Published online Aug 28, 2015. doi: 10.3748/wjg.v21.i32.9534
Peer-review started: March 16, 2015
First decision: May 18, 2015
Revised: June 1, 2015
Accepted: July 8, 2015
Article in press: July 8, 2015
Published online: August 28, 2015
Processing time: 167 Days and 0 Hours
AIM: To investigate how the saturated and unsaturated fatty acid composition influences the susceptibility of developing acute pancreatitis.
METHODS: Primary pancreatic acinar cells were treated with low and high concentrations of different saturated and unsaturated fatty acids, and changes in the cytosolic Ca2+ signal and the expression of protein kinase C (PKC) were measured after treatment.
RESULTS: Unsaturated fatty acids at high concentrations, including oleic acid, linoleic acid, palmitoleic acid, docosahexaenoic acid, and arachidonic acid, induced a persistent rise in cytosolic Ca2+ concentrations in acinar cells. Unsaturated fatty acids at low concentrations and saturated fatty acids, including palmitic acid, stearic acid, and triglycerides, at low and high concentrations were unable to induce a rise in Ca2+ concentrations in acinar cells. Unsaturated fatty acids at high concentrations but not saturated fatty acids induced intra-acinar cell trypsin activation and cell damage and increased PKC expression.
CONCLUSION: At sufficiently high concentrations, unsaturated fatty acids were able to induce acinar cells injury and promote the development of pancreatitis. Unsaturated fatty acids may play a distinctive role in the pathogenesis of pancreatitis through the activation of PKC family members.
Core tip: The mechanism by which severe hypertriglyceridemia precipitates acute pancreatitis remains unknown. Abnormal sustained elevated cytosolic Ca2+ signals, which cause abnormal intracellular enzyme activation, are crucial in the initiation of acute pancreatitis. Unsaturated fatty acids at high concentrations induced a persistent rise in cytosolic Ca2+ concentrations in acinar cells and caused intra-acinar cell trypsin activation and cell damage. Unsaturated fatty acids at low concentrations and saturated fatty acids and triglycerides at low and high concentrations were unable to induce a rise in Ca2+ concentrations in acinar cells. Unsaturated fatty acids at high concentrations may play a crucial and distinctive role in the pathogenesis of hypertriglyceridemic pancreatitis.
- Citation: Chang YT, Chang MC, Tung CC, Wei SC, Wong JM. Distinctive roles of unsaturated and saturated fatty acids in hyperlipidemic pancreatitis. World J Gastroenterol 2015; 21(32): 9534-9543
- URL: https://www.wjgnet.com/1007-9327/full/v21/i32/9534.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i32.9534
Hypertriglyceridemia (HTG) is associated with acute pancreatitis (AP) and is observed in 3%-38% of patients with AP[1-6]. HTG is the third-most frequent etiology of AP in Taiwan[7]. Although the mortality rate of hyperlipidemic pancreatitis (HLP) does not differ from that of AP due to other etiologies, the disease severity and complication rates of HLP are higher than those of other forms of AP[8,9].
The mechanism by which severe HTG precipitates AP remains unknown. Havel proposed that pancreatitis results from toxic injury to the capillary endothelium and pancreatic acinar cells through the liberation of free fatty acids (FFA) by pancreatic lipase[10]. This hypothesis was supported by experimental studies by Saharia et al[11]; perfusion of the pancreas with FFA induced edema and hemorrhage of the pancreatic preparation. FFA has been reported to induce activation of trypsinogen and to initiate AP[12]. Furthermore, in AP animal models, HTG has been shown to contribute to and accelerate the inflammatory cascade[13]. Previous animal studies revealed that HTG can intensify the course of AP in rats[14] and impair the hemorheology in lipoprotein lipase-deficient mice[15]. However, previous studies are inconclusive with respect to predicting which patients with HTG will develop pancreatitis and why some patients with HTG seldom develop pancreatitis despite the markedly elevated TG level. Further research to understand the pathogenesis of HLP is necessary for the development of specific treatments.
Ca2+ signals in the pancreatic acinar cells are generated by the second messengers inositol trisphosphate (IP3), nicotinic acid adenine dinucleotide phosphate (NAADP) and cyclic ADP-ribose (cADPR). Ca2+ signals are modulated in a precise spatiotemporal manner, which is necessary for normal acinar cell secretory function[16]. Abnormal, prolonged elevation of cytosolic Ca2+ is the critical trigger of pancreatitis[16]. Abnormal, global, and sustained elevation of cytosolic Ca2+ signals cause abnormal intracellular enzyme activation, vacuolization and necrosis, which are crucial in the initiation of AP[17,18]. Another pathological signal that appears to be linked to AP is the activation of protein kinase C (PKC) isoforms[19]. PKC isoforms have been linked to NF-κB expression and zymogen activation[20,21]. Although the involvement of PKC isoforms in NF-κB activation by cholecystokinin (CCK) has been addressed, their roles in the pathogenesis of HLP have not been thoroughly studied.
A previous study demonstrated that different serum FFA compositions in patients with AP were related to the severity and complications of AP[22]. Unsaturated fatty acids, mainly linoleic acid, may be involved in the development of AP complications[22]. In addition, palmitoleic acid causes a dose-dependent increase in cytosolic calcium concentrations[23], and oleic acid at high concentrations induces lactate dehydrogenase leakage from acinar cells and causes an increase in cytosolic calcium concentration[24]. We hypothesized that triglyceride composition, i.e., the saturated and unsaturated fatty acid composition, may influence AP susceptibility in HTG patients. To investigate this hypothesis, we used primary pancreatic acinar cell culture to measure the change in cytosolic Ca2+ in acinar cells after treatment with different saturated and unsaturated fatty acids. In this study, we also investigated whether saturated or unsaturated fatty acids activate PKC isoforms in isolated pancreatic acinar cells.
Most of the chemicals, including cerulein, triglyceride, palmitic acid, stearic acid, oleic acid, linoleic acid, palmitoleic acid, docosahexaenoic acid (DHA), arachidonic acid, caffeine, digitonin, rhodamine 100, bis-(CBZ-L-isoleucyl-L-prolyl-L-arginine amide) dihydrochloride, and U-73122, were obtained from Sigma Co Ltd (St Louis, MO, United States). Xestospongin C was obtained from Calbiochem (EMD Millipore Corporation, Billerica, MA, United States). Fura-2 AM was obtained from Invitrogen (Molecular Probes, Eugene, Oregon, United States). The phospho-PKC antibody sampler kit was obtained from Cell Signaling (Danvers, MA, United States).
This study was conducted at the animal facility of National Taiwan University College of Medicine after approval by the National Taiwan University College of Medicine and College of Public Health Institutional Animal Care and Use Committee (IACUC) (IACUC Approval No. 20080297). Eight-week-old male BALB/c mice were used for this study. All animals were obtained from an inbred population of BALB/c mice maintained at the animal facility of National Taiwan University. Pancreatic tissue was aseptically removed from the BALB/c mice, which were sacrificed humanely by means of CO2 asphyxiation after overnight fasting. The pancreatic tissue was dissected free of mesenteric fat, minced, washed with Hanks balanced salts solution (HBSS, pH 7.4) with 0.01% soybean trypsin inhibitor and then incubated with 10 mL 0.05% trypsin and 0.25% EDTA for 5 min at 37 °C. After centrifugation at 200 g for 5 min, the tissue was rinsed with F-12K Nutrient Mixture (Invitrogen/Life Technologies, California, United States) with 1% antibiotics [100 U/mL penicillin-G, 100 μg/mL streptomycin and 0.25 μg/mL FUNGIZONE (Gemini Bio-Products)], 5 mg/mL bovine serum albumin (BSA) and 0.1 mg/mL soybean trypsin inhibitor. The tissue was centrifuged at 200 g for 5 min, and the pellet was resuspended in 12.5 mL of digestive solution containing 1 mg/ml collagenase V (Sigma Chemical Co.) and 0.2 mg/mL BSA in HBSS for 30 min at 37 °C. The digested tissue was centrifuged at 200 g for 5 min and resuspended in F-12K medium with 10% fetal bovine serum (FBS) (Invitrogen/Life Technologies) twice. The obtained pancreatic acinar cells were resuspended in F-12K medium with 10% FBS and 100 U/mL penicillin-G, 100 μg/mL streptomycin and 0.25 μg/mL FUNGIZONE (Gemini Bio-Products) at 37 °C in an atmosphere of 5% CO2 and 95% air.
Freshly isolated mouse pancreatic acinar cells were loaded with 2.5 μmol/L fura-2/AM in loading buffer containing 140 mmol/L NaCl, 5 mmol/L KCl, 1 mmol/L MgCl2, 1 mmol/L CaCl2, 10 mmol/L HEPES, 0.1% BSA and 0.2% glucose (pH 7.4) for 30 min at room temperature. The cell suspension was washed twice and resuspended in loading buffer. The cells were aliquoted into cuvettes in loading buffer containing 1000 mg/dL triglyceride, 10 nmol/L cerulein, saturated fatty acids (0.1 mmol/L palmitic acid and 1.0 mmol/L stearic acid) or unsaturated fatty acids (0.1 mmol/L or 1.0 mmol/L of the following: oleic acid, linoleic acid, palmitoleic acid, DHA, or arachidonic acid). To dissolve the fatty acids, 0.25% chloroform was added to the buffer. Fluorescence was recorded in a mechanically stirred cuvette using an SLM◎AMINCO-8100 spectrophotofluorometer (Spectronic Instruments, Rochester, NY). For measurements of [Ca2+], the excitation wavelength was alternated between 340 and 380 nm every second, and fluorescence intensity was monitored at an emission wavelength of 510 nm. The signal ratio (340:380) was determined after autofluorescence correction, and the free intracellular Ca2+ concentration was calculated.
For pharmacological analysis, we added caffeine (20 mmol/L), xestospongin C (10 μmol/L), and U-73122 (10 μmol/L) to the cells for 5 min before adding different fatty acids as inhibitors; this allowed us to delineate the targets of the different fatty acids in the calcium signaling pathway of the acinar cells.
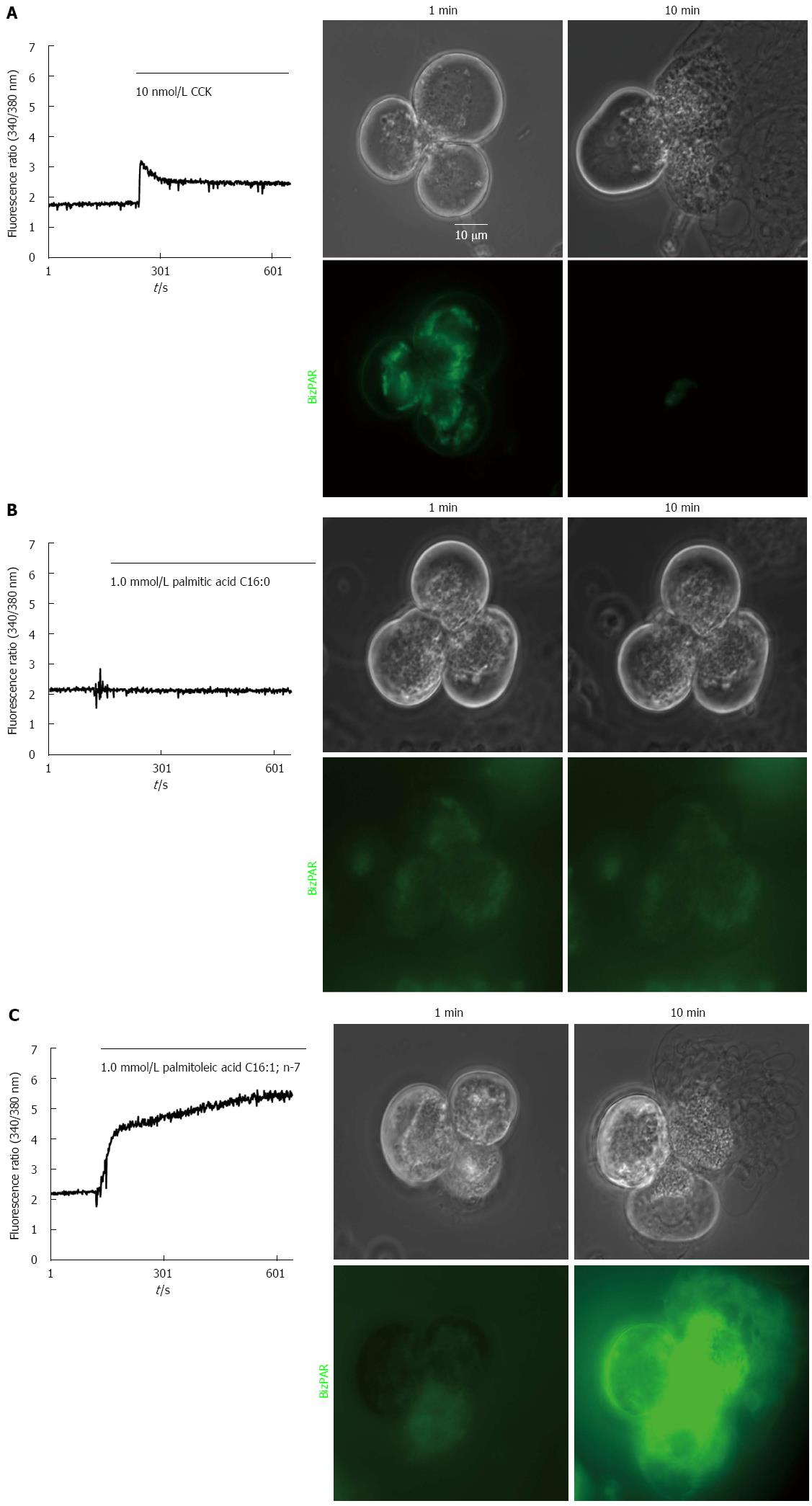
The acinar cells were loaded with 10 μmol/L rhodamine 110 bis-(CBZ-L-isoleucyl-L-prolyl-L-arginine amide) dihydrochloride (BZiPAR) at 37 °C for 60 min, which is a specific substrate for the serine protease trypsin that becomes fluorescent after cleavage of the two oligopeptide side chains. Activation can be observed by fluorescence of rhodamine 110 at an excitation wavelength of 485 nm. To ensure that the tryptic activity observed was from intracellular enzymes only and not from trypsin released into the extracellular fluid, the cells for these experiments were prepared in solution containing 5 μmol/L soybean trypsin inhibitor. The localization of the trypsin activity was investigated by confocal microscopy (Zeiss, LSM510, Germany).
After treatment with different saturated or unsaturated fatty acids for 30 min, the pancreatic acinar cells were lysed on ice in TM buffer and a cocktail of protease inhibitors (Biochain Newark, United States). The protein concentration of the extracts was determined using the Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, CA). Equal amounts of protein were fractionated by 10% SDS-PAGE and electrophoretically transferred to nitrocellulose membranes. The membranes were blocked with Tris-buffered saline supplemented with 5% nonfat dry milk and antibodies against phospho-PKC-α, -δ, -ε, and -ζ (1:1,000 dilution each) for 1 h at room temperature. The membranes were incubated with secondary antibodies conjugated with horseradish peroxidase for 1 h at room temperature. The blots were developed using an enhanced chemiluminescence detection kit (Pierce, Thermo SCIENTIFIC, Rockford, United States).
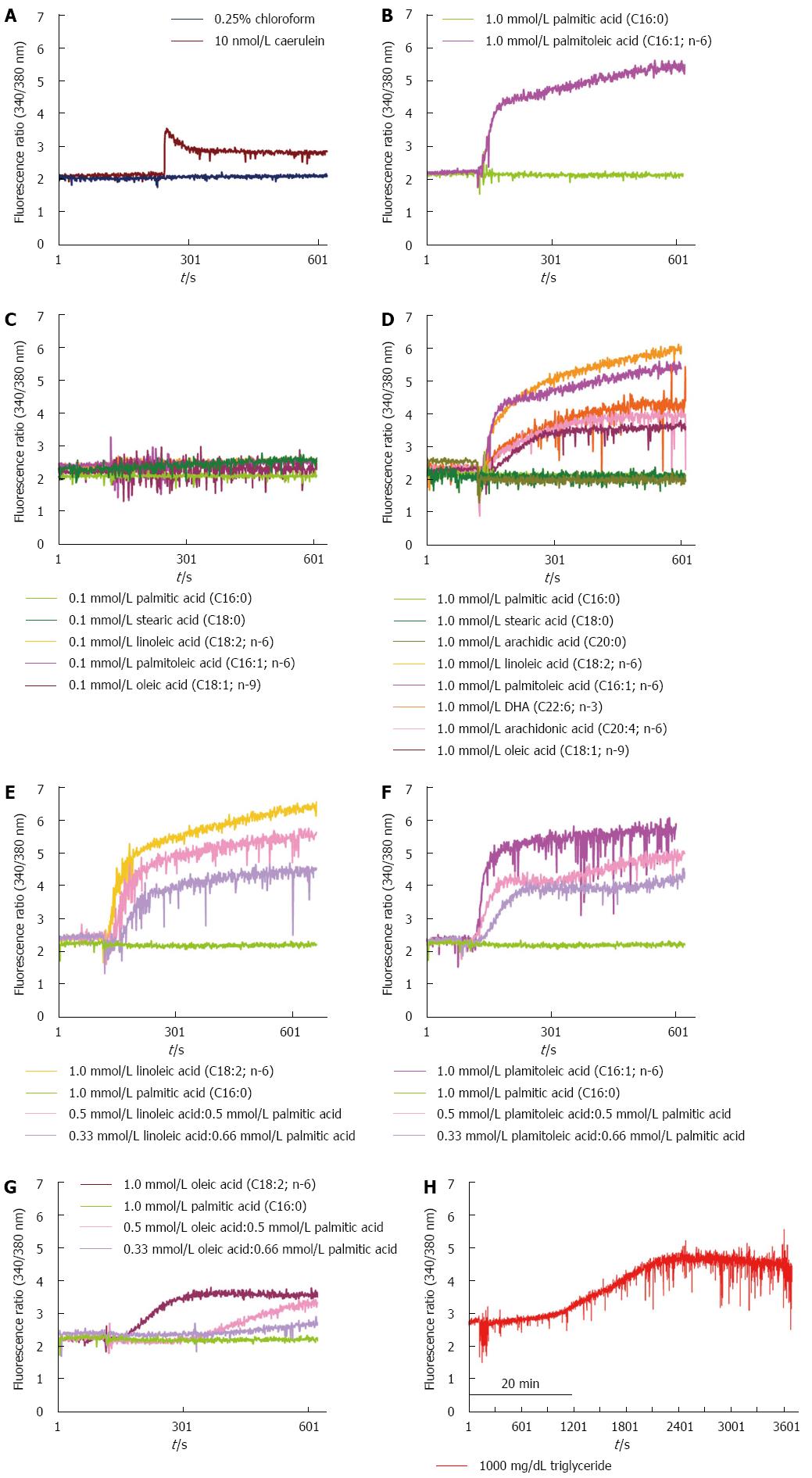
In our experimental acinar cell model, we observed that high concentrations (1 mmol/L) of unsaturated fatty acids, including oleic acid, linoleic acid, palmitoleic acid, DHA, and arachidonic acid, induced a persistent rise in cytosolic Ca2+ concentrations in acinar cells. This rise was similar to that observed with supra-maximal concentrations of CCK in isolated pancreatic acinar cells (Figure 1A-C). However, neither low concentrations (0.1 mmol/L) of unsaturated fatty acids, including oleic acid, linoleic acid, palmitoleic acid, DHA, and arachidonic acid, nor low nor high concentrations of saturated fatty acids, including palmitic acid and stearic acid, were able to induce a rise in Ca2+ concentrations in acinar cells (Figure 1B-D). The elevation of cytosolic Ca2+ concentrations in acinar cells was dose-dependent on the ratio of unsaturated/saturated fatty acids (Figure 1E-G). High concentrations of triglycerides initially did not cause a rise in cytosolic Ca2+ concentrations in acinar cells; however, after long-term incubation with high concentrations of triglycerides, the cytosolic Ca2+ concentration in the acinar cells gradually increased (Figure 1H). All experiments were repeated at least three times with the same results.
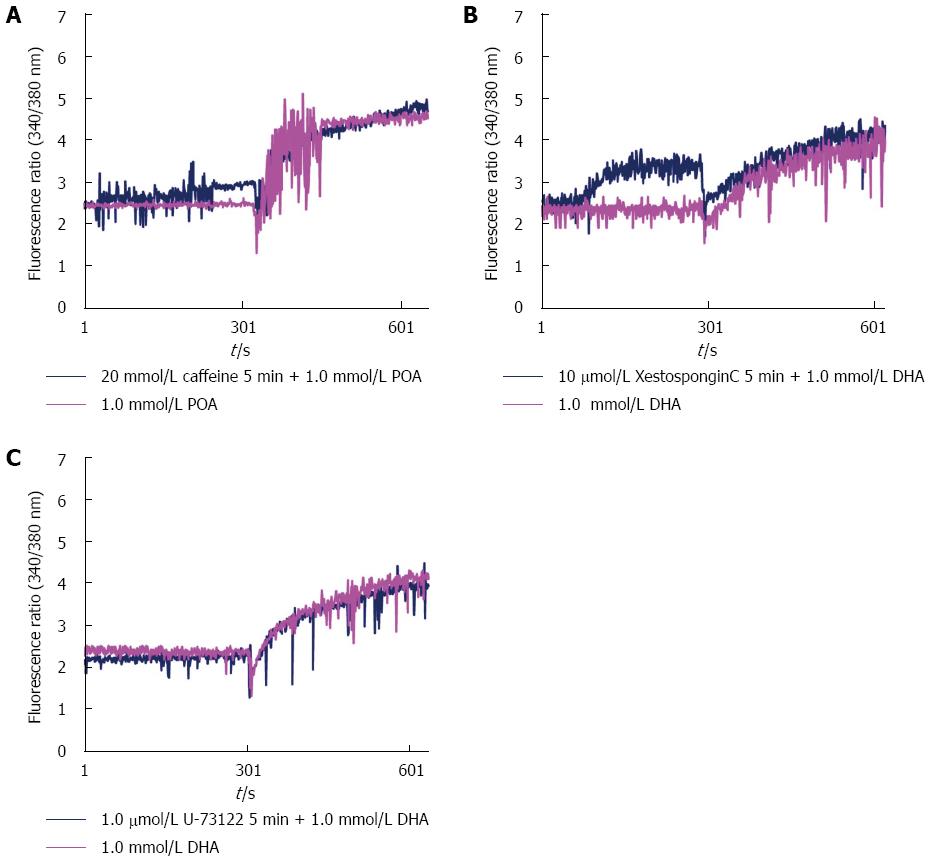
Unsaturated fatty acids at high concentrations induced a persistent rise in cytosolic Ca2+ concentrations in acinar cells, similar to that observed with CCK, but this process was not inhibited by the IP3R antagonists caffeine, xestospongin C or U-73122, which inhibit the hydrolysis of PPI to IP3 (Figure 2).
High but not low concentrations of unsaturated fatty acids induced intra-acinar cell trypsin activation, as observed with CCK, whereas neither low nor high concentrations of saturated fatty acids were able to induce this activation and subsequent cell damage and death (Figure 3).
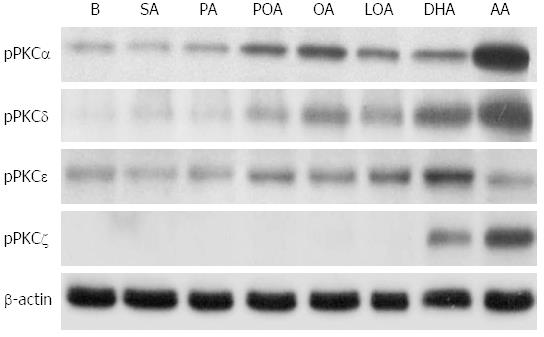
Treatment of acinar cells with high concentrations of fatty acids, induced the expression of PKC-α, -δ, and -ε. This effect was strongest upon treatment with oleic acid, linoleic acid, palmitoleic acid, DHA, and arachidonic acid and weaker with palmitic acid and stearic acid (Figure 4). PKC-ζ expression was only induced by DHA and arachidonic acid (Figure 4).
The mechanism by which HTG leads to pancreatitis is not well understood, and it is difficult to predict which hyperlipidemic patients are likely to develop an episode of pancreatitis. Hydrolysis of triglycerides by pancreatic lipase with localized release of large quantities of FFAs has been proposed to be the pathogenic mechanism by which HTG causes pancreatitis. An early feature of AP is the premature intracellular activation of trypsinogen within pancreatic acinar cells. This activation requires a rise in cytosolic Ca2+ from intracellular compartments[16]. A previous study reported that different serum FFA compositions in patients with AP were related to the severity and complications of AP[22,25]. Unsaturated fatty acids, mainly linoleic acid, may be involved in the development of complications of AP[22]. In addition, palmitoleic acid causes a dose-dependent increase in cytosolic calcium concentrations[23], and high concentrations of oleic acid induce lactate dehydrogenase leakage from acinar cells as well as an increase in cytosolic calcium concentrations[24]. Our data demonstrate for the first time that high but not low concentrations of unsaturated fatty acids and neither high nor low concentrations of saturated fatty acids induced an elevation of cytosolic Ca2+ concentrations in acinar cells as well as intra-acinar cell trypsin activation, cell damage and death. This elevation of cytosolic Ca2+ concentrations in acinar cells is dose-dependently related to the ratio of unsaturated/saturated fatty acids. Triglycerides are unable to induce a rise in cytosolic Ca2+ concentrations in acinar cells. These results indicate that triglycerides cannot induce an attack of AP. Only when triglycerides are hydrolyzed by lipase into FFAs and the concentration of unsaturated fatty acids is sufficiently high do acinar cells become injured, thereby resulting in pancreatitis. These results provide a potential explanation for why only a portion of HTG patients develop HLP clinically. High levels of unsaturated fatty acids may play a crucial role in the pathogenesis of HLP.
In pancreatic acinar cells, Ca2+ release from the endoplasmic reticulum (ER) is mediated by IP3, cADPR and NAADP and primarily occurs through the activation of inositol trisphosphate receptors (IP3Rs) and ryanodine receptors (RyRs)[16]. Caffeine and xestospongin C exhibit inhibitory effects on IP3Rs on the ER membranes of calcium stores, and U-73122 inhibits the hydrolysis of PPI to IP3. However, pretreatment of acinar cells with these inhibitors failed to block the persistent elevation of cytosolic Ca2+ observed in acinar cells exposed to high concentrations of unsaturated fatty acids. These results imply that the elevation of cytosolic Ca2+ concentrations in acinar cells induced by high concentrations of unsaturated fatty acids does not occur through activation of IP3Rs. Criddle et al[26] reported that the unsaturated fatty acid palmitoleic acid can elicit prolonged elevation of the global cytosolic Ca2+ concentrations and that this elevation cannot be inhibited by IP3 receptor blockade, largely due to the inhibition of mitochondrial ATP production. The mechanism by which unsaturated fatty acids induce pathological Ca2+ release in acinar cells from the ER or other acidic stores requires further investigation.
Several studies have demonstrated that PKC isoforms modulate pathological secretion during AP and also regulate the expression of inflammatory mediators[19,20,27]. In pancreatic acinar cells, the conventional PKC-α, the novel PKC-δ and -ε and the atypical PKC-ζ isoforms have been identified[28]. PKC-δ has been shown to participate in premature zymogen activation within pancreatic acinar cells and in activation of the transcription factor NF-κB during experimental pancreatitis[19,29]. PKC-δ is activated and translocated to the plasma membrane and participates in amylase secretion[30], regulates protease activation[31] and modulates inflammatory molecule expression in pancreatic acinar cells[32]. The initiation of AP requires both zymogen activation and the retention of active enzymes in acinar cells. Fatty acids have been shown to increase PKC activity in HTG and AP animal models[33]. In our study, we found that unsaturated fatty acids at high concentrations upregulated the expression of the PKC isoforms PKC-α, PKC-δ, PKC-ε and atypical PKC-ζ in mouse pancreatic acinar cells. Previous studies reported that supraphysiological concentrations of CCK cause activation of the novel PKC isoforms δ and ε and the atypical PKC isoform ζ as well as the activation of NF-κB[34]. Unsaturated fatty acids at high concentrations have a similar effect to that of CCK at supraphysiological concentrations with respect to the activation of PKC-α, -δ and -ζ, implying that HLP may be triggered by unsaturated fatty acids and through the activation of specific isoforms of PKC. In a recent study, Cui et al[35] reported that FFAs induced ER stress by enhancing Ca2+ influx in pancreatic β cells, which contributed to β cell dysfunction and cell apoptosis. In addition, in an arginine model of experimental AP, ER stress sensing and signaling mechanisms were reported to be activated in acinar cells early in the development of AP[36]. Furthermore, HTG was reported to aggravate ER stress[37]. Further studies of the roles and effects of unsaturated fatty acids at high concentrations in key pathogenic cellular events, such as calcium release from acidic stores, mitochondrial dysfunction, ER stress, autophagy, impaired trafficking, and lysosomal and secretory responses in AP are needed.
In conclusion, our in vitro results provide an explanation for the clinical observation that only a portion of HTG patients develop AP and that some patients with HTG seldom develop pancreatitis despite marked elevation of triglyceride level. Triglycerides were unable to induce an attack of AP. Only when triglycerides are hydrolyzed by lipase into FFAs and the concentration of unsaturated fatty acids is sufficiently high do acinar cells become injured, thereby resulting in pancreatitis. Unsaturated fatty acids may play a distinctive role in the pathogenesis of HLP through the activation of PKC family members. Further analysis of the composition of unsaturated/saturated fatty acids in acute phase sera of patients with HLP are needed. In addition, consumption of food containing different types of fat might be a strategy to reduce the risk of the development of AP in HTG patients.
Hypertriglyceridemia (HTG) is the third-most frequent etiology of acute pancreatitis (AP) in Taiwan. The detailed mechanism by which severe HTG precipitates AP remains unknown. Clinically, it is difficult to predict which patients with HTG will develop pancreatitis and why some patients with HTG seldom develop pancreatitis despite the markedly elevated TG level.
Different serum free fatty acid (FFA) compositions in patients with AP were related to the severity and complications of AP. Unsaturated fatty acids, mainly linoleic acid, may be involved in the development of AP complications.
When triglycerides are hydrolyzed by lipase into FFAs and the concentration of unsaturated fatty acids is sufficiently high do acinar cells become injured, thereby resulting in pancreatitis. Unsaturated fatty acids may play a distinctive role in the pathogenesis of HLP.
Consumption of food containing different types of fat might be a strategy to reduce the risk of the development of AP in HTG patients.
HTG is defined by fasting serum triglyceride level of > 150 mg/dL. HTG is considered a risk for pancreatitis when levels are > 1000 mg/dL.
This is a well conducted study that has resulted in a well written manuscript that helps answer a question about the aetiology of hypertryglyceride induced induced acute pancreatitis. The manuscript is worthy of publication but the introduction and discussion could both be reduced in length without any adverse effect on the paper. The authors might also wish to consider how they can demonstrate similar processes are occurring in a human population.
P- Reviewer: Bramhall S, Du YQ, Li SD, Zhang ZM S- Editor: Ma YJ L- Editor: A E- Editor: Ma S
| 1. | Farmer RG, Winkelman EI, Brown HB, Lewis LA. Hyperlipoproteinemia and pancreatitis. Am J Med. 1973;54:161-165. [PubMed] |
| 2. | Yadav D, Pitchumoni CS. Issues in hyperlipidemic pancreatitis. J Clin Gastroenterol. 2003;36:54-62. [PubMed] |
| 3. | Cameron JL, Capuzzi DM, Zuidema GD, Margolis S. Acute pancreatitis with hyperlipemia: the incidence of lipid abnormalities in acute pancreatitis. Ann Surg. 1973;177:483-489. [PubMed] |
| 4. | Dominguez-Muñoz JE, Malfertheiner P, Ditschuneit HH, Blanco-Chavez J, Uhl W, Büchler M, Ditschuneit H. Hyperlipidemia in acute pancreatitis. Relationship with etiology, onset, and severity of the disease. Int J Pancreatol. 1991;10:261-267. [PubMed] |
| 5. | Toskes PP. Hyperlipidemic pancreatitis. Gastroenterol Clin North Am. 1990;19:783-791. [PubMed] |
| 6. | Valdivielso P, Ramírez-Bueno A, Ewald N. Current knowledge of hypertriglyceridemic pancreatitis. Eur J Intern Med. 2014;25:689-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 290] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 7. | Chang MC, Su CH, Sun MS, Huang SC, Chiu CT, Chen MC, Lee KT, Lin CC, Lin JT. Etiology of acute pancreatitis--a multi-center study in Taiwan. Hepatogastroenterology. 2003;50:1655-1657. [PubMed] |
| 8. | Tsuang W, Navaneethan U, Ruiz L, Palascak JB, Gelrud A. Hypertriglyceridemic pancreatitis: presentation and management. Am J Gastroenterol. 2009;104:984-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 276] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 9. | Navarro S, Cubiella J, Feu F, Zambón D, Fernández-Cruz L, Ros E. [Hypertriglyceridemic acute pancreatitis. Is its clinical course different from lithiasic acute pancreatitis?]. Med Clin (Barc). 2004;123:567-570. [PubMed] |
| 10. | Havel RJ. Pathogenesis, differentiation and management of hypertriglyceridemia. Adv Intern Med. 1969;15:117-154. [PubMed] |
| 11. | Saharia P, Margolis S, Zuidema GD, Cameron JL. Acute pancreatitis with hyperlipemia: studies with an isolated perfused canine pancreas. Surgery. 1977;82:60-67. [PubMed] |
| 12. | Halangk W, Lerch MM, Brandt-Nedelev B, Roth W, Ruthenbuerger M, Reinheckel T, Domschke W, Lippert H, Peters C, Deussing J. Role of cathepsin B in intracellular trypsinogen activation and the onset of acute pancreatitis. J Clin Invest. 2000;106:773-781. [PubMed] |
| 13. | Kimura W, Mössner J. Role of hypertriglyceridemia in the pathogenesis of experimental acute pancreatitis in rats. Int J Pancreatol. 1996;20:177-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 106] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 14. | Hofbauer B, Friess H, Weber A, Baczako K, Kisling P, Schilling M, Uhl W, Dervenis C, Büchler MW. Hyperlipaemia intensifies the course of acute oedematous and acute necrotising pancreatitis in the rat. Gut. 1996;38:753-758. [PubMed] |
| 15. | Zhao T, Guo J, Li H, Huang W, Xian X, Ross CJ, Hayden MR, Wen Z, Liu G. Hemorheological abnormalities in lipoprotein lipase deficient mice with severe hypertriglyceridemia. Biochem Biophys Res Commun. 2006;341:1066-1071. [PubMed] |
| 16. | Gerasimenko JV, Gerasimenko OV, Petersen OH. The role of Ca2+ in the pathophysiology of pancreatitis. J Physiol. 2014;592:269-280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 114] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 17. | Ward JB, Petersen OH, Jenkins SA, Sutton R. Is an elevated concentration of acinar cytosolic free ionised calcium the trigger for acute pancreatitis? Lancet. 1995;346:1016-1019. [PubMed] |
| 18. | Petersen OH, Sutton R. Ca2+ signalling and pancreatitis: effects of alcohol, bile and coffee. Trends Pharmacol Sci. 2006;27:113-120. [PubMed] |
| 19. | Satoh A, Gukovskaya AS, Nieto JM, Cheng JH, Gukovsky I, Reeve JR, Shimosegawa T, Pandol SJ. PKC-delta and -epsilon regulate NF-kappaB activation induced by cholecystokinin and TNF-alpha in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol. 2004;287:G582-G591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 119] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 20. | Satoh A, Gukovskaya AS, Reeve JR, Shimosegawa T, Pandol SJ. Ethanol sensitizes NF-kappaB activation in pancreatic acinar cells through effects on protein kinase C-epsilon. Am J Physiol Gastrointest Liver Physiol. 2006;291:G432-G438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Gorelick FS, Thrower E. The acinar cell and early pancreatitis responses. Clin Gastroenterol Hepatol. 2009;7:S10-S14. [PubMed] |
| 22. | Sztefko K, Panek J. Serum free fatty acid concentration in patients with acute pancreatitis. Pancreatology. 2001;1:230-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 62] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 23. | Criddle DN, Raraty MG, Neoptolemos JP, Tepikin AV, Petersen OH, Sutton R. Ethanol toxicity in pancreatic acinar cells: mediation by nonoxidative fatty acid metabolites. Proc Natl Acad Sci USA. 2004;101:10738-10743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 173] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 24. | Wang Y, Sternfeld L, Yang F, Rodriguez JA, Ross C, Hayden MR, Carriere F, Liu G, Hofer W, Schulz I. Enhanced susceptibility to pancreatitis in severe hypertriglyceridaemic lipoprotein lipase-deficient mice and agonist-like function of pancreatic lipase in pancreatic cells. Gut. 2009;58:422-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 25. | Acharya C, Navina S, Singh VP. Role of pancreatic fat in the outcomes of pancreatitis. Pancreatology. 2014;14:403-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 26. | Criddle DN, Murphy J, Fistetto G, Barrow S, Tepikin AV, Neoptolemos JP, Sutton R, Petersen OH. Fatty acid ethyl esters cause pancreatic calcium toxicity via inositol trisphosphate receptors and loss of ATP synthesis. Gastroenterology. 2006;130:781-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 198] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 27. | Tapia JA, García-Marin LJ, Jensen RT. Cholecystokinin-stimulated protein kinase C-delta kinase activation, tyrosine phosphorylation, and translocation are mediated by Src tyrosine kinases in pancreatic acinar cells. J Biol Chem. 2003;278:35220-35230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 28. | Bastani B, Yang L, Baldassare JJ, Pollo DA, Gardner JD. Cellular distribution of isoforms of protein kinase C (PKC) in pancreatic acini. Biochim Biophys Acta. 1995;1269:307-315. [PubMed] |
| 29. | Thrower EC, Osgood S, Shugrue CA, Kolodecik TR, Chaudhuri AM, Reeve JR, Pandol SJ, Gorelick FS. The novel protein kinase C isoforms -delta and -epsilon modulate caerulein-induced zymogen activation in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1344-G1353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 30. | Li C, Chen X, Williams JA. Regulation of CCK-induced amylase release by PKC-delta in rat pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol. 2004;287:G764-G771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 31. | Thrower EC, Wang J, Cheriyan S, Lugea A, Kolodecik TR, Yuan J, Reeve JR, Gorelick FS, Pandol SJ. Protein kinase C delta-mediated processes in cholecystokinin-8-stimulated pancreatic acini. Pancreas. 2009;38:930-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 32. | Ramnath RD, Sun J, Adhikari S, Zhi L, Bhatia M. Role of PKC-delta on substance P-induced chemokine synthesis in pancreatic acinar cells. Am J Physiol Cell Physiol. 2008;294:C683-C692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 33. | Wang YJ, Sun JB, Li F, Zhang SW. Hyperlipidemia intensifies cerulein-induced acute pancreatitis associated with activation of protein kinase C in rats. World J Gastroenterol. 2006;12:2908-2913. [PubMed] |
| 34. | Gorelick F, Pandol S, Thrower E. Protein kinase C in the pancreatic acinar cell. J Gastroenterol Hepatol. 2008;23 Suppl 1:S37-S41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 35. | Cui W, Ma J, Wang X, Yang W, Zhang J, Ji Q. Free fatty acid induces endoplasmic reticulum stress and apoptosis of β-cells by Ca2+/calpain-2 pathways. PLoS One. 2013;8:e59921. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 36. | Kubisch CH, Sans MD, Arumugam T, Ernst SA, Williams JA, Logsdon CD. Early activation of endoplasmic reticulum stress is associated with arginine-induced acute pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2006;291:G238-G245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 93] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 37. | Zeng Y, Wang X, Zhang W, Wu K, Ma J. Hypertriglyceridemia aggravates ER stress and pathogenesis of acute pancreatitis. Hepatogastroenterology. 2012;59:2318-2326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (2)] |