Published online Sep 14, 2015. doi: 10.3748/wjg.v21.i34.9945
Peer-review started: March 7, 2015
First decision: April 24, 2015
Revised: May 12, 2015
Accepted: July 18, 2015
Article in press: July 18, 2015
Published online: September 14, 2015
Processing time: 192 Days and 17.1 Hours
AIM: To explore the potential of β-elemene as a radiosensitizer for gastric cancer cells and the underlying mechanisms.
METHODS: SGC7901, MKN45, MKN28, N87, and AGS human gastric cancer cell lines were used to screen for radioresistant gastric cancer cell lines. A 3-(4,5-dimeth-ylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) assay was used to determine the effects of β-elemene and IPA-3 on cell viability in MKN45 and SGC7901 gastric cancer cell lines. A clonogenic survival assay and annexin V-FITC/PI apoptosis detection assay were used to evaluate cellular radiosensitivity and radiation-induced cell death, respectively. A proteomic method, isobaric tags for relative and absolute quantitation (iTRAQ), was employed to screen the proteins regulated by β-elemene pretreatment prior to ionizing radiation (IR) in SGC7901 gastric cancer cell line. IPA-3 was used as a specific small molecule inhibitor of p21-activated protein kinase 1 (Pak1) to target Pak1 signaling. Protein levels of PAK1IP1 (p21-activated protein kinase-interacting protein 1), total Pak1 (t-Pak1), phospho-Pak1 (T423), phospho-ERK1/2 (Thr202/Tyr204), and cleaved caspase-3 (17 kDa) were assessed by western blotting.
RESULTS: MKN45 and SGC7901 gastric cancer cell lines were relatively more resistant to IR. β-elemene pretreatment decreased clonogenic survival following IR in MKN45 and SGC7901 gastric cancer cell lines. Additionally, β-elemene pretreatment prior to IR increased radiation-induced cell death compared with IR alone in MKN45 (10.4% ± 0.9% vs 34.8% ± 2.8%, P < 0.05) and SGC7901 (11.6% ± 0.9% vs 46.7% ± 5.2%, P < 0.05) human gastric cancer cell lines, respectively, consistent with the level of cleaved caspase-3 (17 kDa). Through iTRAQ analysis and western blot validation, we found that β-elemene upregulated PAK1IP1 and downregulated phospho-Pak1 (T423) and phospho-ERK1/2 in SGC7901 gastric cancer cells. IR increased the level of phospho-Pak1 (T423). Pretreatment with β-elemene decreased radiation-induced Pak1 and ERK1/2 phosphorylation. Inhibition of Pak1 using IPA-3 decreased clonogenic survival following IR. In addition, IPA-3 increased radiation-induced cell death in MKN45 (13.4% ± 0.3% vs 26.6% ± 1.0%, P < 0.05) and SGC7901 (16.0% ± 0.6% vs 37.3% ± 1.7%, P < 0.05) gastric cancer cell lines, respectively, consistent with the level of cleaved caspase-3 (17 kDa). Western blotting showed that IPA-3 decreased radiation-induced Pak1 and ERK1/2 phosphorylation.
CONCLUSION: This is the first demonstration that β-elemene enhances radiosensitivity of gastric cancer cells, and that the mechanism involves inhibition of Pak1 signaling.
Core tip: In this study, we explore the potential of β-elemene, a novel anti-cancer drug isolated from the Chinese traditional herb Curcuma wenyujin, as a radiosensitizer for gastric cancer cells, and the underlying mechanisms. β-elemene pretreatment decreased clonogenic survival and increased apoptosis in response to ionizing radiation in SGC7901 and MKN45 gastric cancer cell lines. This is the first demonstration that β-elemene enhances radiosensitivity of gastric cancer cells. The underlying mechanism involves inhibition of p21-activated protein kinase 1 (Pak1) signaling. We also provide direct evidence for targeting Pak1 signaling as a means to sensitize cancer cells to ionizing radiation.
- Citation: Liu JS, Che XM, Chang S, Qiu GL, He SC, Fan L, Zhao W, Zhang ZL, Wang SF. β-elemene enhances the radiosensitivity of gastric cancer cells by inhibiting Pak1 activation. World J Gastroenterol 2015; 21(34): 9945-9956
- URL: https://www.wjgnet.com/1007-9327/full/v21/i34/9945.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i34.9945
Gastric cancer is a malignancy with a high incidence[1], and its prognosis remains unfavorable in most part of the world[2-4]. Since the publication of INT0116 study in 2001[5], accumulated evidence has demonstrated the efficacy of adjuvant chemoradiotherapy for promoting survival and local control in gastric cancer patients, and radiotherapy (RT) has a recognized role in the management of advanced gastric cancers with high risk[6-8]. However, radioresistance, as well as acute and late toxicity such as late renal toxicity, endocrine pancreatic insufficiency, and a reduction in quality of life resulting from RT, have limited the use of RT in clinical practice[9-12]. Therefore, it is urgent and necessary to search for an effective radiosensitizer to improve the efficacy of RT and to reduce its toxicity in tissues and organs surrounding the radiation field.
The radiosensitivity of cancer cells depends on several aspects, such as apoptosis sensitivity, cell cycle distribution of irradiated cells, the levels of various oncogenes or cancer suppressor genes, the repair of sublethal DNA damage, and the signaling pathways altered by ionizing radiation (IR)[13-15]. In recent studies, p21-activated protein kinase 1 (Pak1) has been found to be a new molecule that closely relates to the radiation response in cancer cells. Pak1 belongs to a serine/threonine kinase family comprising 6 isoforms (Pak1-6) which regulate diverse cellular behaviors, including proliferation, survival, invasiveness, metastasis, and transcription in cancer[16,17]. The kinase activity and catalytic function of Pak1 are regulated through phosphorylation of multiple amino acid residues, of which threonine 423 (T423) is a critical determinant for its complete activated state[18]. Numerous studies have shown that Pak1 and other Pak family members are overexpressed or hyperactivated in a broad spectrum of cancers, and perform critical roles in tumorigenesis and metastasis[19]. In a recent study, Pak1 was found to be activated by IR and participated in DNA damage repair after IR[20]. More recently, Motwani et al[21] reported that the genes regulated by Pak1 in response to IR were mainly involved in DNA damage responsive events, such as cell cycle arrest and apoptosis. These preliminary results indicate that Pak1 signaling is a potential target for enhancing tumor radiosensitivity.
The drugs that target the radiation-related signaling pathway and induce cellular apoptosis and inhibit DNA damage repair are potential radiosensitizers for cancer. Extracts of some natural plants, including Chinese herbs, have provided plenty of anti-cancer ingredients for cancer therapy[22]. β-elemene (1-methyl-1-vinyl-2,4-diisopropenyl-cyclohexane) is a novel anti-cancer ingredient isolated from the Chinese traditional herb Curcuma wenyujin[23]. Our group and others have found its anti-cancer potential in a variety of cancer cells[24-27]. The involved mechanisms include inhibition of multiple oncogenic signaling pathways, such as ERK and PI3K/Akt signaling, and thus induction of apoptosis and cell cycle arrest[24,25]. These signaling pathways and cellular events have been demonstrated to be vital for regulating the radiosensitivity of cancer cells[14]. In a previous study, we found that β-elemene inhibited the viability of gastric cancer cells and induced apoptosis in a dose-dependent manner[27]. Then, through a proteomic screening method, we found that β-elemene increased the expression of Pak1-interacting protein 1 (PAK1IP1) in gastric cancer cells. PAK1IP1 is a specific negative modulator of Pak1[28]. By sequence analysis, Xia and his colleagues found that PAK1IP1 shares a homologic sequence with the fission yeast PAK (Shk1)-binding protein Skb15. Then, through an immunoprecipitation assay and western blot analysis, they demonstrated that PAK1IP1 interacts with Pak1 in vitro and in vivo, and specifically binds to the regulatory domain of Pak1. Overexpression of PAK1IP1 in mammalian cells inhibits the activation of the Pak1 signaling pathway[28]. Other researchers are in agreement with the results of the study[19,29]. So, we assumed that β-elemene could inhibit Pak1 activation through upregulation of PAK1IP1, and then alter the radiation response and radiosensitivity of gastric cancer cells.
In the current study, we found that β-elemene pretreatment decreased clonogenic survival and increased apoptosis in response to IR in SGC7901 and MKN45 gastric cancer cell lines. Then, through iTRAQ proteomic screening assay and western blot validation, we found that β-elemene increased the expression of PAK1IP1 in gastric cancer cells, and that pretreatment with β-elemene inhibited Pak1 activation and sensitized gastric cancer cells to IR. Finally, we employed IPA-3, another specific molecular inhibitor of Pak1, and confirmed that the selective inhibition of Pak1 activation could enhance the radiosensitivity of gastric cancer cells.
β-elemene was obtained from Jingang Pharmaceutical Co. Ltd (Dalian, China). IPA-3 was purchased from Abcam (#ab141014, United Kingdom). Annexin V-FITC/PI Apoptosis Detection Kit was obtained from 7Sea Pharmatech Co. Ltd (#A005, Shanghai, China). Anti-PAK1IP1 antibody (#ab67348) and anti-Pak1 antibody (#ab40852) were purchased from Abcam. Anti-phospho-Pak1 (T423) antibody was from Abcam (#ab2477) and KeyGEN BioTECH (#KG11165, Nanjing, China). Anti-phospho-ERK1/2 (Thr202/Tyr204) antibody was from KeyGEN BioTECH (#KG30108, Nanjing, China). Anti-caspase-3 (cleaved, 17 kDa) antibody was from Biosynthesis Biotechnology Co. Ltd (#bs-0081R, Beijing, China).
The MKN45 and SGC7901 human gastric cancer cell lines were obtained from the Laboratory Animal Centre of The Fourth Military Medical University (Xi’an, China). The MKN28 and AGS cell lines were obtained from the Central Laboratory of Xi’an Jiaotong University School of Medicine (Xi’an, China). N87 cell line was obtained from Typical Culture Preservation Commission Cell Bank, Chinese Academy of Sciences (Shanghai, China). All the cells were cultured in RPMI1640 medium (HyClone, #SH30809.01B) supplemented with 10% fetal bovine serum (FBS) (Tianhang Biotechnology Co. Ltd) at 37 °C with 5% CO2.
Cell viability was measured using the 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT) assay. The cells were seeded in 24-well plates at 5 × 104-10 × 104 per well and incubated overnight, followed by application of different doses of drug for 24 or 48 h. Then, 50 μL MTT (5 mg/mL) (Sigma-Aldrich, United States) was added to each well and incubated for another 3-4 h. Then the supernatant was removed gently and 500 μL DMSO (Sigma-Aldrich, United States) was added to each well. The absorbance was measured using a microplate reader at 490 nm and cell viability was calculated according to the following formula. Cell viability = (Absorbance of the experimental group)/(Absorbance of the control group) × 100%.
Cells were seeded into 6-well plates and treated with a drug or not after 4-6 h attachment. Then, cells were exposed to different doses of IR on the following day. Cells were incubated for 10-14 d to form colonies, then fixed with methyl alcohol and stained with 1% crystal violet solution. Colonies containing no less than 50 cells were counted. Survival fractions were normalized according to the non-irradiated subgroup to eliminate cytotoxicity generated by drug pretreatment. Cell survival curves were obtained using Graphpad Prism 5.1 software (United States) according to the multi-target single hit model. The radiation-associated parameters of mean lethal dose (D0), quasi-threshold dose (Dq), fitted surviving fraction at 2 Gy radiation (SF2), and sensitization enhancement ratio (SER) were calculated to evaluate the effect of drug pretreatment on the radiosensitivity of gastric cancer cells.
The cells were seeded into 6-well-plates at 3 × 105 per well and incubated overnight followed by drug pretreatment and IR according to the experimental groups. Then, cells were collected 24 h post IR and manipulated according to the manufacturer’s instructions, incubated with 5 μL Annexin V-FITC for 15 min and 10 μL PI for 5 min, then analyzed using flow cytometry (FCM) (BD Biosciences Clontech, United States) within 30 min.
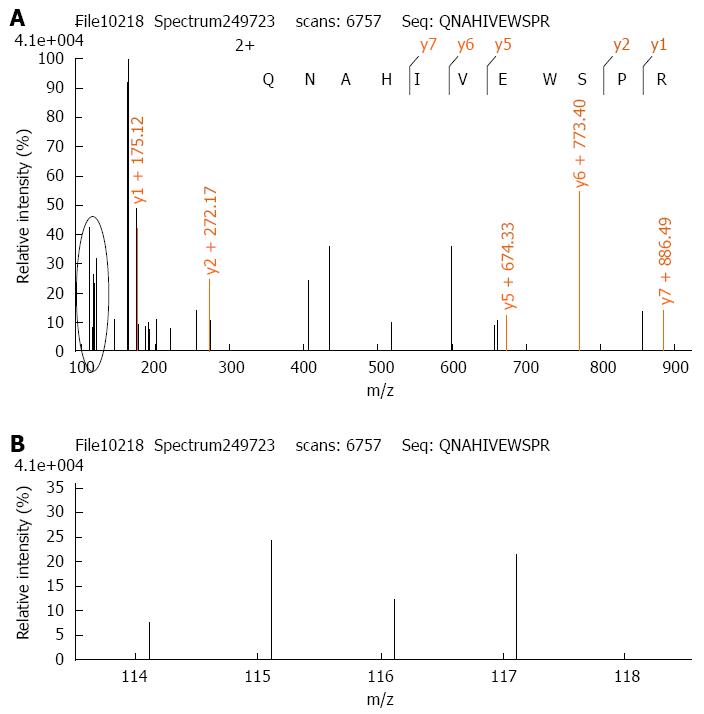
The iTRAQ proteomic method has been described previously[27]. Thus, 100 μg protein from each experimental group was digested with trypsin and the peptides of each group were labeled with iTRAQ reagents according to the manufacturer’s protocol (Applied Biosystems, New York, NY, United States) (control-114 tag, β-elemene treated-115 tag, IR-116 tag, β-elemene combined with IR-117 tag). Thereafter, the labeled peptide samples were mixed together and fractionated using Strong-Cation Exchange (SCX) chromatography (Shimadzu LC-20AB HPLC Pump system and the 4.6 mm × 250 mm Ultremex SCX column). Mass spectrometric analysis of the labeled peptides was done using a Q-EXACTIVE (ThermoFisher Scientific, San Jose, CA, United States) coupled online to the high performance liquid chromatography (HPLC). Data processing of LC-MS/MS samples was searched against the International Protein Index (IPI) human protein database v 3.87 fasta (91464 sequences) using Mascot 2.3.02 software (Matrix Science, United Kingdom). When protein abundance changed by more than 1.2-fold, with P < 0.05, we defined the protein as a differentially expressed protein.
Equal amounts of protein samples were loaded onto SDS-PAGE. Proteins were transferred to the nitrocellulose (NC) membranes and blocked with 5%-10% skimmed milk for 1-3 h at room temperature. Thereafter, the NC membranes were sequentially incubated with corresponding primary antibodies and secondary antibodies. The bands of proteins were visualized using an electrochemiluminescence (ECL) detection kit (#CW0049, CWBIO, Beijing, China).
Data are presented as mean ± SD. Statistical analysis was performed using the Student t-test and SPSS 17.0 software (SPSS Inc., Chicago, IL, United States). P < 0.05 was considered statistically significant.
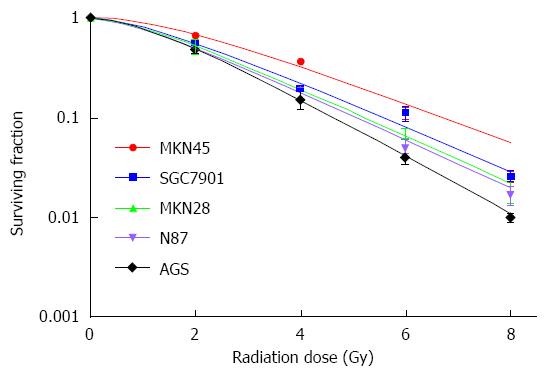
In the present study, we examined 5 gastric cancer cell lines to determine their relative sensitivity to IR by the clonogenic survival assay. The result showed that MKN45 and SGC7901 gastric cancer cell lines were relatively more resistant to IR, with higher D0 and SF2 (Figure 1 and Table 1). The 2 cell lines were selected to evaluate the radiosensitization effects of β-elemene in gastric cancer cells in the subsequent study.
| Cell line | D0 | Dq | SF2 |
| MKN45 | 2.16 | 1.81 | 68.89% |
| SGC7901 | 1.92 | 1.24 | 56.30% |
| MKN28 | 1.82 | 1.08 | 52.06% |
| N87 | 1.79 | 1.00 | 49.78% |
| AGS | 1.51 | 1.23 | 50.13% |
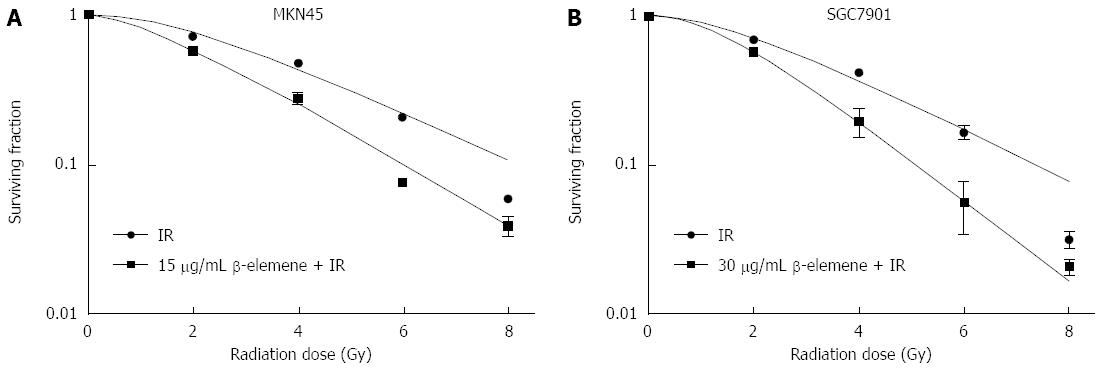
According to our previous study, β-elemene reduced the viability of gastric cancer cells in a dose-dependent manner[27]. The concentrations that led to less than 20% inhibition of cell viability for MKN45 and SGC7901 gastric cancer cell lines were about 15 μg/mL and 30 μg/mL, respectively. Thus, we chose 15 μg/mL and 30 μg/mL β-elemene pretreatment to evaluate whether β-elemene could enhance the radiosensitivity of gastric cancer cells. As shown in Figure 2, β-elemene pretreatment decreased clonogenic survival of gastric cancer cells in response to IR. The SF2 decreased from 76.47% to 59.44% in the MKN45 cell line and decreased from 71.15% to 57.71% in the SGC7901 cell line. The D0 value decreased from 2.62 Gy to 2.08 Gy in the MKN45 cell line and from 2.43 Gy to 1.59 Gy in the SGC7901 cell line. The Dq decreased from 2.19 Gy to 1.30 Gy in the MKN45 cell line and decreased from 1.86 Gy to 1.49 Gy in the SGC7901 cell line. The SER was 1.26 and 1.53 for MKN45 and SGC7901 gastric cancer cells, respectively. These data suggest that β-elemene enhances the radiosensitivity of gastric cancer cells, and that the effect is relatively greater in SGC7901 gastric cancer cells than that in MKN45 cells.
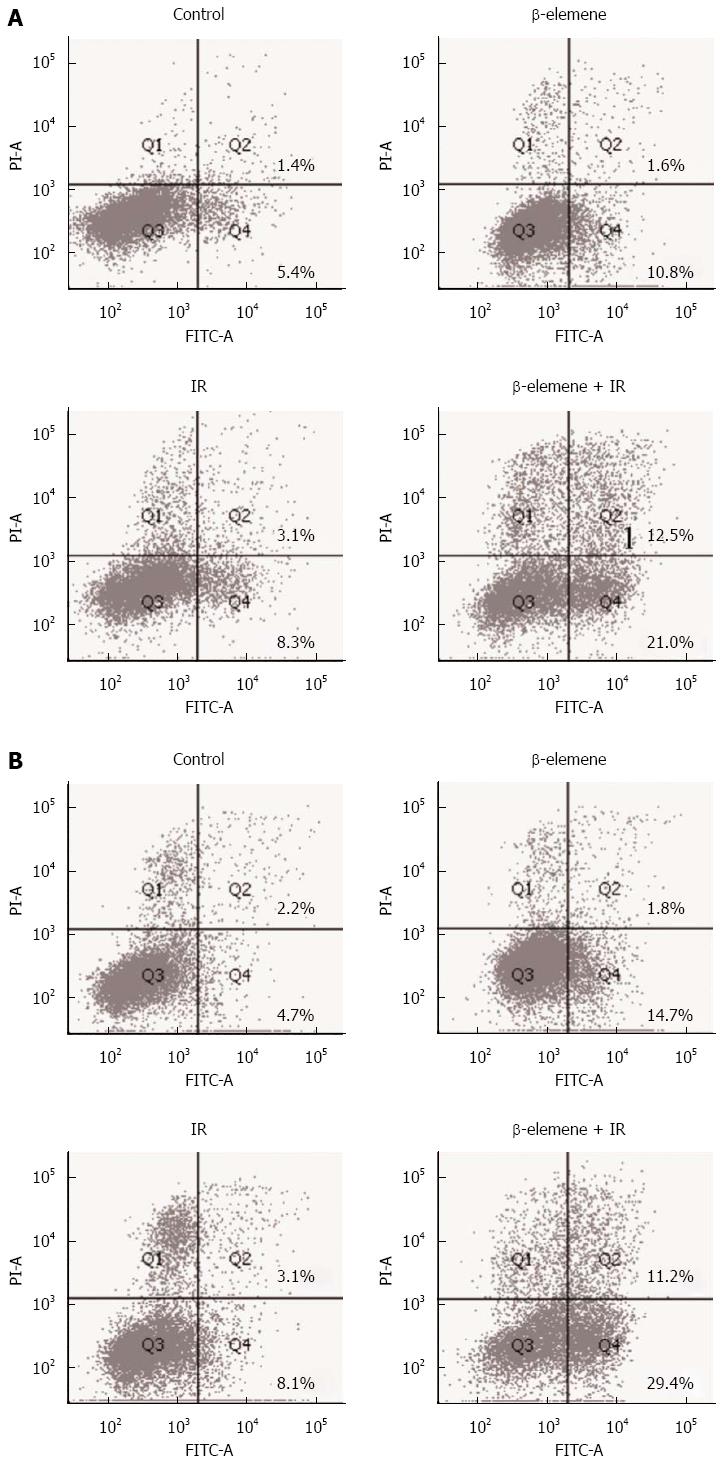
Along with the clonogenic survival assay, we investigated whether β-elemene could increase radiation-induced cell death. β-elemene pretreatment significantly increased radiation-induced cell death compared with IR alone in MKN45 (10.4% ± 0.9% vs 34.8% ± 2.8%, P < 0.05) and SGC7901 (11.6% ± 0.9% vs 46.7% ± 5.2%, P < 0.05) gastric cancer cell lines (Figure 3). The level of cleaved caspase-3 (17 kDa) was consistent with the cellular results. These results suggest that β-elemene increases the cell killing effect of IR in gastric cancer cells.
The cytological data suggested that β-elemene enhanced the radiosensitivity of gastric cancer cells. Then, we adopted an iTRAQ proteomic method to investigate the potential proteins underlying the mechanisms. Considering that β-elemene exhibited greater radiosensitization in the SGC7901 cell line than in the MKN45 cell line, we chose to conduct proteomic screening in SGC7901 cells. Through a comparison of protein expression among different experimental groups, we found that PAK1IP1 was the most upregulated protein by β-elemene pretreatment (Figure 4, the detailed lists of altered proteins are not shown). PAK1IP1 is a negative modulator of Pak1 and selectively inhibits the activation of Pak1 and its downstream signaling[28]. So, we assumed that β-elemene inhibited Pak1 activation through upregulation of PAK1IP1, and then validated the levels by western blotting.
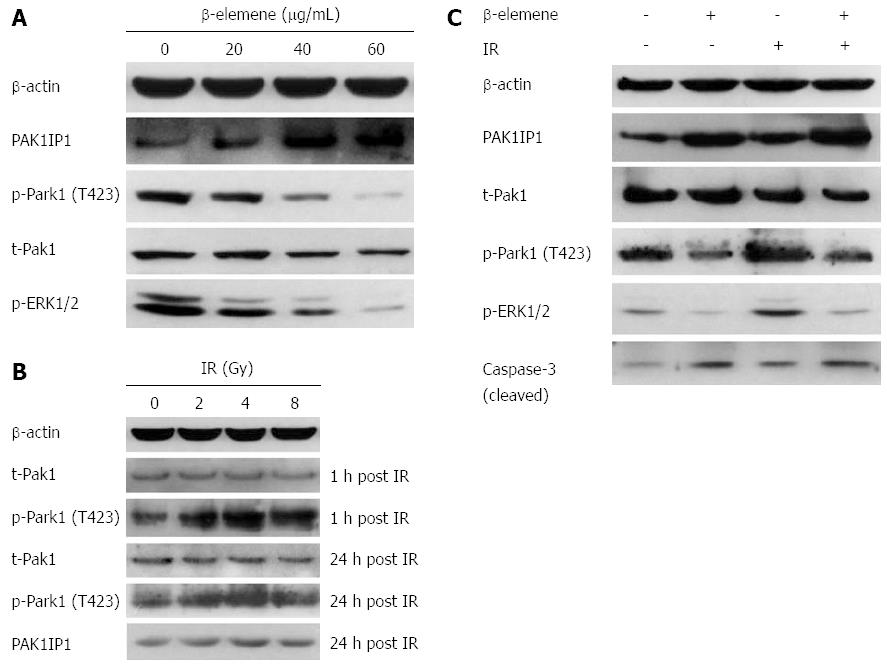
We examined the levels of PAK1IP1 and phospho-Pak1 (T423) in response to treatment dose. We found that β-elemene increased the expression of PAK1IP1 in a dose-dependent manner in SGC7901 gastric cancer cells (Figure 5A). The trend was consistent with the iTRAQ proteomic results. Meanwhile, β-elemene decreased the levels of phospho-Pak1 (T423) and phospho-ERK1/2 (Thr202/Tyr204). The results suggest that β-elemene increases PAK1IP1 expression and decreases Pak1 activation.
We found upregulation of phospho-Pak1 (T423) 1 h and 24 h after IR, but no changes in total Pak1 expression (Figure 5B). IR also led to a slight increase in PAK1IP1 expression, consistent with the iTRAQ result. β-elemene pretreatment inhibited IR-induced Pak1 (T423) phosphorylation 1h after IR (Figure 5C). β-elemene pretreatment also decreased phospho-ERK1/2 post IR.
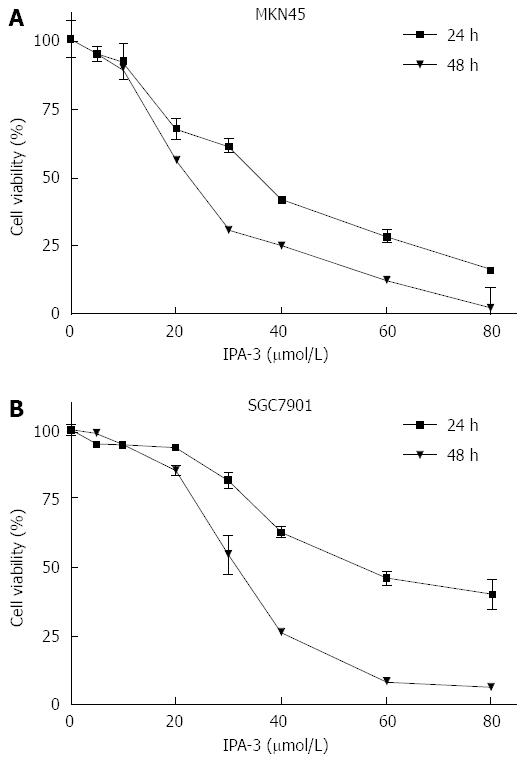
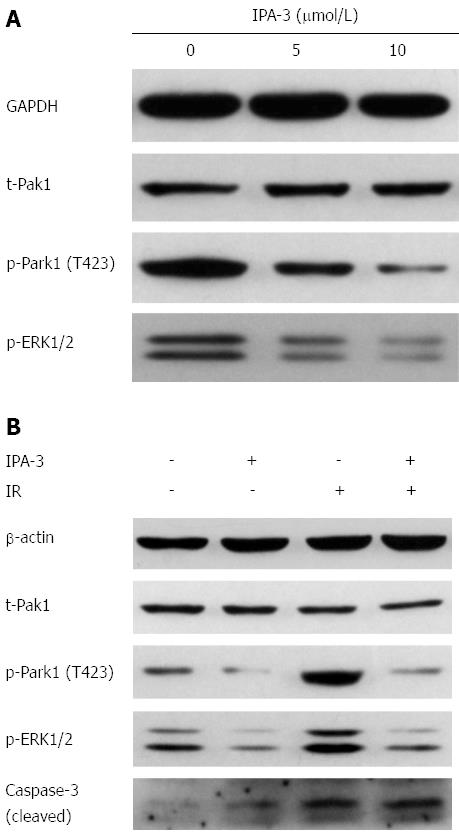
IPA-3 is a potent and specific small molecule inhibitor of Pak1[30,31]. We chose IPA-3 pretreatment to confirm whether inhibition of Pak1 activation could sensitize gastric cancer cells to IR. We first evaluated the effects of IPA-3 on the viability of gastric cancer cells. Our data showed that IPA-3 reduced the viability of gastric cancer cells in a dose- and time-dependent manner (Figure 6). These results suggest that IPA-3 inhibits the viability of gastric cancer cells.
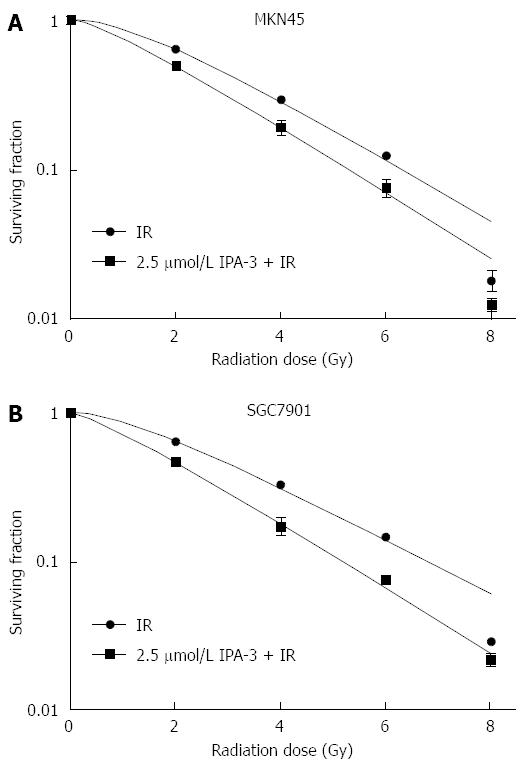
IPA-3 (2.5 μmol/L) pretreatment decreased clonogenic survival of gastric cancer cells in response to IR (Figure 7). The SF2 decreased from 64.64% to 46.53% in SGC7901 gastric cancer cells. The D0 value decreased from 2.34 Gy to 1.99 Gy, and the Dq decreased from 1.47 Gy to 0.63 Gy. The SER was 1.18 for SGC7901 gastric cancer cells. A similar effect was seen in MKN45 gastric cancer cells. These data suggest that IPA-3 enhances the radiosensitivity of gastric cancer cells.
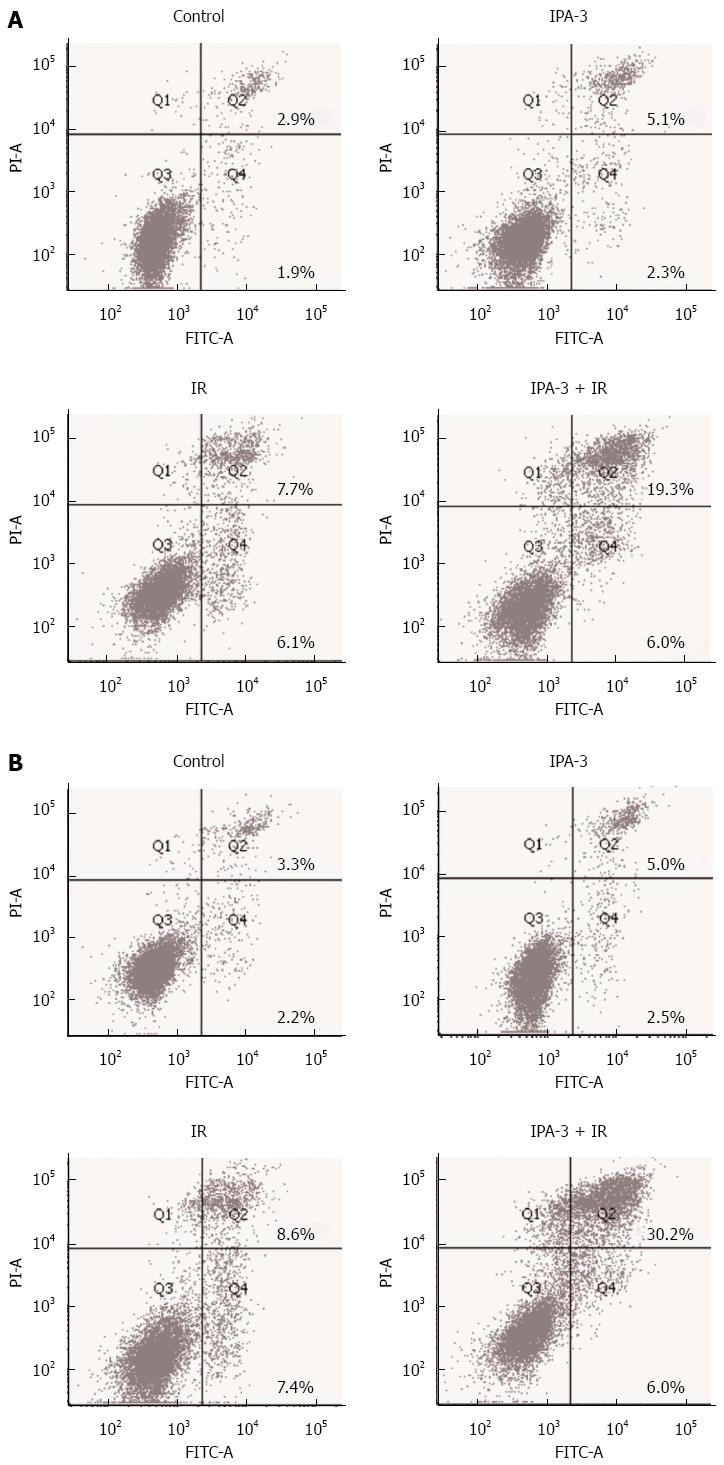
Along with the clonogenic survival assay, we investigated whether IPA-3 could increase radiation-induced cell death. As a result, IPA-3 (10 μmol/L) pretreatment dramatically increased IR-induced cell death compared with IR alone in MKN45 (13.4% ± 0.3% vs 26.6% ± 1.0%, P < 0.05) and SGC7901 (16.0% ± 0.6% vs 37.3% ± 1.7%, P < 0.05) gastric cancer cell lines (Figure 8). The level of cleaved caspase-3 (17 kDa) was consistent with the cytological results (Figure 9B). These results suggest that IPA-3 increases radiation-induced cell death in gastric cancer cells.
To confirm the signaling that altered the radiosensitivity, we evaluated the phosphorylation of Pak1 and its downstream ERK1/2 in response to IR after IPA-3 pretreatment. First, we confirmed that IPA-3 decreased the level of Pak1 phosphorylation (T423) in a dose-dependent manner (Figure 9A). Meanwhile, the level of phospho-ERK1/2, the downstream substrate of Pak1, was also reduced. This further supported that Pak1 kinase activity was reduced by IPA-3. Then, we found that IPA-3 pretreatment decreased the level of phospho-Pak1 (T423) and phospho-ERK1/2 induced by IR (Figure 9B).
Gastric cancer is a malignancy with high incidence and unfavorable prognosis, and requires multi-disciplinary therapeutic strategies, including radiotherapy. The search for an effective radiosensitizer is an important and urgent issue for the treatment of gastric cancer. β-elemene is a plant-derived ingredient and has been used in clinical practice for the treatment of several types of cancers[32]. It is noteworthy that basic research and clinical practice have demonstrated its efficacy and safety in cancer treatment[32,33]. Previous studies revealed that β-elemene exhibited its anti-cancer activities by targeting major signaling pathways[25]. In the present study, we investigated whether β-elemene could enhance the efficacy of IR in gastric cancer cells. We found that β-elemene pretreatment decreased clonogenic survival and increased radiation-induced cell death in gastric cancer cells. β-elemene pretreatment inhibited the activation of Pak1 and its downstream ERK1/2 signaling in response to IR. Furthermore, selective inhibition of Pak1 activation by IPA-3 presented similar radiosensitization effects as β-elemene in gastric cancer cells, decreasing clonogenic survival and increasing radiation-induced cell death. Our data indicate that β-elemene enhances the radiosensitivity of gastric cancer cells. Moreover, the underlying mechanism involves inhibition of Pak1 and its downstream ERK1/2 signaling.
Signaling transduction after IR is vital for mediating damage repair and thus controls the fate of cancer cells receiving IR. Some signaling events, such as ERK1/2 activation, facilitate the damage repair process in cancer cells and thus provide protection from IR damage and improve cell survival[34]. Therefore, it is an attractive goal to develop a radiosensitizer that intervenes in the radiation-associated signaling pathways. Pak1 is a serine/threonine kinase that is overexpressed and/or hyperactivated in many types of cancers including gastric cancer, and modulates diverse biological functions and signaling pathways[17,35]. In 1999, Roig and Traugh first reported that group I Pak members could be activated by IR[36]. Little attention was focused on the role of Pak members in the radiation response until recently, when Li et al[20] reported that Pak1 activation participated in DNA damage repair after IR. More recently, Motwani et al[21] reported that the genes altered by Pak1 in radiation scenarios were mainly involved in DNA damage responsive events, such as cell cycle arrest and apoptosis. In the current study, we found that β-elemene could inhibit the activation of Pak1 and demonstrated that specific inhibition of Pak1 by a selective inhibitor could sensitize gastric cancer cells to IR. This result is consistent with previous studies. Our results indicate that β-elemene is a potential radiosensitizer for gastric cancer cells. In addition, we provide direct evidence for radiosensitization effect of targeting Pak1 in cancer cells.
Previous studies have proved that Pak1 is located upstream of ERK1/2 signaling and can activate ERK1/2 in a kinase-dependent or -independent manner[19,37]. Numerous studies have demonstrated the activation of ERK1/2 signaling in response to IR and its vital role in promoting cancer cell survival after IR, and inhibition of ERK1/2 signaling is a means of sensitizing cancer cells to IR[34,38]. In the present study, ERK1/2 signaling was also inhibited when Pak1 was inhibited. Considering their relationship in signaling transduction, this result is consistent with previous studies and supports the possibility of targeting Pak1 to develop a radiosensitizer.
To our knowledge, this is the first demonstration that β-elemene enhances radiosensitivity of gastric cancer cells. The underlying mechanism involves inhibition of Pak1/ERK1/2 signaling. We also provide direct evidence for targeting Pak1 signaling as a means to sensitize cancer cells to IR.
The therapeutic use of β-elemene in clinical cancer treatment has proven efficacy, and has the advantage of being clinically safe and non-toxic to normal cells[32]. However, little is known about the direct molecular targets of β-elemene to induce the anti-cancer effects seen by our group and others. It remains an issue requiring further study. In addition, further in vivo studies and clinical trials are needed to confirm the effect of β-elemene in combination with IR in the management of gastric cancer. Our study lays the ground for continued research.
Gastric cancer (GC) is a malignancy with a high incidence and poor prognosis. Radiotherapy (RT) is a part of the multi-disciplinary therapeutic strategies for GC. However, radioresistance and radiation toxicity limited the use of RT in clinical practice. It is urgent and necessary to identify an effective radiosensitizer for GC.
Recent studies have shown that β-elemene has diverse anti-cancer activities, such as inhibiting proliferation and inducing apoptosis of cancer cells, enhancing tumor chemosensitivity, and overcoming drug resistance in a variety of cancers. The involved mechanisms include interacting with various oncogenic signaling pathways.
This is the first demonstration that β-elemene enhances the radiosensitivity of gastric cancer cells. The underlying mechanism involved inhibition of Pak1 signaling, which was confirmed by targeting Pak1 using the selective small molecule inhibitor, IPA-3. This also provided direct evidence that targeting Pak1 signaling is a means of sensitizing cancer cells to ionizing radiation.
This study highlights a new therapeutic possibility for improving the efficacy of RT and for reducing radiation toxicity, and may contribute to prolongation of patient survival and improvement in their quality of life.
β-elemene (1-methyl-1-vinyl-2,4-diisopropenyl-cyclohexane) is a novel anti-cancer compound isolated from the Chinese traditional herb Curcuma wenyujin and has diverse anti-cancer activities. Radiosensitivity is a key determinant of the radiation response and affects the efficacy of radiotherapy in cancer.
This is an interesting work. The authors present data suggesting that β-elemene enhances the radiosensitivity of gastric cancer cells via upregulation of PAK1IP1 (p21-activated protein kinase-interacting protein 1) and downregulation of phospho-Pak1 (T423) and phospho-ERK1/2. The therapeutic potential of β-elemene as a radiosensitizer for gastric cancer may be worth pursuing.
Biostatistics statement: The statistical methods of this study were reviewed by Prof. Shao-Nong Dang from Department of Epidemiology and Health Statistics, School of Public Health, Xi’an Jiaotong University.
P- Reviewer: Gao SY, Wei Q, Wu XP S- Editor: Ma YJ L- Editor: Cant MR E- Editor: Wang CH
| 1. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11128] [Cited by in RCA: 11834] [Article Influence: 845.3] [Reference Citation Analysis (4)] |
| 2. | Verdecchia A, Francisci S, Brenner H, Gatta G, Micheli A, Mangone L, Kunkler I. Recent cancer survival in Europe: a 2000-02 period analysis of EUROCARE-4 data. Lancet Oncol. 2007;8:784-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 649] [Cited by in RCA: 669] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 3. | Dassen AE, Lemmens VE, van de Poll-Franse LV, Creemers GJ, Brenninkmeijer SJ, Lips DJ, Vd Wurff AA, Bosscha K, Coebergh JW. Trends in incidence, treatment and survival of gastric adenocarcinoma between 1990 and 2007: a population-based study in the Netherlands. Eur J Cancer. 2010;46:1101-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 120] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 4. | Zhang H, Sun LL, Meng YL, Song GY, Hu JJ, Lu P, Ji B. Survival trends in gastric cancer patients of Northeast China. World J Gastroenterol. 2011;17:3257-3262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 5. | Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, Haller DG, Ajani JA, Gunderson LL, Jessup JM. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2465] [Cited by in RCA: 2436] [Article Influence: 101.5] [Reference Citation Analysis (0)] |
| 6. | Macdonald JS. Clinical overview: adjuvant therapy of gastrointestinal cancer. Cancer Chemother Pharmacol. 2004;54 Suppl 1:S4-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Kim S, Lim DH, Lee J, Kang WK, MacDonald JS, Park CH, Park SH, Lee SH, Kim K, Park JO. An observational study suggesting clinical benefit for adjuvant postoperative chemoradiation in a population of over 500 cases after gastric resection with D2 nodal dissection for adenocarcinoma of the stomach. Int J Radiat Oncol Biol Phys. 2005;63:1279-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 207] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 8. | Coburn NG, Govindarajan A, Law CH, Guller U, Kiss A, Ringash J, Swallow CJ, Baxter NN. Stage-specific effect of adjuvant therapy following gastric cancer resection: a population-based analysis of 4,041 patients. Ann Surg Oncol. 2008;15:500-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Takahashi T, Saikawa Y, Fukuda K, Wada N, Kawakubo H, Takeuchi H, Fukada J, Kawaguchi O, Takaishi H, Shigematsu N. [Chemoradiotherapy for advanced gastric cancer]. Gan To Kagaku Ryoho. 2012;39:2464-2468. [PubMed] |
| 10. | Jansen EP, Saunders MP, Boot H, Oppedijk V, Dubbelman R, Porritt B, Cats A, Stroom J, Valdés Olmos R, Bartelink H. Prospective study on late renal toxicity following postoperative chemoradiotherapy in gastric cancer. Int J Radiat Oncol Biol Phys. 2007;67:781-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 71] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 11. | Kassam Z, Mackay H, Buckley CA, Fung S, Pintile M, Kim J, Ringash J. Evaluating the impact on quality of life of chemoradiation in gastric cancer. Curr Oncol. 2010;17:77-84. [PubMed] |
| 12. | Gemici C, Sargin M, Uygur-Bayramicli O, Mayadagli A, Yaprak G, Dabak R, Kocak M. Risk of endocrine pancreatic insufficiency in patients receiving adjuvant chemoradiation for resected gastric cancer. Radiother Oncol. 2013;107:195-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Peltenburg LT. Radiosensitivity of tumor cells. Oncogenes and apoptosis. Q J Nucl Med. 2000;44:355-364. [PubMed] |
| 14. | Ding M, Zhang E, He R, Wang X. Newly developed strategies for improving sensitivity to radiation by targeting signal pathways in cancer therapy. Cancer Sci. 2013;104:1401-1410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | Pawlik TM, Keyomarsi K. Role of cell cycle in mediating sensitivity to radiotherapy. Int J Radiat Oncol Biol Phys. 2004;59:928-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 669] [Cited by in RCA: 787] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 16. | Manser E, Leung T, Salihuddin H, Zhao ZS, Lim L. A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature. 1994;367:40-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1189] [Cited by in RCA: 1266] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 17. | Kumar R, Gururaj AE, Barnes CJ. p21-activated kinases in cancer. Nat Rev Cancer. 2006;6:459-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 477] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 18. | Zenke FT, King CC, Bohl BP, Bokoch GM. Identification of a central phosphorylation site in p21-activated kinase regulating autoinhibition and kinase activity. J Biol Chem. 1999;274:32565-32573. [PubMed] |
| 19. | Dummler B, Ohshiro K, Kumar R, Field J. Pak protein kinases and their role in cancer. Cancer Metastasis Rev. 2009;28:51-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 211] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 20. | Li DQ, Nair SS, Ohshiro K, Kumar A, Nair VS, Pakala SB, Reddy SD, Gajula RP, Eswaran J, Aravind L. MORC2 signaling integrates phosphorylation-dependent, ATPase-coupled chromatin remodeling during the DNA damage response. Cell Rep. 2012;2:1657-1669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 117] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 21. | Motwani M, Li DQ, Horvath A, Kumar R. Identification of novel gene targets and functions of p21-activated kinase 1 during DNA damage by gene expression profiling. PLoS One. 2013;8:e66585. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Gan T, Wu Z, Tian L, Wang Y. Chinese herbal medicines for induction of remission in advanced or late gastric cancer. Cochrane Database Syst Rev. 2010;CD005096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Tan W, Lu J, Huang M, Li Y, Chen M, Wu G, Gong J, Zhong Z, Xu Z, Dang Y. Anti-cancer natural products isolated from chinese medicinal herbs. Chin Med. 2011;6:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 243] [Cited by in RCA: 262] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 24. | Zhang F, Xu L, Qu X, Zhao M, Jin B, Kang J, Liu Y, Hu X. Synergistic antitumor effect of β-elemene and etoposide is mediated via induction of cell apoptosis and cell cycle arrest in non-small cell lung carcinoma cells. Mol Med Rep. 2011;4:1189-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Zhan YH, Liu J, Qu XJ, Hou KZ, Wang KF, Liu YP, Wu B. β-Elemene induces apoptosis in human renal-cell carcinoma 786-0 cells through inhibition of MAPK/ERK and PI3K/Akt/ mTOR signalling pathways. Asian Pac J Cancer Prev. 2012;13:2739-2744. [PubMed] |
| 26. | Liu J, Zhang Y, Qu J, Xu L, Hou K, Zhang J, Qu X, Liu Y. β-Elemene-induced autophagy protects human gastric cancer cells from undergoing apoptosis. BMC Cancer. 2011;11:183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 109] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 27. | Liu JS, He SC, Zhang ZL, Chen R, Fan L, Qiu GL, Chang S, Li L, Che XM. Anticancer effects of β-elemene in gastric cancer cells and its potential underlying proteins: a proteomic study. Oncol Rep. 2014;32:2635-2647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 28. | Xia C, Ma W, Stafford LJ, Marcus S, Xiong WC, Liu M. Regulation of the p21-activated kinase (PAK) by a human Gbeta -like WD-repeat protein, hPIP1. Proc Natl Acad Sci USA. 2001;98:6174-6179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 59] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 29. | Bokoch GM. Biology of the p21-activated kinases. Annu Rev Biochem. 2003;72:743-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 846] [Cited by in RCA: 865] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 30. | Deacon SW, Beeser A, Fukui JA, Rennefahrt UE, Myers C, Chernoff J, Peterson JR. An isoform-selective, small-molecule inhibitor targets the autoregulatory mechanism of p21-activated kinase. Chem Biol. 2008;15:322-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 308] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 31. | Wong LL, Lam IP, Wong TY, Lai WL, Liu HF, Yeung LL, Ching YP. IPA-3 inhibits the growth of liver cancer cells by suppressing PAK1 and NF-κB activation. PLoS One. 2013;8:e68843. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 32. | Xu HB, Zheng LP, Li L, Xu LZ, Fu J. Elemene, one ingredient of a Chinese herb, against malignant tumors: a literature-based meta-analysis. Cancer Invest. 2013;31:156-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 33. | Wang G, Li X, Huang F, Zhao J, Ding H, Cunningham C, Coad JE, Flynn DC, Reed E, Li QQ. Antitumor effect of beta-elemene in non-small-cell lung cancer cells is mediated via induction of cell cycle arrest and apoptotic cell death. Cell Mol Life Sci. 2005;62:881-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 176] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 34. | Dent P, Yacoub A, Fisher PB, Hagan MP, Grant S. MAPK pathways in radiation responses. Oncogene. 2003;22:5885-5896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 457] [Cited by in RCA: 474] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 35. | He H, Baldwin GS. p21-activated kinases and gastrointestinal cancer. Biochim Biophys Acta. 2013;1833:33-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 36. | Roig J, Traugh JA. p21-activated protein kinase gamma-PAK is activated by ionizing radiation and other DNA-damaging agents. Similarities and differences to alpha-PAK. J Biol Chem. 1999;274:31119-31122. [PubMed] |
| 37. | Wang Z, Fu M, Wang L, Liu J, Li Y, Brakebusch C, Mei Q. p21-activated kinase 1 (PAK1) can promote ERK activation in a kinase-independent manner. J Biol Chem. 2013;288:20093-20099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 38. | Qiao L, Yacoub A, McKinstry R, Park JS, Caron R, Fisher PB, Hagan MP, Grant S, Dent P. Pharmocologic inhibitors of the mitogen activated protein kinase cascade have the potential to interact with ionizing radiation exposure to induce cell death in carcinoma cells by multiple mechanisms. Cancer Biol Ther. 2002;1:168-176. [PubMed] |