Published online Sep 7, 2015. doi: 10.3748/wjg.v21.i33.9765
Peer-review started: April 9, 2015
First decision: May 18, 2015
Revised: June 2, 2015
Accepted: July 8, 2015
Article in press: July 8, 2015
Published online: September 7, 2015
Processing time: 151 Days and 5.8 Hours
AIM: To investigate the relationship among pretreatment serum CXC chemokine ligand 10 (CXCL10), thyroid peroxidase antibody (TPOAb) levels and thyroid dysfunction (TD) in Chinese hepatitis C patients.
METHODS: One hundred and thirty-nine treatment-naive genotype 1 chronic hepatitis C patients with no history of TD or treatment with thyroid hormones were enrolled in this study. Patients underwent peginterferon alfa-2a/ribavirin (PegIFNα-2a/RBV) treatment for 48 wk, followed by detection of clinical factors at each follow-up point. Hepatitis C virus (HCV) antibodies were analyzed using microsomal chemiluminescence, and serum HCV RNA was measured by real-time PCR assay at 0, 4, 12, 24 and 48 wk after the initiation of therapy and 24 wk after the end of therapy. To assess thyroid function, serum thyroid stimulating hormone (TSH), free thyroxine (FT4), free triodothyronine (FT3) and TPOAb/thyroglobulin antibody (TGAb) levels were determined using chemiluminescent immunoassays every 3 mo. Serum CXCL10 levels were determined at baseline.
RESULTS: The prevalence of TD was 18.0%. Twenty-one (84.0%) out of twenty-five patients exhibited normal thyroid function at week 24 after therapy. The rate of sustained virological response to PegIFNα-2a/RBV in our study was 59.0% (82/139), independent of thyroid function. Pretreatment serum CXCL10 levels were significantly increased in patients with euthyroid status compared with patients with TD (495.2 ± 244.2 pg/mL vs 310.0 ± 163.4 pg/mL, P = 0.012). Patients with TD were more frequently TPOAb-positive than non-TD (NTD) patients (24.2% vs 12.3%, P = 0.047) at baseline. Three of the one hundred and fifteen patients without TPOAb at baseline developed TD at the end of treatment (37.5% vs 2.6%, P = 0.000). Female patients exhibited an increased risk for developing TD compared with male patients (P = 0.014).
CONCLUSION: Lower pretreatment serum CXCL10 levels are associated with TD, and TD prevalence increases in female patients and patients who are positive for TPOAb at baseline.
Core tip: We present novel data on the influence of peginterferon alfa-2a/ribavirin (PegIFNα-2a/RBV) on thyroid function in Chinese genotype 1 hepatitis C virus (HCV)-infected patients over a 48-wk treatment period. The results demonstrate that the prevalence of thyroid dysfunction (TD) was 18.0%. Lower pretreatment serum CXCL10 levels were associated with PegIFNα-2a/RBV induced TD in genotype 1 HCV-infected patients, and female patients exhibited an increased risk for developing TD compared with male patients. Baseline TPOAb positivity may also be a risk factor for TD development. However, most (84%) of the TD cases were reversible. To our knowledge, this is the first study to investigate the association of CXCL10 levels with PegIFNα-2a/RBV induced TD in genotype 1 HCV-infected patients in China.
- Citation: Zhang RW, Shao CP, Huo N, Li MR, Xi HL, Yu M, Xu XY. Thyroid dysfunction in Chinese hepatitis C patients: Prevalence and correlation with TPOAb and CXCL10. World J Gastroenterol 2015; 21(33): 9765-9773
- URL: https://www.wjgnet.com/1007-9327/full/v21/i33/9765.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i33.9765
Of the estimated 185 million people infected with hepatitis C virus (HCV) worldwide, 350000 die each year[1,2]. Currently, the standard treatment for chronic hepatitis C (CHC) patients in China is peginterferon and ribavirin in combination (PegIFNα-2a/RBV), with sustained virological response (SVR) rates of 54% to 80%[3,4].
Despite its success, interferon-alpha (IFN-α) has a well-documented side effect profile, including influenza-like symptoms, and hematologic abnormalities lead to dose reductions in up to 40% of patients and drug discontinuation in 14% of patients[5]. Thyroid diseases, such as the emergence of thyroid autoantibodies (TAs) and thyroid dysfunction (TD), are common in CHC patients and represent extrahepatic manifestations of HCV infection[6,7]. Subclinical thyroiditis occurs in 20% to 40% of CHC patients, and clinical thyroiditis occurs in 5% to 10% of CHC patients[8]. TD may result from IFN-based therapy. In some cases, IFN-induced TD may lead to the discontinuation of IFN therapy, thus representing a major clinical problem in hepatitis C patients receiving IFN-α therapy[8]. IFN-α-related TD has been widely investigated, and preliminary studies have suggested that there are at least two different models by which IFN-α may induce TD: immune-mediated effects or direct toxicity to the thyroid. IFN-α exerts various effects on the immune system, many of which may lead to the development of autoimmunity. Upon culture with human thyroid follicular cells, type I IFNs inhibit thyroid-stimulating hormone (TSH) -induced gene expression of thyroglobulin (TG), thyroperoxidase (TPO), and sodium iodide symporter (NIS). This study assessed TSH receptor, TG, and TPO gene expression levels in a rat thyroid cell line, and the results demonstrated that IFN-α has a direct toxic effect on the thyroid. Chronic HCV infection appears to play a significant role in triggering thyroiditis among IFN α-treated patients[8,9].
CXC chemokine ligand 10 (CXCL10 or IP-10), a member of the CXC chemokine family, is expressed in the liver of CHC patients and selectively recruits activated T cells to inflammatory sites[10]. Evidence also indicates that circulating CXCL10 levels increase in HCV-infected patients with autoimmune thyroiditis[11], potentially because CXCL10 recruits T-helper (Th) 1 lymphocytes. These cells secrete IFN-γ and tumor necrosis factor (TNF), promoting further CXCL10 secretion and perpetuating the autoimmune process[12,13].
Although most thyroid autoimmunity cases exhibit no clinical symptoms, they are often characterized by the expression of thyroid antibodies (TAs), including thyroperoxidase antibody (TPOAb) and thyroglobulin antibody (TGAb). Data from pooled studies revealed that the risk of developing TD in CHC patients with baseline TAs positivity was 46.1%; whereas this risk was only 5.4% in TAs-negative CHC patients[14]. Our preliminary results indicate that the positive TPOAb IgG2 subclass was a risk factor for TD in untreated HCV patients, and may play an important role in TD development in CHC patients[15]. The appearance of TPOAb before treatment was a strong indicator of subsequent TD for CHC patients receiving PegIFNα-2a/RBV combination therapy. Female and TAs-positive patients were also more likely to develop TD during IFNα/RBV therapy[9].
Previous investigations showed that the addition of RBV to IFN-α therapy in HCV patients could increase the risk of developing hypothyroidism[16]. However, it is not clear whether the addition of RBV affects the emergence of other TDs. Most studies have focused on the effects of combination therapy with standard IFN-α and RBV on the thyroid gland and demonstrated that the risk for developing TD during IFN-α therapy is closely correlated with mixed HCV genotype infection and lower HCV RNA levels, female gender, and pretreatment positivity for TAs (particularly TPOAb)[9]. Corresponding data on PegIFNα-2a/RBV induced TD in genotype 1 HCV-infected patients in China are rare and the related factors have not yet been fully elucidated.
In the present study, we investigated the relationship among TPOAb, pretreatment serum CXCL10 levels and the occurrence of PegIFNα-2a/RBV induced TD in patients with genotype 1 HCV infection in China.
This study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the ethics committee of Peking University People Hospital. Written informed consent was obtained from all participant subjects. Biological and behavioral information was linked anonymously to protect the participants’ privacy. This procedure was approved by the ethics committee.
Two hundred and sixty CHC patients who visited the Department of Infectious Diseases, Peking University First Hospital from September 2009 to June 2011 were included in this study. These patients came from five different regions of China (Beijing, Hebei province, Henan province, Heilongjiang province and Shanxi province), and the criteria for CHC diagnosis followed the Guideline of Prevention and Treatment of Hepatitis C[17]. All patients had compensated liver disease without cirrhosis, but never received hepatitis C treatment. Patients with hepatitis B virus (HBV) infection, or human immunodeficiency virus (HIV) infection and those who were pregnant or using amiodarone or lithium were excluded. HCV patients with other autoimmune disorders or treated with immuno-modulant drugs were also excluded. Further screening excluded 25 patients with a history of thyroid gland dysfunction, 80 patients who previously received IFN-α treatment and 26 patients who were not infected with genotype 1 HCV. A total of 139 HCV genotype 1 treatment-naïve patients were enrolled in the final study. All participants included had euthyroid status and never received thyroid hormone treatment.
All enrolled CHC genotype-1 patients received a weekly 180 μg subcutaneous dose of PegIFNα-2a and a daily 600-1000 mg (according to body weight) dose of RBV for 48 wk.
All patients fasted for 12 h prior to blood tests. Alanine aminotransferase (ALT), aspartate aminotransferase (AST), total and direct bilirubin (TBIL and DBIL), and albumin (ALB) were determined using an automatic biochemical analyzer[18]. HCV antibodies were analyzed using microsomal chemiluminescence (Abbott Diagnostics Division)[19], and serum HCV RNA was measured by real-time PCR assay (COBAS Taqman HCV Test; Roche Molecular Systems, Pleasanton, CA) at 0, 4, 12, 24 and 48 wk after the initiation of therapy and 24 wk after the end of therapy.
To assess thyroid function, serum TSH, TPOAb/TGAb, free thyroxine (FT4) and free triodothyronine (FT3) levels were determined using chemiluminescent immunoassays every 3 mo. Briefly, assay kits for TSH, FT3, and FT4 were purchased from ADVIA Centaur (Bayer Healthcare Diagnostics) and the kit for TPOAb/TGAb was from IMMULITE 1000 (Diagnostic Products Corporation, Los Angeles, CA, United States). The normal range of each assay was as follows: TSH, 0.35-5.5 μIU/mL; FT3, 3.50-6.50 pmol/L; and FT4, 11.48-22.70 pmol/L.
Clinical hypothyroidism was defined as a serum TSH level greater than 5.5 μIU/mL and a FT4 level less than 11.48 pmol/L. Clinical hyperthyroidism was diagnosed when TSH was less than 0.35 μIU/mL and FT4 was greater than 22.7 pmol/L and/or FT3 was greater than 6.5 pmol/L. Subclinical hypothyroidism or hyperthyroidism was diagnosed when serum TSH levels were greater than 5.5 μIU/mL or less than 0.35 μIU/mL, respectively, with normal FT3 and FT4 levels. TAs were considered positive when TPOAb ≥ 35 IU/mL or TGAb ≥ 40 IU/mL[15].
Serum CXCL10 levels were measured prior to treatment using the Quantikine human CXCL10 immunoassay (RD Systems, Minneapolis, MN, United States). All blood samples were stored at -80 °C until use in assays. These samples were diluted 1:2 with Calibrator Diluent RD6Q solution and analyzed in duplicate. The linear dynamic range for CXCL10 measurement in this assay was 7.8 to 500 pg/mL.
Categorical variables were compared between the groups using the χ2 test or the Fisher’s exact test. Continuous variables were assessed using Student’s t-test or the Mann-Whitney U test. Differences with a two-tailed P-value < 0.05 were considered statistically significant. Statistical analyses were conducted using SPSS version 16.0 (SPSS Inc, Chicago, IL, United States).
The demographic characteristics of the 139 CHC patients enrolled in the study are presented in Table 1. In total, 70 male and 69 female CHC patients, with a mean age of 46.8 ± 14.0 years, participated in this study. Twenty-four (17.3%) patients were TPOAb and/or TGAb positive (TPOAb: 15.8%; TGAb: 8.6%). Nine out of twenty-five patients with TD were TAs positive, and the ratio of TPOAb to TGAb was 8:3.
The overall prevalence of thyroid abnormities was 18.0% (5.0% in men and 13.0% in women) during the therapy. Following 48 wk of exposure to PegIFNα-2a/RBV, 25 out of 139 patients developed TD, including 7 (6 females and 1 male) with subclinical hyperthyroidism, 16 (10 females and 6 males) with subclinical hypothyroidism and 2 female patients with hypothyroidism. Table 2 summarizes the findings from the patients who had chronic hepatitis C and developed PegIFNα-2a/RBV-induced TD. Clinical hypothyroidism (1.4%) and subclinical hypothyroidism (11.5%) were more frequent than clinical hyperthyroidism and subclinical hyperthyroidism (5.0%). Of these 25 patients, 15 developed TD at 24 wk of the therapy, including 9 with subclinical hypothyroidism, 5 with subclinical hyperthyroidism and 1 with hypothyroidism. An additional 2 patients developed subclinical hyperthyroidism at week 36 of the therapy. At 48 wk of the therapy, 3 patients with subclinical hypothyroidism and 1 patient with overt hypothyroidism were observed. In patients with overt hypothyroidism, we began L-thyroxine treatment, which halted TD progression. In 21 (84.0%) out of 25 patients, normal thyroid function was restored at 24 wk after the end of therapy. PegIFNα-2a/RBV SVR rate was 59.0% (82/139), independent of thyroid function.
| No. | Gender | Age(yr) | Diagnosis time | FT3 | FT4 | TSH | Diagnosis | Outcome |
| (3.50-6.50 pmol/L) | (11.48-22.70 pmol/L) | (0.35-5.50 μIU/mL) | ||||||
| 1 | Female | 53 | 12th wk | 4.81 | 11.66 | 6.62 | Sub hypo | Normal |
| 2 | Female | 61 | 48th wk | 4.67 | 12.19 | 19.61 | Sub hypo | Normal |
| 3 | Female | 42 | 24th wk | 6.02 | 11.56 | 7.13 | Sub hypo | Normal |
| 4 | Female | 40 | 24th wk | 5.88 | 11.71 | 8.95 | Sub hypo | Normal |
| 5 | Female | 46 | 24th wk | 4.13 | 14.13 | 6.25 | Sub hypo | Normal |
| 6 | Female | 56 | 24th wk | 5.46 | 24.55 | 11.01 | Sub hypo | Normal |
| 7 | Female | 56 | 48th wk | 5.12 | 12.89 | 8.45 | Sub hypo | Normal |
| 8 | Female | 44 | 48th wk | 4.00 | 24.36 | 6.31 | Sub hypo | Normal |
| 9 | Female | 41 | 12th wk | 6.19 | 13.48 | 5.58 | Sub hypo | Sub hyper |
| 10 | Female | 45 | 12th wk | 5.27 | 19.24 | 6.15 | Sub hypo | Normal |
| 11 | Male | 39 | 24th wk | 5.36 | 13.45 | 23.19 | Sub hypo | Normal |
| 12 | Male | 28 | 24th wk | 5.44 | 15.27 | 6.66 | Sub hypo | Normal |
| 13 | Male | 53 | 24th wk | 3.79 | 14.67 | 8.71 | Sub hypo | Normal |
| 14 | Male | 50 | 12th wk | 6.20 | 11.85 | 37.42 | Sub hypo | Normal |
| 15 | Male | 51 | 24th wk | 5.15 | 12.47 | 6.46 | Sub hypo | Normal |
| 16 | Male | 18 | 24th wk | 4.95 | 13.99 | 6.16 | Sub hypo | Normal |
| 17 | Female | 43 | 24th wk | 6.34 | 13.89 | 0.32 | Sub hyper | Sub hyper |
| 18 | Female | 21 | 36th wk | 4.37 | 21.24 | 0.29 | Sub hyper | Normal |
| 19 | Female | 38 | 36th wk | 5.72 | 12.97 | 0.24 | Sub hyper | Normal |
| 20 | Female | 28 | 24th wk | 4.19 | 21.24 | 0.32 | Sub hyper | Normal |
| 21 | Female | 42 | 24th wk | 4.67 | 16.47 | 0.01 | Sub hyper | Normal |
| 22 | Female | 33 | 24th wk | 5.31 | 11.58 | 0.12 | Sub hyper | Normal |
| 23 | Male | 70 | 24th wk | 5.77 | 16.70 | 0.02 | Sub hyper | Normal |
| 24 | Female | 21 | 24th wk | 5.90 | 5.97 | 6.16 | Hypo | Therapy |
| 25 | Female | 66 | 48th wk | 6.03 | 0.97 | 6.58 | Hypo | Therapy |
Table 3 presents the baseline characteristics of the CHC patients with TD and NTD. Female patients exhibited an increases risk for TD development compared with male patients. Serum AST levels and the frequency of TPOAb positivity in TD patients were significantly increased compared with NTD patients (AST levels: P = 0.018; TPOAb positivity: 24.2% vs 12.3%, P = 0.047). However, no significant differences in ALT, TBIL, DBIL, ALB, HCV RNA levels, or the percentage of TGAb-positive patients were noted between the TD and NTD groups (P > 0.05).
| TDIFN | NTDIFN | P value | |
| Gender (%, male/female) | 28.0/72.0 | 55.3/45.7 | 0.014 |
| Age (yr)1 | 43.40 ± 13.60 | 47.50 ± 14.0 | 0.183 |
| HCV RNA (log10, IU/mL)2 | 6.23 (3.54, 7.52) | 6.36 (3.0, 7.74) | 0.919 |
| ALT (IU/L)2 | 81.0 (26, 416) | 55.0 (10, 527) | 0.162 |
| AST (IU/L)2 | 67.0 (26, 248) | 41.5 (16, 264) | 0.018 |
| TBIL(μmol/L)2 | 17.20 (8.9, 36.4) | 16.0 (5.0, 150.0) | 0.732 |
| DBIL(μmol/L)2 | 4.57 (0.69, 13.80) | 4.5 (0.48, 108) | 0.447 |
| ALB (g/L)1 | 42.30 ± 4.24 | 42.62 ± 4.15 | 0.764 |
| TPOAb positivity | 24.2% | 12.3% | 0.047 |
| TGAb positivity | 12.0% | 7.9% | 0.469 |
| CXCL10 level (pg/mL)1 | 310.0 ± 163.4 | 495.20 ± 244.2 | 0.012 |
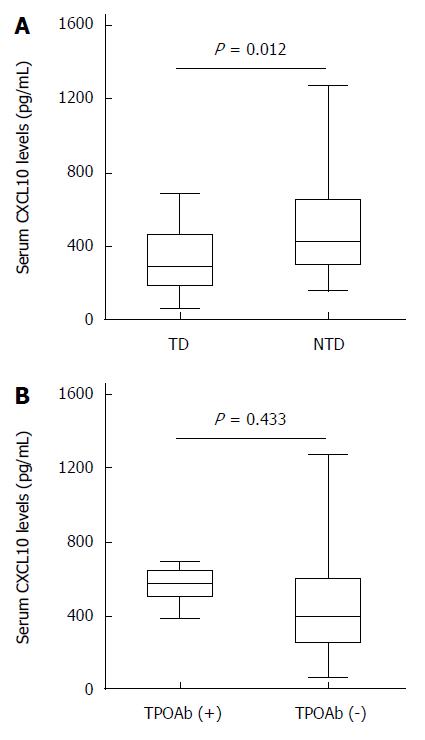
In our study, pretreatment serum CXCL10 levels were significantly increased in patients with euthyroid status compared with TD patients (495.2 ± 244.2 pg/mL vs 310.0 ± 163.4 pg/mL, P = 0.012) (Figure 1A). Although pretreatment serum CXCL10 levels were increased in TPOAb-positive vs TPOAb-negative patients, no significant differences were detected. (TPOAb positive/negative: 542.5 ± 107.2 pg/mL vs 442.3 ± 249.8 pg/mL, P = 0.433) (Figure 1B).
The percentages of patients positive for TPOAb and/or TGAb were 17.3% (24/139) at baseline and 22.3% (31/139) at the end of treatment (Table 4). Nine of twenty-four patients with TPOAb/TGAb at baseline developed TD. By contrast, three (one male and two females) of one hundred and fifteen patients without TPOAb/TGAb at baseline developed TD at the end of the treatment (37.5% vs 2.6%, P = 0.000).
| TPOAb (+) only | TGAb (+) only | Both (+) | Both (-) | |
| At baseline | 12 | 7 | 5 | 115 |
| At the end of treatment | 13 | 10 | 8 | 108 |
We present novel data regarding the influence of PegIFNα-2a combined with RBV on thyroid function in Chinese adult genotype 1 HCV-infected patients over a 48-wk treatment period. The results demonstrate that the prevalence of thyroid abnormities was 18.0%, and lower pretreatment serum CXCL10 levels were associated with PegIFNα-2a/RBV induced TD. The prevalence of TD was increased in female patients and those who were TPOAb-positive at baseline. However, most (84%) of the TD cases were reversible. To our knowledge, this is the first study to investigate the association of CXCL10 levels with PegIFNα-2a/RBV-induced TD in genotype 1 HCV-infected patients in China.
In our study, the PegIFNα-2a/RBV SVR rate was 59.0% (82/139), independent of thyroid function. After 48 wk of PegIFNα-2a/RBV treatment, 25 out of 139 patients developed TD, including 16 patients with subclinical hypothyroidism, 7 with subclinical hyperthyroidism and 2 with hypothyroidism. Although a previous study reported that hypothyroidism was the most common type of TD induced by IFN[20,21], subclinical hypothyroidism was most prevalent in our study. This discrepancy may be explained by differences in patient ethnicities, genetic backgrounds and the type of IFN used.
IFN-associated thyroid disease was first reported in 1985 when three cases of hypothyroidism were observed in breast cancer patients who received IFN α treatment[22]. Studies report an incidence of TD during IFN-α plus RBV combination therapy of 4.7% to 27.8%[23], which may result from immune activation mediated by IFN. Jami et al[24] demonstrated that patients who used pegylated IFN had a higher risk of TD than those using conventional IFN (14% vs 7%, P = 0.038). However, in a meta-analysis, Tran et al[25] found that pegylated IFN in combination with RBV did not cause more thyroid diseases in HCV-infected patients than classical IFN plus RBV. This variation may be explained by the differences in the race of the patients. In our study, 18.0% (25/139) of Chinese HCV-infected patients developed TD during PegIFNα-2a/RBV therapy. The incidence of TD in our subjects was increased compared with a large Australian cohort, in which approximately 14% of patients developed TD during PegIFN/RBV therapy[24]. The difference between these two studies may result from differences in the race of the included patient populations and the virus genotypes.
RBV can modulate the Th1 and Th2 subset balance by activating type 1 cytokines in the HCV-specific immune response. Furthermore, RBV could also enhance the non-virus-induced immune response, suggesting that RBV, as a type 1-inducing agent, can trigger autoimmune phenomena in predisposed patients[26]. In previous studies, the incidence of TD induced by IFN monotherapy in CHC patients was 4% to 18%, with a mean incidence of approximately 6% in a meta-analysis study[27]. Earlier studies reveal that the mean incidence of TD in patients treated with combination therapy is increased compared with those treated with IFN alone[16].
In the present study, the enrolled genotype-1 patients received PegIFNα-2a/RBV treatment for 48 wk, and the prevalence of thyroid abnormities was 18.0%. Some studies have suggested that higher doses of IFN-α and longer durations of therapy are risk factors for the development of IFN induced TD[14]. It is therefore possible that the longer time period of 48 wk of therapy in our study increased the likelihood that patients developed TD. Fifteen patients developed TD at 24 wk of therapy, including 9 with subclinical hypothyroidism, 5 with subclinical hyperthyroidism and 1 with hypothyroidism. An additional 2 patients developed subclinical hyperthyroidism at 36 wk of therapy. At 48 wk of therapy, 3 patients with subclinical hypothyroidism and 1 patient with overt hypothyroidism were noted. L-thyroxine treatment was initiated in patients with overt hypothyroidism. It is worth noting that, by the end of the therapy, TD did not further progress among these patients.
Whether the long-term evolution of TD is induced by IFN-α therapy remains controversial. Some studies indicate that TD is reversible in all patients, whereas others report that TD is only reversible in a proportion of the patients[28,29]. In our study, at week 24 post-treatment, normal thyroid function was restored in 21 (84.0%) out of 25 patients. Such a discrepancy may result from the short time period of the follow-up study and a subsequently incomplete evaluation of TD status. There also may have been additional factors, such as differences in study designs, the time period of the follow-up, the population races, and individual variations.
Our data revealed that 17.3% of patients were TAs-positive, whereas previous studies reported TAs incidence rates in HCV-infected patients ranging from 10% to 45%; this discrepancy may be related to differences in the population race, genetic variations, geographical distribution, and environmental factors[16]. Data from the pooled studies indicate that the risk of TD in patients who were positive for TAs at baseline was increased compared with those without TAs at baseline[14]. Female gender and TAs positivity were shown to be the predictive factors of TD development during IFN-α/RBV therapy[24,30]. In our study, female patients had a higher risk for the development of TD than male patients. The baseline TPOAb-positivity have been suggested to be a risk factor for TD development secondarily to PegIFNα-2a/RBV treatment. We also demonstrated that the percentage of patients with elevated autoantibody levels developing TD was significantly higher than that of patients with normal autoantibody levels before treatment.
A previous large-scale study in patients receiving combination therapy demonstrated that TGAb was present in 91.7% of patients, whereas TPOAb was present in 83.3% of those with overt hypothyroidism[31]. Thus, in combination therapy, TAs play an important role in predicting the emergence of TD. Analyzing TAs levels before combination therapy may therefore identify patients at risk for developing PegIFNα-2a/RBV-associated TD.
Many studies have noted the Th1 immune response and changes in CXCL10 chemokine level during HCV infection. It was recently reported that HCV-infected patients who developed IFN-induced dysfunction exhibited Th1 polarization in their innate immune responses. The Th1 immune response is characterized by increased IFN-γ and TNF-α production by Th1 lymphocytes. These chemokines subsequently stimulate CXCL10 secretion from the hepatocytes in chronic HCV infection, thus perpetuating the immune cascade[32]. Elevated serum CXCL10 levels are not only associated with the development of autoimmunity, but also lead to thyroid follicular destruction and hypothyroidism. Antonelli et al[33] demonstrated that the development of TD during the IFN-α therapy correlated with significantly reduced CXCL10 serum levels, both before and during the treatment. A prospective study found that CXCL10 increased in HCV-infected patients, with no associated TD development, even after matching for sex and age[34]. We demonstrated that pretreatment serum CXCL10 levels were significantly increased in patients with euthyroid status compared with patients with TD. Although pretreatment serum CXCL10 levels were higher in TPOAb-positive than in TPOAb-negative patients, no significant difference was detected. However, the prevalence of TD was increased in patients who were TPOAb-positive at baseline than patients who were not TPOAb-positive at baseline.
Evidence also indicates that circulating CXCL10 levels increase in HCV-infected patients with autoimmune thyroiditis[11], potentially because CXCL10 recruits T-helper (Th) 1 lymphocytes. Indeed, it is reasonable to hypothesize that the changes in serum CXCL10 may be more evident in patients developing overt TD, who show a microenvironment much more enriched in Th1 molecules[33]. In our study, although high standard deviation was observed for both categories of patients, there was an important variation of the CXCL10 levels in HCV patients with or without TD. At least in the studied population, the values of CXCL10 were significantly lower in patients who developed TD. Maybe the reason is that the number of patients with overt TD was too small (only two) in our studied populations. Therefore, our results should be confirmed by studies with a much larger sample size.
We studied the occurrence of TD in genotype 1 HCV-infected patients, without examining other genotypes. Our findings must be confirmed by studies using a larger sample size with a longer follow-up period.
In conclusion, low pretreatment serum CXCL10 levels were associated with PegIFNα-2a/RBV induced TD in genotype 1 HCV-infected patients in China. The prevalence of TD was increased in female patients and patients who were TPOAb-positive at baseline. The appearance of TPOAb before treatment is predictive of subsequent TD for CHC patients receiving PegIFNα-2a/RBV combination therapy. Screening for TPOAb and CXCL10 before combination therapy may identify high-risk patients who are more likely to develop PegIFNα-2a/RBV-associated TD. Further studies are needed to elucidate the characteristics and mechanisms involved in PegIFNα-2a/RBV-induced TD in HCV-infected patients.
Currently, the standard treatment for chronic hepatitis C (CHC) in China is combination peginterferon and ribavirin (RBV) therapy, and the sustained virological response rates are 54% to 80%. Despite its success, interferon (IFN)-α has a well-documented side effect profile, including thyroid diseases. The emergence of thyroid dysfunction (TD) may result from IFN-based therapy. In some cases, IFN-induced TD may cause the discontinuation of IFN therapy. Most studies have focused on the effects of combination therapy with standard IFN-α and ribavirin (RBV) on the thyroid gland and demonstrated that the risk for developing TD during IFN-α therapy is closely correlated with female gender and pretreatment TAs positivity (particularly TPOAb). Evidence indicates that circulating CXCL10 levels are increased in HCV-infected patients with autoimmune thyroiditis. The relationship among the pretreatment serum CXCL10 levels, TPOAb levels and the occurrence of peginterferon alfa-2a (PegIFNα-2a)/RBV-induced TD in patients with genotype 1 HCV infection in China is unclear.
CXCL10 recruits Th1 lymphocytes, which secrete IFN-γ and tumor necrosis factor, leading to further CXCL10 secretion and potentially the development of the autoimmunity. Data from pooled studies revealed that the risk of developing TD in CHC patients who were TAs-positive (TPOAb and TGAb) at baseline was 46.1%. By contrast, this risk was only 5.4% in CHC patients who were TAs-negative at baseline. The preliminary results indicate that the TPOAb IgG2 subclass was a risk factor for TD in untreated HCV patients, and may play an important role in TD development in CHC patients. The appearance of TPOAb before treatment is predictive of subsequent thyroid dysfunction for CHC patients receiving PegIFNα-2a/RBV combination therapy.
Lower pretreatment serum CXCL10 levels are associated with PegIFNα-2a/RBV- induced TD in genotype 1 HCV-infected patients in China. The frequency of TD is increased in female patients and patients who are TPOAb-positive at baseline. However, most (84%) of the TD cases were reversible. This is the first study to investigate the association of CXCL10 levels with PegIFNα-2a/RBV- induced TD in genotype 1 HCV-infected patients in China.
The study results indicate that screening for TPOAb and CXCL10 before combination therapy may identify the patients who are at high risk for developing PegIFNα-2a/RBV-associated thyroid dysfunction.
Clinical hypothyroidism was defined by serum TSH levels greater than 5.5 μIU/mL and FT4 less than 11.48 pmol/L; whereas clinical hyperthyroidism was diagnosed when TSH levels were less than 0.35 μIU/mL and FT4 was greater than 22.7 pmol/L and/or FT3 was greater than 6.5 pmol/L. Subclinical hypothyroidism or hyperthyroidism were defined by serum TSH levels higher than 5.5 μIU/mL or lower than 0.35 μIU/mL, respectively, with normal levels of FT3 and FT4. The patients was considered to be positive for TAs when TPOAb was greater than or equal to 35 IU/mL or TGAb was greater than or equal to 40 IU/mL.
Well-written and with valuable data, this manuscript reinforces the recommendation that HCV-infected patients should be screened for the presence of thyroid dysfunction markers before undergoing IFN-a/ribavirin treatment, because such treatment may increase the prevalence of TD. The value of TPOAb positivity as a marker for treatment-induced TD in HCV infected patients is also suggested by several studies. There is variation in CXCL10 levels in HCV patients with or without TD, as demonstrated by a high standard deviation observed for both categories of patients.
P- Reviewer: Florea D, Hughes E, Keppeke GD S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Ma S
| 1. | Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57:1333-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1770] [Cited by in RCA: 1847] [Article Influence: 153.9] [Reference Citation Analysis (3)] |
| 2. | Lavanchy D. The global burden of hepatitis C. Liver Int. 2009;29 Suppl 1:74-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 933] [Cited by in RCA: 939] [Article Influence: 58.7] [Reference Citation Analysis (1)] |
| 3. | McHutchison JG, Gordon SC, Schiff ER, Shiffman ML, Lee WM, Rustgi VK, Goodman ZD, Ling MH, Cort S, Albrecht JK. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. Hepatitis Interventional Therapy Group. N Engl J Med. 1998;339:1485-1492. [PubMed] |
| 4. | Yu ML, Dai CY, Huang JF, Hou NJ, Lee LP, Hsieh MY, Chiu CF, Lin ZY, Chen SC, Hsieh MY. A randomised study of peginterferon and ribavirin for 16 versus 24 weeks in patients with genotype 2 chronic hepatitis C. Gut. 2007;56:553-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 232] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 5. | Russo MW, Fried MW. Side effects of therapy for chronic hepatitis C. Gastroenterology. 2003;124:1711-1719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 193] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 6. | Hsieh MY, Dai CY, Lee LP, Huang JF, Tsai WC, Hou NJ, Lin ZY, Chen SC, Wang LY, Chang WY. Antinuclear antibody is associated with a more advanced fibrosis and lower RNA levels of hepatitis C virus in patients with chronic hepatitis C. J Clin Pathol. 2008;61:333-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | Huang JF, Chuang WL, Yu ML, Yu SH, Huang CF, Huang CI, Yeh ML, Hsieh MH, Yang JF, Lin ZY. Hepatitis C virus infection and metabolic syndrome---a community-based study in an endemic area of Taiwan. Kaohsiung J Med Sci. 2009;25:299-305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Tomer Y, Blackard JT, Akeno N. Interferon alpha treatment and thyroid dysfunction. Endocrinol Metab Clin North Am. 2007;36:1051-1066; x-xi. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 95] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 9. | Carella C, Mazziotti G, Amato G, Braverman LE, Roti E. Clinical review 169: Interferon-alpha-related thyroid disease: pathophysiological, epidemiological, and clinical aspects. J Clin Endocrinol Metab. 2004;89:3656-3661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 83] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Abe T, Fukuhara T, Wen X, Ninomiya A, Moriishi K, Maehara Y, Takeuchi O, Kawai T, Akira S, Matsuura Y. CD44 participates in IP-10 induction in cells in which hepatitis C virus RNA is replicating, through an interaction with Toll-like receptor 2 and hyaluronan. J Virol. 2012;86:6159-6170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Antonelli A, Fallahi P, Ferrari SM, Sebastiani M, Manfredi A, Mazzi V, Fabiani S, Centanni M, Marchi S, Ferri C. Circulating CXCL11 and CXCL10 are increased in hepatitis C-associated cryoglobulinemia in the presence of autoimmune thyroiditis. Mod Rheumatol. 2012;22:659-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Antonelli A, Fallahi P, Rotondi M, Ferrari SM, Romagnani P, Grosso M, Ferrannini E, Serio M. Increased serum CXCL10 in Graves’ disease or autoimmune thyroiditis is not associated with hyper- or hypothyroidism per se, but is specifically sustained by the autoimmune, inflammatory process. Eur J Endocrinol. 2006;154:651-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 13. | Antonelli A, Ferri C, Ferrari SM, Colaci M, Sansonno D, Fallahi P. Endocrine manifestations of hepatitis C virus infection. Nat Clin Pract Endocrinol Metab. 2009;5:26-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 82] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 14. | Mandac JC, Chaudhry S, Sherman KE, Tomer Y. The clinical and physiological spectrum of interferon-alpha induced thyroiditis: toward a new classification. Hepatology. 2006;43:661-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 116] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 15. | Shao C, Huo N, Zhao L, Gao Y, Fan X, Zheng Y, Wang L, Lu H, Xu X, Guo X. The presence of thyroid peroxidase antibody of IgG2 subclass is a risk factor for thyroid dysfunction in chronic hepatitis C patients. Eur J Endocrinol. 2013;168:717-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Carella C, Mazziotti G, Morisco F, Rotondi M, Cioffi M, Tuccillo C, Sorvillo F, Caporaso N, Amato G. The addition of ribavirin to interferon-alpha therapy in patients with hepatitis C virus-related chronic hepatitis does not modify the thyroid autoantibody pattern but increases the risk of developing hypothyroidism. Eur J Endocrinol. 2002;146:743-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 49] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Asian Pacific Association for the Study of the Liver (APASL) Hepatitis C Working Party; McCaughan GW, Omata M, Amarapurkar D, Bowden S, Chow WC, Chutaputti A, Dore G, Gane E, Guan R, Hamid SS, Hardikar W, Hui CK, Jafri W, Jia JD, Lai MY, Wei L, Leung N, Piratvisuth T, Sarin S, Sollano J, Tateishi R. Asian Pacific Association for the Study of the Liver consensus statements on the diagnosis, management and treatment of hepatitis C virus infection. J Gastroenterol Hepatol. 2007;22:615-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 124] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 18. | Wang LF, Wu CH, Shan Y, Fan XH, Huo N, Lu HY, Xu XY. Prevalence of abnormal glycometabolism in patients with chronic hepatitis C and related risk factors in China. Chin Med J (Engl). 2011;124:183-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 19. | Bai L, Feng ZR, Lu HY, Li WG, Yu M, Xu XY. Prevalence of antinuclear and anti-liver-kidney-microsome type-1 antibodies in patients with chronic hepatitis C in China. Chin Med J (Engl). 2009;122:5-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 20. | Schultz M, Müller R, von zur Mühlen A, Brabant G. Induction of hyperthyroidism by interferon-alpha-2b. Lancet. 1989;1:1452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Gisslinger H, Gilly B, Woloszczuk W, Mayr WR, Havelec L, Linkesch W, Weissel M. Thyroid autoimmunity and hypothyroidism during long-term treatment with recombinant interferon-alpha. Clin Exp Immunol. 1992;90:363-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 79] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Fentiman IS, Thomas BS, Balkwill FR, Rubens RD, Hayward JL. Primary hypothyroidism associated with interferon therapy of breast cancer. Lancet. 1985;1:1166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 88] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Costelloe SJ, Wassef N, Schulz J, Vaghijiani T, Morris C, Whiting S, Thomas M, Dusheiko G, Jacobs M, Vanderpump MP. Thyroid dysfunction in a UK hepatitis C population treated with interferon-alpha and ribavirin combination therapy. Clin Endocrinol (Oxf). 2010;73:249-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Jamil KM, Leedman PJ, Kontorinis N, Tarquinio L, Nazareth S, McInerney M, Connelly C, Flexman J, Burke V, Metcalf C. Interferon-induced thyroid dysfunction in chronic hepatitis C. J Gastroenterol Hepatol. 2009;24:1017-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Tran HA, Attia JR, Jones TL, Batey RG. Pegylated interferon-alpha2beta in combination with ribavirin does not aggravate thyroid dysfunction in comparison to regular interferon-alpha2beta in a hepatitis C population: meta-analysis. J Gastroenterol Hepatol. 2007;22:472-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Tam RC, Pai B, Bard J, Lim C, Averett DR, Phan UT, Milovanovic T. Ribavirin polarizes human T cell responses towards a Type 1 cytokine profile. J Hepatol. 1999;30:376-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 231] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 27. | Hsieh MC, Yu ML, Chuang WL, Shin SJ, Dai CY, Chen SC, Lin ZY, Hsieh MY, Liu JF, Wang LY. Virologic factors related to interferon-alpha-induced thyroid dysfunction in patients with chronic hepatitis C. Eur J Endocrinol. 2000;142:431-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 52] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 28. | Huang JF, Chuang WL, Dai CY, Chen SC, Lin ZY, Lee LP, Lee PL, Wang LY, Hsieh MY, Chang WY. The role of thyroid autoantibodies in the development of thyroid dysfunction in Taiwanese chronic hepatitis C patients with interferon-alpha and ribavirin combination therapy. J Viral Hepat. 2006;13:396-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Baudin E, Marcellin P, Pouteau M, Colas-Linhart N, Le Floch JP, Lemmonier C, Benhamou JP, Bok B. Reversibility of thyroid dysfunction induced by recombinant alpha interferon in chronic hepatitis C. Clin Endocrinol (Oxf). 1993;39:657-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 82] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 30. | Gelu-Simeon M, Burlaud A, Young J, Pelletier G, Buffet C. Evolution and predictive factors of thyroid disorder due to interferon alpha in the treatment of hepatitis C. World J Gastroenterol. 2009;15:328-333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 31. | Bini EJ, Mehandru S. Incidence of thyroid dysfunction during interferon alfa-2b and ribavirin therapy in men with chronic hepatitis C: a prospective cohort study. Arch Intern Med. 2004;164:2371-2376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 41] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 32. | Mazziotti G, Sorvillo F, Piscopo M, Morisco F, Cioffi M, Stornaiuolo G, Gaeta GB, Molinari AM, Lazarus JH, Amato G. Innate and acquired immune system in patients developing interferon-alpha-related autoimmune thyroiditis: a prospective study. J Clin Endocrinol Metab. 2005;90:4138-4144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 33. | Antonelli A, Rotondi M, Fallahi P, Romagnani P, Ferrari SM, Buonamano A, Ferrannini E, Serio M. High levels of circulating CXC chemokine ligand 10 are associated with chronic autoimmune thyroiditis and hypothyroidism. J Clin Endocrinol Metab. 2004;89:5496-5499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 84] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 34. | Rotondi M, Minelli R, Magri F, Leporati P, Romagnani P, Baroni MC, Delsignore R, Serio M, Chiovato L. Serum CXCL10 levels and occurrence of thyroid dysfunction in patients treated with interferon-alpha therapy for hepatitis C virus-related hepatitis. Eur J Endocrinol. 2007;156:409-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |