]. Recently, the lymphocyte to monocyte ratio (LMR), which also reflects the degree of systemic inflammation, has been reported to correlate with survival in various types of malignancies. However, the prognostic value of the LMR has been investigated mainly in patients with hematological malignancies and there have been only a few reports focusing on patients with solid tumors, such as colon, bladder, and lung cancers[15-22]. Moreover, to the best of our knowledge, no studies regarding the prognostic significance of the LMR in patients with unresectable metastatic CRC are available. The aim of this retrospective study was to evaluate the prognostic significance of the LMR in patients with unresectable metastatic CRC.
We retrospectively reviewed a database of 104 patients who underwent palliative combination chemotherapy for unresectable metastatic colorectal cancer at the Department of Surgical Oncology of Osaka City University between 2005 and 2010.
The patient characteristics are listed in Table 1. The patient population consisted of 59 males and 45 females, with a median age of 64 years (range: 27-86). According to the definition of the Eastern Cooperative Oncology Group performance status (PS), 96 patients were classified as having a PS of 0, 6 patients were classified as having a PS of 1, and 2 patients was classified as having a PS of 2. Sixty patients had primary tumors located in the colon and 44 had primary tumors located in the rectum. A total of 42 patients had metachronous unresectable cancer, and 62 patients had synchronous unresectable cancer. Fifty-eight patients had only one organ affected by metastasis and 46 patients had more than one organ affected by metastasis. Among the 104 patients, 88 underwent resection of a primary tumor. All patients underwent combination chemotherapy with oxaliplatin or irinotecan plus 5-fluorouracil/leucovorin or a prodrug of 5-fluorouracil as first-line chemotherapy. There was no initiation of palliative chemotherapy for recurrence while undergoing adjuvant chemotherapy. 64 patients received 5-fluorouracil + leucovorin + oxaliplatin (FOLFOX), 26 patients received capecitabine + oxaliplatin (CapeOX), nine patients received 5-fluorouracil + leucovorin + irinotecan (FOLFIRI), and five patients received S-1 + oxaliplatin (SOX). Seventy-six patients underwent chemotherapy combined with molecular targeted therapy. The median follow-up period in the survivors was 22.4 mo (range: 2.6-69.5). During the follow-up period, a total of 67 patients died.
| Age (yr) | |
| Median (range) | 64 (27-86) |
| Gender | |
| Male | 59 |
| Female | 45 |
| Performance status | |
| 0 | 96 |
| 1 | 6 |
| 2 | 2 |
| Location of primary tumor | |
| Colon | 60 |
| Rectum | 44 |
| Histological type | |
| Well, moderately | 81 |
| Poorly, mucinous | 14 |
| KRAS | |
| Wild type | 25 |
| Mutant type | 25 |
| Unknown | 54 |
| Detection of unresectable tumor | |
| Synchronous | 62 |
| Metachronous | 42 |
| Number of organs affected by metastasis | |
| One organ | 58 |
| More than one organ | 46 |
| Resection of primary tumor | |
| No | 16 |
| Yes | 88 |
| Regimen of first-line chemotherapy | |
| FOLFOX | 64 |
| CapeOX | 26 |
| FOLFIRI | 9 |
| SOX | 5 |
| Molecular targeted therapy | |
| No | 28 |
| Yes | 76 |
| Pre-treatment LMR (mean ± SD) | 4.548 ± 2.314 |
| Pre-treatment NLR (mean ± SD) | 3.204 ± 2.284 |
Response evaluations were performed every eight weeks. A variation of approximately one week was regarded as an allowable error. All patients were followed up with a physical examination, blood tests, and tumor marker level measurements [i.e., carcinoembryonic antigen (CEA), computed tomography, and ultrasonography].
We adopted the response evaluation criteria in solid tumors (RESIST)[23] to classify the treatment response of each patient as one of the following: complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD). An objective response was defined as either CR or PR, while disease control was defined as CR, PR, or SD.
Pre-treatment blood samples were obtained within one week before the initiation of chemotherapy and post-treatment blood samples were obtained eight weeks after the initiation of chemotherapy. The differential white blood cell count was analyzed using an XE-5000 hematology analyzer (Sysmex, Kobe, Japan) based on the manufacturer’s protocol. In each case, the LMR was calculated from a blood sample by dividing the absolute lymphocyte count by the absolute monocyte count. The neutrophil to lymphocyte ratio (NLR) was calculated from a blood sample by dividing the absolute neutrophil count by the absolute lymphocyte count.
The significance of correlations between the pre-treatment LMR and the clinicopathological characteristics were analyzed using the χ2 test, t-test, and Mann-Whitney U-test. The duration of survival was calculated according to the Kaplan-Meier method. Differences in the survival curves were assessed using the log-rank test. A multivariate analysis was performed according to the Cox proportional hazards model. All statistical analyses were conducted using the SPSS software package for Windows (SPSS Japan, Tokyo, Japan). Statistical significance was set at a value of P < 0.05.
This research conformed to the provisions of the Declaration of Helsinki established in 1995. All patients were informed of the investigational nature of the study and provided their written informed consent. This retrospective study was approved by the ethics committee of Osaka City University.
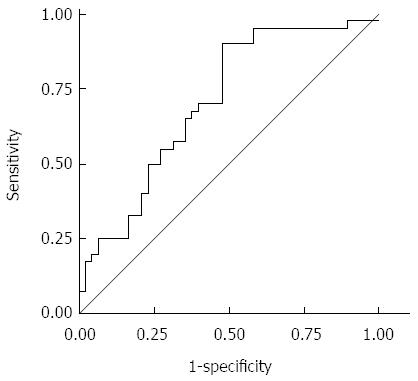
We used the LMR, a continuous variable, as the test variable and the 24.8-mo survival (median survival time: 24.8 mo) as the state variable. When we investigated the cut-off value for the LMR using the receiver operating characteristic (ROC) curve, we found that the appropriate cut-off value for the LMR was 3.38 (sensitivity: 90.0%; specificity: 52.1%) (Figure 1). We therefore set 3.38 as the cut-off value and the patients were classified into high-LMR (n = 66) and low-LMR (n = 38) groups. We also set the cut-off value for the NLR at 2.8 in accordance with the findings of a previous report[10].
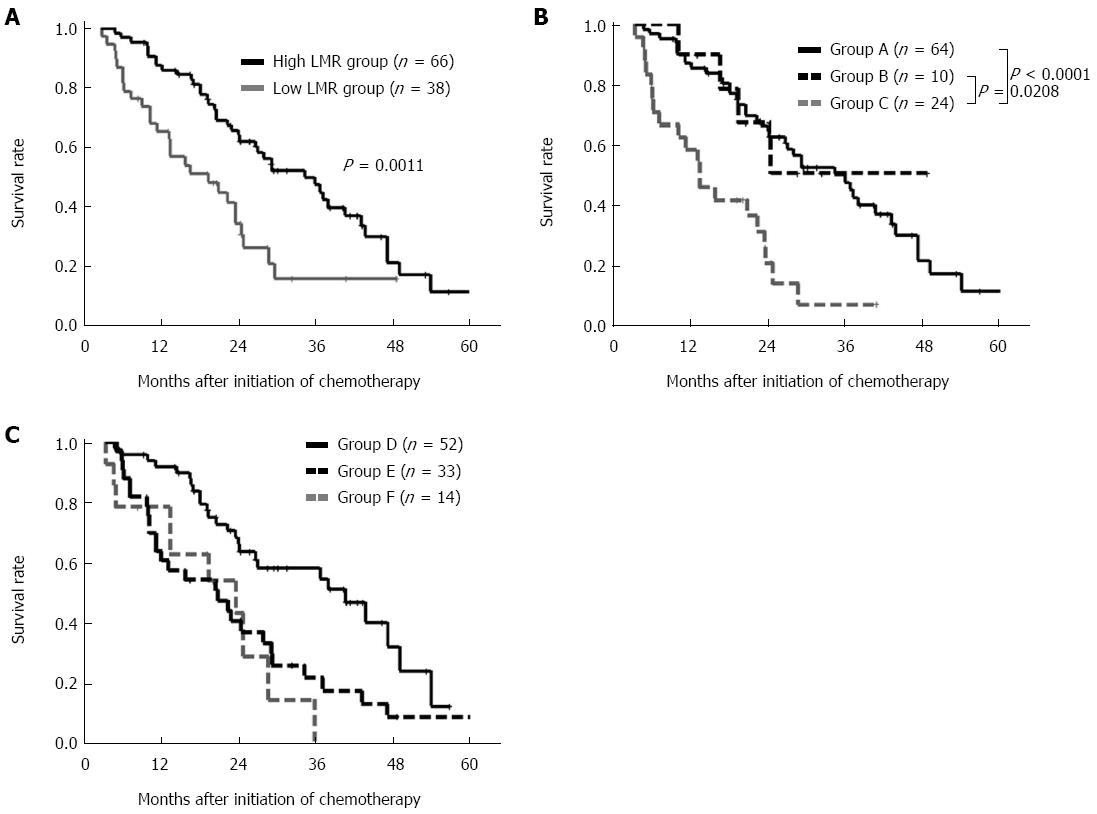
The overall survival rate was significantly worse in the low pre-treatment LMR group than in the high pre-treatment LMR group (P = 0.0011) (Figure 2A).
Correlations between the pre-treatment LMR and clinicopathological factors are shown in Table 2. The pre-treatment LMR had no significant relationship with any of the clinicopathological factors, with the exception of the pre-treatment NLR.
| Pre-treatment LMR | |||
| High | Low | P value | |
| Performance status | |||
| 0 | 62 | 34 | |
| 1, 2 | 4 | 4 | 0.459 |
| Location of primary tumor | |||
| Colon | 39 | 21 | |
| Rectum | 27 | 17 | 0.837 |
| Detection of unresectable tumor | |||
| Synchronous | 39 | 23 | |
| Metachronous | 27 | 15 | 1.000 |
| Resection of primary tumor | |||
| No | 8 | 8 | |
| Yes | 58 | 30 | 0.264 |
| Histological type | |||
| Well, moderately | 51 | 30 | |
| Poorly, mucinous | 10 | 4 | 0.764 |
| KRAS | |||
| Wild type | 15 | 10 | |
| Mutant type | 15 | 10 | 1.000 |
| Peritoneal dissemination | |||
| Negative | 53 | 32 | |
| Positive | 13 | 6 | 0.793 |
| Number of organs affected by metastasis | |||
| One organ | 39 | 19 | |
| More than one organ | 27 | 19 | 0.416 |
| Pre-treatment CEA (ng/mL) | |||
| ≤ 5 | 10 | 3 | |
| > 5 | 54 | 35 | 0.362 |
| Average relative dose intensity (%) | |||
| Median (range) | 100 (60.0-100) | 96.2 (50.0-100) | 0.697 |
| Molecular targeted therapy | |||
| No | 15 | 13 | |
| Yes | 51 | 25 | 0.253 |
| Pre-treatment NLR | |||
| < 2.8 | 48 | 5 | |
| ≥ 2.8 | 18 | 33 | < 0.001 |
The distribution of the chemotherapeutic response after the administration of first-line chemotherapy with reference to the LMR/NLR subgroup is shown in Table 3. The objective response rate did not differ according to the LMR (34.4% vs 28.9%, P = 0.664). However, the disease control rate of the high-LMR group was significantly higher than in that of the low-LMR group (82.8% vs 63.2%, P = 0.033). On the other hand, there was no significant relationship between the NLR and the chemotherapeutic response.
| Response | LMR | NLR | ||||
| High (n = 64) | Low (n = 38) | P value | High (n = 51) | Low (n = 51) | P value | |
| CR | 2 | 2 | 2 | 2 | ||
| PR | 20 | 9 | 12 | 17 | ||
| SD | 31 | 13 | 22 | 22 | ||
| PD | 11 | 14 | 15 | 10 | ||
| Objective response rate | 34.4% | 28.9% | 0.664 | 27.5% | 37.3% | 0.397 |
| Disease control rate | 82.8% | 63.2% | 0.033 | 70.6% | 80.4% | 0.357 |
The correlations between overall survival and the various clinicopathological factors are shown in Table 4. According to the results of a univariate analysis, overall survival exhibited significant relationships with performance status (P < 0.001), number of organs affected by metastasis (P = 0.045), response to molecular targeted therapy (P = 0.011), response to chemotherapy (P = 0.006), pre-treatment LMR (P = 0.002), and pre-treatment NLR (P < 0.001). A multivariate analysis indicated that performance status (HR = 3.345, 95%CI: 1.558-7.182, P = 0.002), response to molecular targeted therapy (HR = 0.462, 95%CI: 0.263-0.813, P = 0.007), and response to chemotherapy (HR = 0.432, 95%CI: 0.244-0.765, P = 0.004) were independent prognostic factors for survival.
| Univariate analysis | Multivariate analysis | |||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Performance status (≥ 1) | 3.821 | 1.805-8.087 | < 0.001 | 3.345 | 1.558-7.182 | 0.002 |
| Location of primary tumor (colon) | 1.405 | 0.857-2.304 | 0.177 | |||
| Detection of unresectable tumor (synchronous) | 1.407 | 0.852-2.312 | 0.182 | |||
| Histological type (poorly, mucinous) | 1.283 | 0.644-2.556 | 0.478 | |||
| Peritoneal dissemination (yes) | 0.981 | 0.534-1.802 | 0.951 | |||
| Number of organs affected by metastasis (≥ 2) | 1.637 | 1.012-2.648 | 0.045 | 1.270 | 0.737-2.187 | 0.389 |
| Pre-treatment CEA (> 5 ng/mL) | 1.949 | 0.841-4.521 | 0.120 | |||
| Resection of primary tumor (no) | 1.716 | 0.948-3.104 | 0.074 | 1.736 | 0.871-3.459 | 0.117 |
| Molecular targeted therapy (yes) | 0.496 | 0.289-0.853 | 0.011 | 0.462 | 0.263-0.813 | 0.007 |
| Response to chemotherapy (CR, PR) | 0.459 | 0.264-0.797 | 0.006 | 0.432 | 0.244-0.765 | 0.004 |
| Pre-treatment LMR (< 3.38) | 2.273 | 1.368-3.777 | 0.002 | 1.734 | 0.942-3.192 | 0.077 |
| Pre-treatment NLR (< 2.8) | 2.578 | 1.569-4.237 | < 0.001 | 1.734 | 0.947-3.178 | 0.075 |
We evaluated the prognostic significance of normalization of the LMR/NLR eight weeks after the initiation of chemotherapy. We categorized the patients into three groups according to the combination of their pre-treatment and post-treatment LMR values. Patients with high pre-treatment LMR were categorized into group A. Patients with low pre-treatment LMR and normalization of the LMR eight weeks after the initiation of chemotherapy were categorized into group B. Patients with low pre-treatment and post-treatment LMR value were categorized into group C. The patients in group C exhibited a worse prognosis than those in groups A and B (A vs C, P < 0.0001; B vs C, P = 0.0308) (Figure 2B). We categorized the patients into three groups according to the combination of their pre-treatment and post-treatment NLR values. Patients with low pre-treatment LMR were categorized into group D. Patients with high pre-treatment NLR and normalization of the NLR eight weeks after the initiation of chemotherapy were categorized into group E. Patients with high pre-treatment and post-treatment NLR value were categorized into group F. There was no significant difference between groups E and F (Figure 2C).
The absolute neutrophil count tended to decrease after chemotherapy. However, the absolute lymphocyte count did not change after chemotherapy, while the absolute monocyte count tended to increase after chemotherapy (Table 5).
| Pre-treatment value | Post-treatment value | P value | |
| All patients | |||
| Neutrophil (mean ± SD) | 4538.5 ± 200.4 | 2798.0 ± 190.0 | < 0.001 |
| Lymphocyte (mean ± SD) | 1664.3 ± 649.1 | 1610.5 ± 671.0 | 0.247 |
| Monocyte (mean ± SD) | 422.1 ± 19.0 | 471.7 ± 24.6 | 0.027 |
| Patients receiving chemotherapy based on oxaliplatin | |||
| Neutrophil (mean ± SD) | 4455.8 ± 1962.6 | 2791.7 ± 1859.9 | < 0.001 |
| Lymphocyte (mean ± SD) | 1694.9 ± 695.4 | 1639.2 ± 677.8 | 0.365 |
| Monocyte (mean ± SD) | 412.4 ± 181.0 | 457.3 ± 248.4 | 0.001 |
| Patients receiving chemotherapy based on irinotecan | |||
| Neutrophil (mean ± SD) | 4567.6 ± 1873.2 | 2879.4 ± 2007.3 | 0.038 |
| Lymphocyte (mean ± SD) | 1307.5 ± 440.8 | 1238.6 ± 342.1 | 0.394 |
| Monocyte (mean ± SD) | 466.9 ± 221.0 | 427.0 ± 134.2 | 0.174 |
In this study, we investigated the prognostic significance of pre-treatment LMR as a marker for predicting chemotherapeutic response and survival time in patients with unresectable metastatic CRC. Moreover, we demonstrated that normalization of the LMR after chemotherapy resulted in improved overall survival. Recently, systemic inflammation has been recognized to correlate with tumor progression and inflammatory markers have been reported to be useful for predicting the prognosis[9-13]. The LMR is an inflammatory marker, and a correlation between the LMR and survival has been reported[14-21]. However, most analyses in previous studies targeted patients with hematological malignancies[14-18]. To the best of our knowledge, this is the first study to assess the prognostic significance of the LMR in patients with unresectable metastatic CRC who received palliative chemotherapy.
Lymphocytes play an important role in the anti-tumor immunity of the host, including cytotoxic cell death and the inhibition of tumor cell proliferation and migration[9,24-26]. The absolute lymphocyte count is assumed to reflect the degree of responsiveness of the immune system of the host[26-28]. A decreased number of lymphocytes is therefore considered to be responsible for an insufficient immunologic reaction to the tumor, thus promoting tumor progression and metastasis[20].
On the other hand, monocytes play an important role in tumor progression and metastasis[9,29]. Tumor-associated macrophages (TAMs), which are derived from circulating monocytes, suppress adaptive immunity and promote angiogenesis, invasion, migration, and tumor growth[9,30-32]. The circulating level of monocytes in the peripheral blood is reported to reflect the formation and/or presence of TAMs[20,22]. Therefore, an increased level of monocytes reflects a high tumor burden in patients with cancer.
As mentioned above, the LMR reflects both the immune status of the host and the degree of tumor progression. Since both a low lymphocyte count and a high monocyte count reflect insufficient anti-tumor immunity and an elevated tumor burden, a low LMR is associated with a poorer prognosis.
In this study, normalization of the LMR eight weeks after the initiation of chemotherapy tended to correlate with an improvement in overall survival. Based on this result, the post-treatment LMR is considered to reflect the responsiveness of chemotherapy. The LMR is therefore a useful marker for monitoring tumor progression in patients with unresectable metastatic CRC who receive palliative chemotherapy.
The NLR, which has been reported to correlate with survival in patients with CRC, is quite similar to the LMR, as both results can be easily obtained from an examination of the peripheral blood. Although the pre-treatment NLR significantly correlated with the pre-treatment LMR and similar results regarding the long-term survival were obtained, only the LMR significantly correlated with the chemotherapeutic response. Moreover, in relation to the normalization of the value after chemotherapy, only the LMR significantly correlated with survival. Since the absolute neutrophil count tends to decrease after chemotherapy, the NLR tends to improve regardless of whether the tumor is controlled. On the other hand, because the absolute monocyte count tends to increase, the LMR tends to worsen, regardless of whether the tumor progresses. The normalization of the LMR after chemotherapy despite such situations is considered to reflect tumor control. This is because the prognostic significance of normalization after chemotherapy varied between the LMR and NLR. Therefore, the LMR is considered to be superior to the NLR.
There are some possible limitations associated with this study. First, we evaluated a relatively small number of patients and the study design was retrospective. Second, factors such as infection, ischemia, and coronary syndrome, which may affect the white blood cell count, were not taken into consideration. Third, the appropriate cut-off value for the LMR was not uniform in previous studies, although we set 3.38 as the cut-off value in the current study based on the ROC curve. Large prospective studies should therefore be performed to confirm our findings.
Despite recent major advances in the development of new cytotoxic and molecular targeted therapies, patients with unresectable metastatic colorectal cancer (CRC) still have a poor prognosis. According to the guidelines of the European Society for Medical Oncology, it is recommended that the treatment of patients with metastatic CRC be individualized based on their tumor- and disease-related characteristics. It is therefore necessary to detect biomarkers for predicting survival.
The lymphocyte to monocyte ratio (LMR) is a useful marker for predicting survival and chemotherapeutic response. This marker can therefore be used for the individualization of treatment in patients with unresectable metastatic CRC. By using this marker, the authors can identify patients with a high risk of a poor prognosis, and thereby choose the most appropriate intensive therapy.
It is difficult to predict the prognosis of patients with unresectable metastatic CRC. A few markers for predicting patient survival have been reported previously. Survival prediction is important for planning an appropriate course of treatment. The LMR was revealed to correlate with both survival and the chemotherapeutic response in the present study. The LMR makes a useful clinical biological marker because its measurement by peripheral blood cell count is a quick and easy assay to perform.
The results of the present study suggest that the LMR is a useful prognostic marker for predicting survival and chemotherapeutic response in patients with unresectable metastatic CRC who undergo palliative chemotherapy.
The LMR was calculated from a blood sample by dividing the absolute lymphocyte count by the absolute monocyte count. The LMR reflects the immune status and systemic inflammatory response of the host. Immune status and systemic inflammation have been reported to correlate with tumor progression, invasion, and metastasis. The LMR is thus considered to correlate with the survival of patients with CRC.
This is a good descriptive study in which the authors evaluated the prognostic significance of the lymphocyte to monocyte ratio in patients with unresectable metastatic colorectal cancer who underwent palliative chemotherapy. The study is well structured and the subject is clear and interesting. The manuscript is correctly written and the conclusions are justified by the results found in the study.
P- Reviewer: Lakatos PL, Liu XE, Nishida T, Wang G S- Editor: Ma YJ L- Editor: Rutherford A E- Editor: Liu XM
| 1. | Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108. [PubMed] |
| 2. | Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, Jandik P, Iveson T, Carmichael J, Alakl M. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355:1041-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2407] [Cited by in RCA: 2381] [Article Influence: 95.2] [Reference Citation Analysis (1)] |
| 3. | de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer G. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938-2947. [PubMed] |
| 4. | Saltz LB, Clarke S, Díaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS, Rivera F. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26:2013-2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2302] [Cited by in RCA: 2269] [Article Influence: 133.5] [Reference Citation Analysis (0)] |
| 5. | Bokemeyer C, Bondarenko I, Makhson A, Hartmann JT, Aparicio J, de Braud F, Donea S, Ludwig H, Schuch G, Stroh C. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2009;27:663-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1218] [Cited by in RCA: 1240] [Article Influence: 72.9] [Reference Citation Analysis (0)] |
| 6. | Heinemann V, von Weikersthal LF, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran SE, Heintges T, Lerchenmüller C, Kahl C, Seipelt G. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:1065-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1117] [Cited by in RCA: 1330] [Article Influence: 120.9] [Reference Citation Analysis (0)] |
| 7. | Grothey A, Sugrue MM, Purdie DM, Dong W, Sargent D, Hedrick E, Kozloff M. Bevacizumab beyond first progression is associated with prolonged overall survival in metastatic colorectal cancer: results from a large observational cohort study (BRiTE). J Clin Oncol. 2008;26:5326-5334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 522] [Cited by in RCA: 509] [Article Influence: 29.9] [Reference Citation Analysis (1)] |
| 8. | Van Cutsem E, Cervantes A, Nordlinger B, Arnold D. Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25 Suppl 3:iii1-iii9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 658] [Cited by in RCA: 798] [Article Influence: 72.5] [Reference Citation Analysis (0)] |
| 9. | Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8581] [Cited by in RCA: 8320] [Article Influence: 489.4] [Reference Citation Analysis (0)] |
| 10. | Shibutani M, Maeda K, Nagahara H, Noda E, Ohtani H, Nishiguchi Y, Hirakawa K. A high preoperative neutrophil-to-lymphocyte ratio is associated with poor survival in patients with colorectal cancer. Anticancer Res. 2013;33:3291-3294. [PubMed] |
| 11. | Maeda K, Shibutani M, Otani H, Nagahara H, Sugano K, Ikeya T, Amano R, Kimura K, Sakurai K, Kubo N. Prognostic value of preoperative inflammation-based prognostic scores in patients with stage IV colorectal cancer who undergo palliative resection of asymptomatic primary tumors. Anticancer Res. 2013;33:5567-5573. [PubMed] |
| 12. | Shimada H, Takiguchi N, Kainuma O, Soda H, Ikeda A, Cho A, Miyazaki A, Gunji H, Yamamoto H, Nagata M. High preoperative neutrophil-lymphocyte ratio predicts poor survival in patients with gastric cancer. Gastric Cancer. 2010;13:170-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 296] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 13. | Xue P, Kanai M, Mori Y, Nishimura T, Uza N, Kodama Y, Kawaguchi Y, Takaori K, Matsumoto S, Uemoto S. Neutrophil-to-lymphocyte ratio for predicting palliative chemotherapy outcomes in advanced pancreatic cancer patients. Cancer Med. 2014;3:406-415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 99] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 14. | Tomita M, Ayabe T, Chosa E, Nakamura K. Prognostic significance of pre- and postoperative glasgow prognostic score for patients with non-small cell lung cancer. Anticancer Res. 2014;34:3137-3140. [PubMed] |
| 15. | Li YL, Gu KS, Pan YY, Jiao Y, Zhai ZM. Peripheral blood lymphocyte/monocyte ratio at the time of first relapse predicts outcome for patients with relapsed or primary refractory diffuse large B-cell lymphoma. BMC Cancer. 2014;14:341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Koh YW, Park CS, Yoon DH, Suh C, Huh J. Should the cut-off values of the lymphocyte to monocyte ratio for prediction of prognosis in diffuse large B-cell lymphoma be changed in elderly patients? Eur J Haematol. 2014;93:340-348. [PubMed] |
| 17. | Kumagai S, Tashima M, Fujikawa J, Iwasaki M, Iwamoto Y, Sueki Y, Fukunaga A, Yanagita S, Nishikori M, Takaori-Kondo A. Ratio of peripheral blood absolute lymphocyte count to absolute monocyte count at diagnosis is associated with progression-free survival in follicular lymphoma. Int J Hematol. 2014;99:737-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Porrata LF, Ristow KM, Habermann TM, Witzig TE, Colgan JP, Inwards DJ, Ansell SM, Micallef IN, Johnston PB, Nowakowski G. Peripheral blood absolute lymphocyte/monocyte ratio during rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone treatment cycles predicts clinical outcomes in diffuse large B-cell lymphoma. Leuk Lymphoma. 2014;55:2728-2738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Koh YW, Kang HJ, Park C, Yoon DH, Kim S, Suh C, Go H, Kim JE, Kim CW, Huh J. The ratio of the absolute lymphocyte count to the absolute monocyte count is associated with prognosis in Hodgkin’s lymphoma: correlation with tumor-associated macrophages. Oncologist. 2012;17:871-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 100] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 20. | Stotz M, Pichler M, Absenger G, Szkandera J, Arminger F, Schaberl-Moser R, Samonigg H, Stojakovic T, Gerger A. The preoperative lymphocyte to monocyte ratio predicts clinical outcome in patients with stage III colon cancer. Br J Cancer. 2014;110:435-440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 237] [Cited by in RCA: 259] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 21. | Temraz S, Mukherji D, Farhat ZA, Nasr R, Charafeddine M, Shahait M, Wehbe MR, Ghaida RA, Gheida IA, Shamseddine A. Preoperative lymphocyte-to-monocyte ratio predicts clinical outcome in patients undergoing radical cystectomy for transitional cell carcinoma of the bladder: a retrospective analysis. BMC Urol. 2014;14:76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Hu P, Shen H, Wang G, Zhang P, Liu Q, Du J. Prognostic significance of systemic inflammation-based lymphocyte- monocyte ratio in patients with lung cancer: based on a large cohort study. PLoS One. 2014;9:e108062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 116] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 23. | Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15860] [Cited by in RCA: 21598] [Article Influence: 1349.9] [Reference Citation Analysis (1)] |
| 24. | Terzić J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138:2101-2114.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1333] [Cited by in RCA: 1508] [Article Influence: 100.5] [Reference Citation Analysis (0)] |
| 25. | Lin EY, Pollard JW. Role of infiltrated leucocytes in tumour growth and spread. Br J Cancer. 2004;90:2053-2058. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 205] [Cited by in RCA: 198] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 26. | Hoffmann TK, Dworacki G, Tsukihiro T, Meidenbauer N, Gooding W, Johnson JT, Whiteside TL. Spontaneous apoptosis of circulating T lymphocytes in patients with head and neck cancer and its clinical importance. Clin Cancer Res. 2002;8:2553-2562. [PubMed] |
| 27. | Kitayama J, Yasuda K, Kawai K, Sunami E, Nagawa H. Circulating lymphocyte is an important determinant of the effectiveness of preoperative radiotherapy in advanced rectal cancer. BMC Cancer. 2011;11:64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 101] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 28. | Cézé N, Thibault G, Goujon G, Viguier J, Watier H, Dorval E, Lecomte T. Pre-treatment lymphopenia as a prognostic biomarker in colorectal cancer patients receiving chemotherapy. Cancer Chemother Pharmacol. 2011;68:1305-1313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 107] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 29. | Evani SJ, Prabhu RG, Gnanaruban V, Finol EA, Ramasubramanian AK. Monocytes mediate metastatic breast tumor cell adhesion to endothelium under flow. FASEB J. 2013;27:3017-3029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 30. | Mantovani A, Bottazzi B, Colotta F, Sozzani S, Ruco L. The origin and function of tumor-associated macrophages. Immunol Today. 1992;13:265-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 735] [Cited by in RCA: 743] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 31. | Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2416] [Cited by in RCA: 2566] [Article Influence: 122.2] [Reference Citation Analysis (0)] |
| 32. | Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1921] [Cited by in RCA: 2059] [Article Influence: 108.4] [Reference Citation Analysis (0)] |