Published online Jan 21, 2015. doi: 10.3748/wjg.v21.i3.888
Peer-review started: April 6, 2014
First decision: June 10, 2014
Revised: July 3, 2014
Accepted: September 5, 2014
Article in press: September 5, 2014
Published online: January 21, 2015
Processing time: 291 Days and 2.5 Hours
AIM: To investigate the feasibility of temporary extracorporeal continuous porta-caval diversion (ECPD) to relieve portal hyperperfusion in “small-for-size” syndrome following massive hepatectomy in pigs.
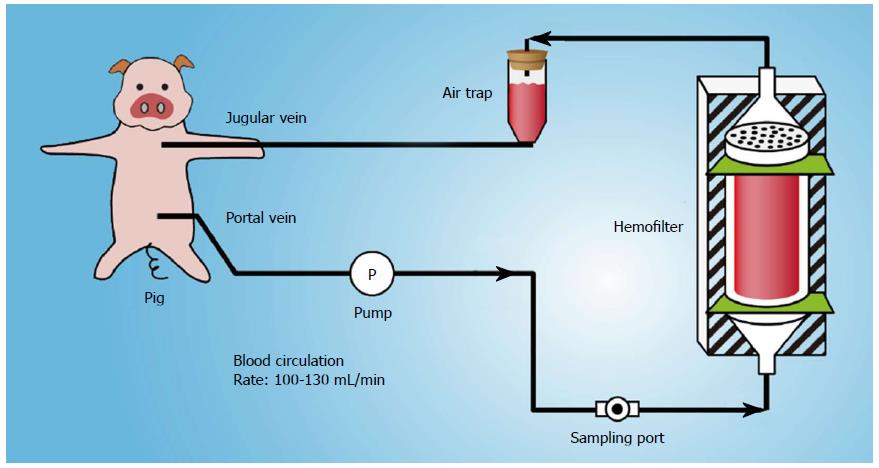
METHODS: Fourteen pigs underwent 85%-90% liver resection and were then randomly divided into the control group (n = 7) and diversion group (n = 7). In the diversion group, portal venous blood was aspirated through the portal catheter and into a tube connected to a centrifugal pump. After filtration, the blood was returned to the pig through a double-lumen catheter inserted into the internal jugular or subclavian vein. With the conversion pump, portal venous inflow was partially diverted to the inferior vena cava through a catheter inserted via the gastroduodenal vein at 100-130 mL/min. Portal hemodynamics, injury, and regeneration in the liver remnant were compared between the two groups.
RESULTS: Compared to the control group, porta-caval diversion via ECPD significantly mitigated excessive portal venous flow and portal vein pressure (PVP); the portal vein flow (PVF), hepatic artery flow (HAF), and PVP in the two groups were not significantly different at baseline; however, the PVF (431.8 ± 36.6 vs 238.8 ± 29.3, P < 0.01; 210.3 ± 23.4 vs 122.3 ± 20.6, P < 0.01) and PVP (13.8 ± 2.6 vs 8.7 ± 1.4, P < 0.01; 15.6 ± 2.1 vs 10.1 ± 1.3, P < 0.05) in the control group were significantly higher than those in the diversion group, respectively. The HAF in the control group was significantly lower than that in the diversion group at 2 h and 48 h post hepatectomy, and ECPD significantly attenuated injury to the sinusoidal lining and hepatocytes, increased the regeneration index of the liver remnant, and relieved damage that the liver remnant suffered due to endotoxin and bacterial translocation.
CONCLUSION: ECPD, which can dynamically modulate portal inflow, can reduce injury to the liver remnant and facilitate liver regeneration, and therefore should replace permanent meso/porta-caval shunts in “small-for-size” syndrome.
Core tip: Meso/porta-caval shunts have usually been adopted to relieve portal hyperperfusion in “small-for-size” syndrome (SFSS) or postoperative liver failure; however, these methods cannot dynamically adjust portal inflow to affect “functional competition”. In this study, extracorporeal continuous porta-caval diversion was temporarily adopted to relieve hyperperfusion, dynamically adjust the effect of portal inflow towards functional competition, and preserve optimal portal inflow. This also reduces injury to the sinusoidal endothelium, decreases endotoxin/bacterial translocation, and facilitates liver regeneration in SFSS after massive hepatectomy, and therefore could replace permanent meso/porta-caval shunts, which have no benefit or harm towards liver regeneration in late stages.
- Citation: Wang DD, Xu Y, Zhu ZM, Tan XL, Tu YL, Han MM, Tan JW. Should temporary extracorporeal continuous portal diversion replace meso/porta-caval shunts in “small-for-size” syndrome in porcine hepatectomy? World J Gastroenterol 2015; 21(3): 888-896
- URL: https://www.wjgnet.com/1007-9327/full/v21/i3/888.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i3.888
When the residual liver volume is below a certain threshold, the liver remnant cannot sustain metabolic, synthetic, and detoxifying functions[1-4], and postoperative liver failure (PLF) or “small-for-size” syndrome (SFSS) will ensue. Portal hypertension and splanchnic pooling following major hepatectomy or a small graft have been reported to contribute to the high postoperative morbidity and mortality rates associated with these procedures[5-10]. Some studies have shown that the placement of a portal-systemic shunt or splenic artery ligation improves survival following subtotal hepatectomy or a small graft, probably as a result of decompression of the portal blood flow. However, excessive diversion or hypoperfusion by the portal-systemic shunt retards regeneration of the liver remnant or small graft[11]. In this study, extracorporeal continuous porta-caval diversion (ECPD) was temporarily adopted to relieve hyperperfusion. This system is theoretically appealing, as it can dynamically adjust portal inflow to affect functional competition between the portal vein and systemic circulation, and preserve optimal portal inflow to allow hypertrophy of the liver remnant. Following the establishment of a stable, critical pig model of 85%-90% hepatectomy, the effect of ECPD in preventing sinusoidal microcirculatory injury from portal hypertension following extended hepatectomy and its advantages was analyzed.
Fourteen male Bama miniature pigs (15-20 kg), 4 to 6 mo of age, were obtained from the Pig and Poultry Production Institute (GuangXi province, China). The pigs were raised in a closed herd and kept under strict quarantine. All animals in this study were treated humanely and in accordance with institutional and national guidelines for ethical treatment of animals. Experiments were conducted in accordance with the Chinese legislation on the protection of animals and “Principles of laboratory animal care” (NIH publication No. 85-23, revised 1985).
The pigs were food-deprived for 8 h before the operation. All pigs were anesthetized by initial sedation with a deep intramuscular injection of ketamine (15-20 mg/kg) and chlorpromazine (6-8 mg/kg), 15 min after the administration of atropine (0.01 mg/kg). An upper-midline incision with right or bilateral subcostal extensions (inverse “L” or Mercedes incision) was performed. A subtotal hepatectomy with less than 60 mL of blood loss and without hepatic pedicle occlusion was performed. The extent of the hepatectomy was referred to bench dissection of 10 pigs, according to a previous study: extended hepatectomy involving approximately 85%-90% of the liver was accomplished by 75%-80% hepatectomy plus removal of the partial posterior segment[12]. A 16-gauge double-lumen catheter was inserted into the main portal vein via the gastroduodenal vein, and was connected to a RM6240 physiology device (Chengdu, China) to measure the portal vein pressure (PVP). Another double-lumen catheter was advanced into the suprahepatic inferior vena cava through the internal jugular vein to monitor central vein pressure (CVP). Two ultrasonic flow probes (3 mm, 10 mm) were connected to a flow meter (HT107, Transonic Systems, Ithaca, NY, United States) to measure hepatic artery flow (HAF) and portal vein flow (PVF), respectively.
The 14 animals that underwent subtotal (85%-90%) hepatectomy with less than 60 mL of blood loss and without hepatic pedicle occlusion were divided into two groups: the control group (n = 7) and the diversion group (n = 7). In the diversion group, hepatectomy was followed by ECPD. The diversion flow was 100-130 mL/min, and the room temperature was 18 to 23 °C.
Portal venous blood was aspirated through the portal catheter and into a tube connected to a centrifugal pump (Plasma Separation Apparatus, Asahi Medical Co., Ltd., Japan) (Figure 1). After filtration, the blood was returned to the pig through a double-lumen catheter inserted into the internal jugular or subclavian vein. The adequacy of anticoagulation was monitored by the activated clotting time (ACT), and heparin was administered as required to maintain an ACT greater than 180 s. Standard monitoring (ECG, arterial line for blood pressure and blood gases, and Foley catheter for urine output) was performed for all pigs. With the conversion pump, the portal venous inflow was partially diverted to the inferior vena cava through a catheter inserted via the gastroduodenal vein at 100-130 mL/min. The portal hemodynamics, liver injury and regeneration were investigated and compared between the two groups.
After the operation, one daily dose of 375 mg penicillin was given intramuscularly to all pigs, and 500 mL of normal saline and 500 mL of a 10% glucose solution were administered during recovery. Thereafter, the pigs were monitored daily until euthanasia at 49-50 h post-hepatectomy (PH). Food and water intake and serum glucose levels were evaluated at each daily postoperative assessment, and animals that had limited or no intake or low serum glucose levels (< 70-80 mg/dL) were administered 50 g of intravenous (IV) glucose (500 mL of a 10% glucose solution). At euthanasia, the liver remnant was removed, weighed, and sampled.
A double-lumen catheter was placed in the internal jugular vein to monitor invasive venous pressure. The catheter was tunneled subcutaneously to exit at the back of the neck for postoperative access. A single-lumen catheter was introduced into the portal vein to monitor portal venous pressure (PVP). Ultrasonic flow probes were connected to a flow meter (HT107, Transonic Systems, Ithaca, NY, United States) to measure hepatic artery flow (HAF) and portal vein flow (PVF). PVP, HAF, and PVF were recorded at baseline, 48 h PH, and 50 h PH (stopping ECPD for 1 h), before euthanasia.
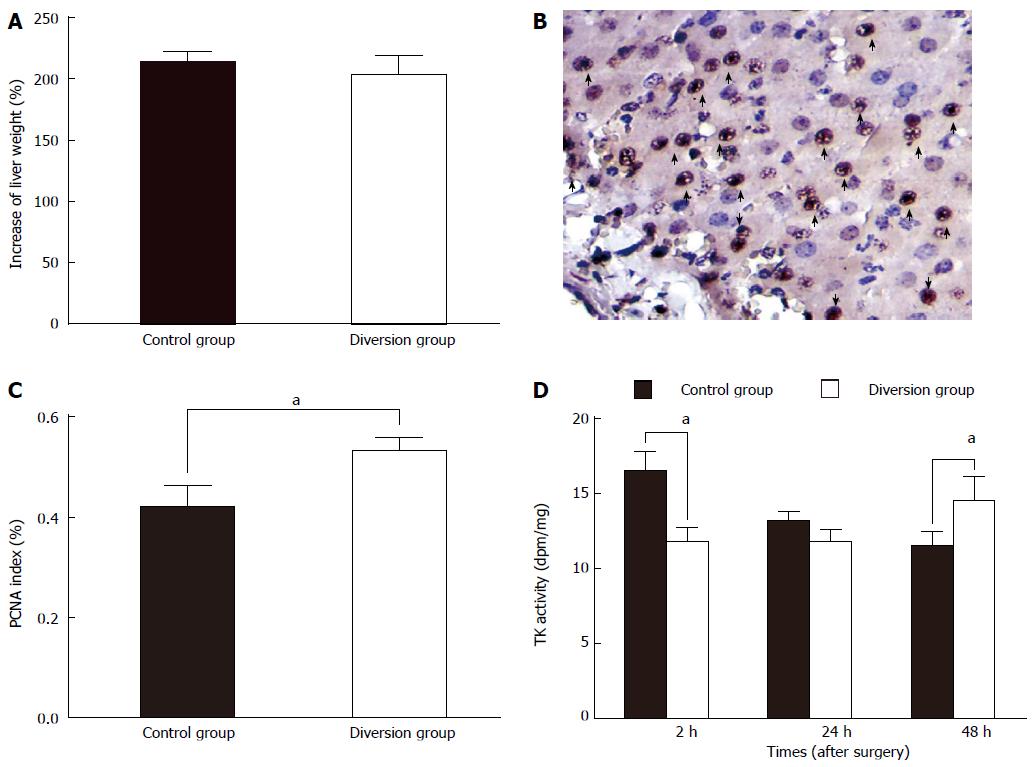
Serum samples were collected pre-hepatectomy and serially during the follow-up period, at 2 h, 24 h and 48 h PH. In these samples, the levels of alanine aminotransferase (ALT), total bilirubin (TB), hyaluronic acid (HA), and thymidine kinase (TK) activity were determined. HA was measured by a radiometric assay with an HA test kit (Pharmacia Diagnostics, Shanghai, China). HA is eliminated mainly in the hepatic sinusoidal endothelium; increased serum HA levels indicate sinusoidal endothelial damage[13,14]. Serum TK activity provides an index of hepatic regeneration[15,16]. TK activity was measured using the LIAISON TK assay (Jingmei Biotech Co. Ltd., Shenzhen, China).
Hepatic tissue was sampled in the two groups at 1 h PH, and each biopsy sample was divided into 2 sections. The tissue specimens for electron microscopy were fixed in 2.5% glutaraldehyde and 2% paraformaldehyde in 0.1 mol/L sodium cacodylate buffer (pH 7.3). The other set of samples was preserved in 10% neutral buffered formalin, embedded in paraffin, and stained with hematoxylin and eosin using standard histological techniques. Clusters of differentiation molecule 31 (CD31) immunoglobulin helps maintain endothelial stability by interacting with other CD31 molecules at the extracellular border of adjacent cells. Sections of hepatic tissue were immunostained with porcine anti-CD31 antibody (Serotec, Oxford, United Kingdom) to evaluate the integrity of the endothelial cells in the hepatic sinusoid, as previously described[17,18].
The pigs were re-opened at 48 h PH and HAF, PVF, and PVP were measured. Porta-cava conversion was stopped in the diversion group, and these parameters were measured again 1 h later. The pigs were then sacrificed, the liver excised, weighed, and processed, and hepatic tissue was sampled again from the two groups.
The increase rate of the liver remnant after hepatectomy was calculated using the following equation: increase rate = regenerated liver volume at sacrifice/estimated remnant liver volume at operation × 100%.
Liver samples at 48 h PH were stained for Proliferating Cell Nuclear Antigen (PCNA), a stable cell-cycle nuclear protein. The rate of DNA synthesis correlates with the rate of cell proliferation. Data are expressed as the percentage of PCNA-stained hepatocytes per total number of hepatocytes. The mean number of PCNA-stained hepatocytes per 10 high-power fields was calculated for the two groups, divided by the total cell number, and then compared.
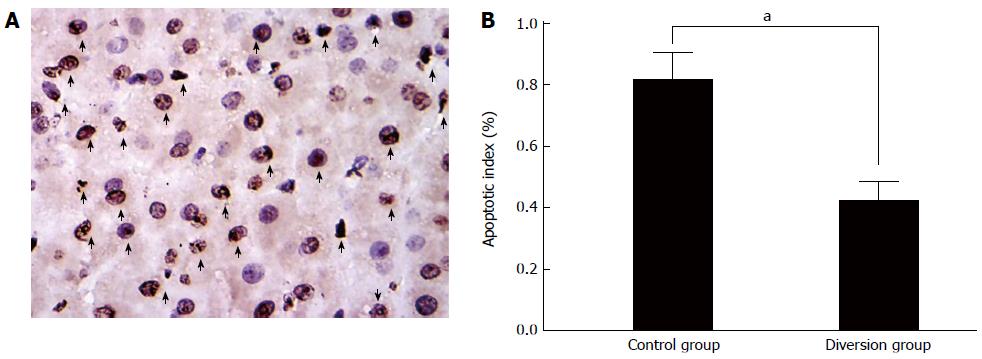
Four-micrometer-thick sections were stained with hematoxylin and eosin and analyzed by in situ terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL) using an apoptosis in situ detection kit (Jiamei Biotech Co. Ltd, Shenzhen, China) according to the manufacturer’s instructions. For each pig, ten consecutive high-power fields were examined for counting at 400 × magnification. The apoptosis index (AI) is defined as the mean number of apoptotic cells per 10 high-power fields, and was measured for the two groups, divided by the total cell number, and then compared.
LPS level: The LPS level was quantified by the limulus amebocyte lysate (LAL) assay based on the methods first introduced by Iwanaga and his colleagues[19] using the commercially available chromogenic LAL endpoint kit (Yihua BioScience Ltd.; Shanghai, China), following the manufacturer’s instructions. A calculated value of 0.1 EU/mL (10 pg/mL) in the specimens was considered the threshold to be considered endotoxin positive. To control for endotoxin contamination, a sterile water sample was used as a negative control. Standards and samples were analyzed in duplicate.
Real-time polymerase chain reaction assay for bacterial DNA: DNA was isolated from the blood using the Fast DNA Spin Kit (Cat. 69506; Qiagen, United States) according to the manufacturer’s instructions. Subsequently, total bacteria quantification was performed with 16S rRNA gene-targeted primers, which have demonstrated uniform success in quantifying a wide range of bacteria (49 different strains). The sequences of the universal primers were 5’-TTCCGGTTGATCCTGCCGGA-3’ (forward) and 5’-GGTTACCTTGTTACGACTT-3’ (reverse)[20]. Serially diluted genomic DNA from selected bacterial isolates was used as a real-time PCR control. Bacterial counts from real-time PCR are expressed as log10 cells per gram tissue (cells/g) mean ± SE[20,21].
All variables are expressed as mean ± SD and were compared between the two groups by the Student’s t-test, using the PASW Statistics 18 software (SPSS Inc., Chicago, IL, United States). P-values < 0.05 were considered significant.
The operative outcomes of the two groups were not significantly different, as shown in Table 1. The evolution of hemodynamic parameters is shown in Table 2. The PVF, HAF, and PVP in the two groups were not significantly different at baseline. However, at other time points, the PVF and PVP in the control group were significantly higher than those in the diversion group (P < 0.01). The HAF in the control group was significantly lower than that in the diversion group (P < 0.05).
| Control group | Diversion group | P value | |
| Body weight (kg) | 17.4 ± 3.3 | 18.1 ± 3.5 | 0.97 |
| Left tri-lobes (g) | 351.2 ± 14.9 | 365.5 ± 15.8 | 0.81 |
| ETL (g) | 442.7 ± 18.4 | 457.0 ± 19.7 | 0.86 |
| WRL (g) | 390.7 ± 19.4 | 401.8 ± 20.4 | 0.79 |
| ERL (g) | 55.7 ± 3.8 | 60.8 ± 4.1 | 0.91 |
| Proportion of ERL (%) | 12.8 ± 2.3 | 13.2 ± 3.5 | 0.87 |
| OT (min) | 115 ± 23 | 121 ± 28 | 0.45 |
| Blood loss (mL) | 35.7 ± 13.8 | 45.1 ± 16.1 | 0.73 |
| Control group | Diversion group | P value | |
| PVF, mL/min per 100 g | |||
| BAS | 61.9 ± 9.6 | 64.1 ± 10.6 | 0.87 |
| 2 h PH | 431.8 ± 36.6 | 238.8 ± 29.3 | < 0.01 |
| 48 h PH (re-open) | 210.3 ± 23.4 | 122.3 ± 20.6 | < 0.01 |
| Pre-EUT1 | 204.5 ± 21.4 | 211.4 ± 26.3 | 0.94 |
| HAF, mL/min per 100 g | |||
| BAS | 19.4 ± 4.5 | 19.9 ± 4.1 | 0.96 |
| 2 h PH | 6.1 ± 2.5 | 14.9 ± 2.5 | < 0.01 |
| 48 h PH (re-open) | 5.5 ± 2.1 | 13.2 ± 4.2 | 0.011 |
| Pre-EUT1 | 5.3 ± 2.0 | 11.2 ± 3.4 | 0.014 |
| P/A | |||
| BAS | 3.1 ± 0.2 | 3.4 ± 0.2 | 0.89 |
| 2 h PH | 74.0 ± 8.1 | 14.8 ± 3.1 | 0.01 |
| 48 h PH | 40.8 ± 6.6 | 9.5 ± 1.8 | < 0.01 |
| Pre-EUT1 | 41.3 ± 6.3 | 19.1 ± 2.5 | < 0.01 |
| PVP, mmHg | |||
| BAS | 6.4 ± 1.8 | 6.0 ± 0.8 | 0.94 |
| 2 h PH | 13.8 ± 2.6 | 8.7 ± 1.4 | < 0.01 |
| 48 h PH | 15.6 ± 2.1 | 10.1 ± 1.3 | 0.021 |
| Pre-EUT1 | 15.1 ± 1.9 | 11.4 ± 1.8 | 0.032 |
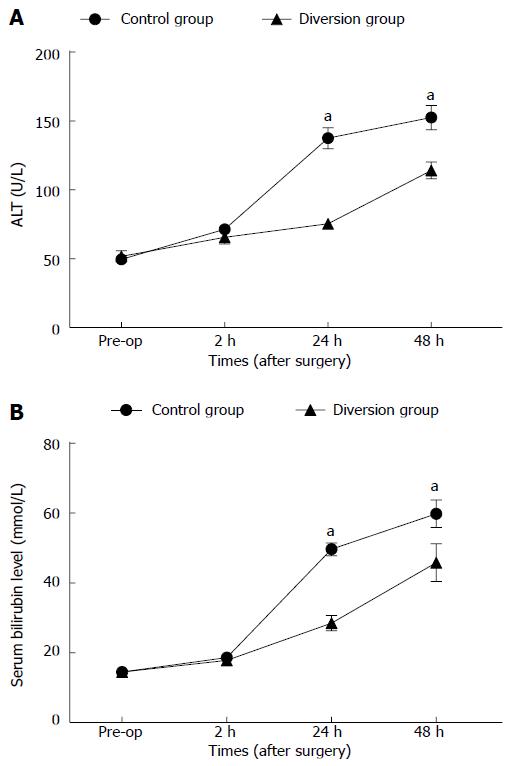
Hepatocellular injury: The preoperative and serial postoperative measurements of serum ALT and total bilirubin (TB) are shown in Figure 2A, B, with significant differences noted. There were no significant differences between the two groups before the operation or at 2 h PH (P > 0.05). At other time points, the control group showed significantly elevated values compared to the diversion group (P < 0.05).
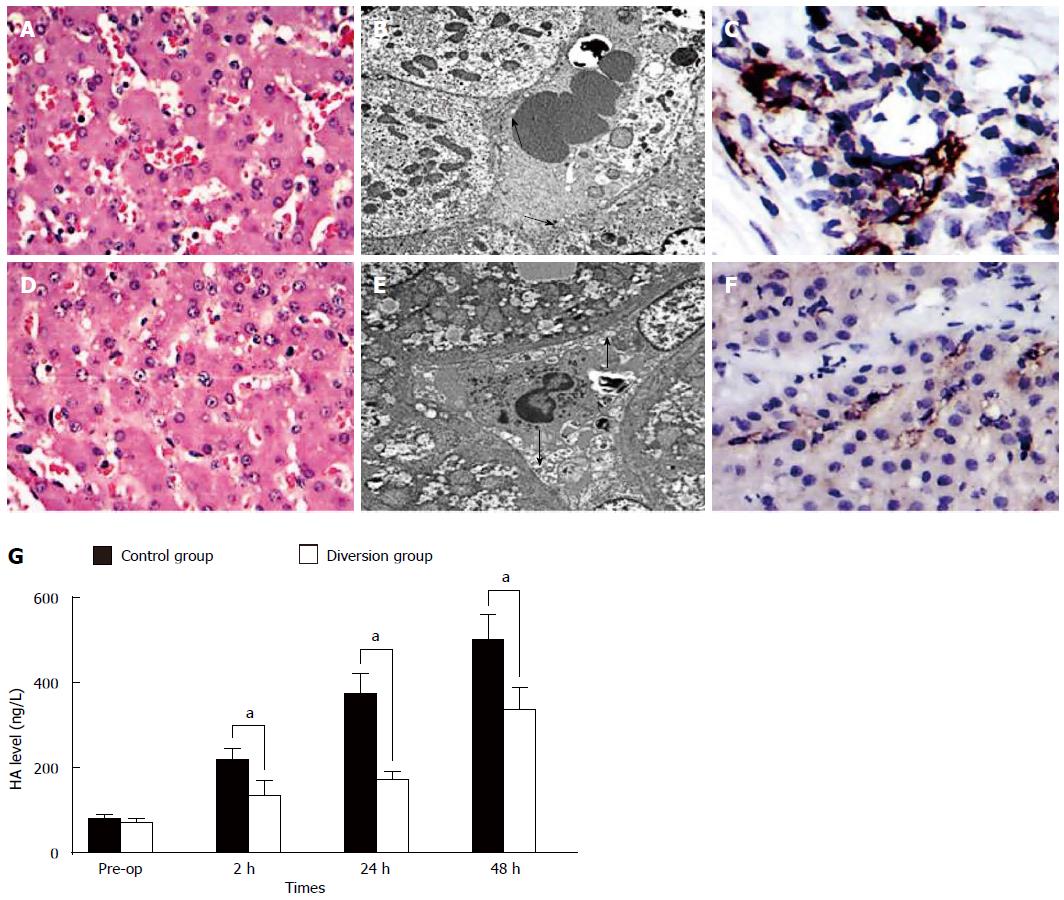
In the control group, HA levels were significantly higher than those in the diversion group (Figure 3, P < 0.05). There was significant endothelial denudation, sinusoidal dilation, hydropic changes in hepatocytes, and hemorrhage into the hepatic parenchyma (Figure 3A). The sinusoidal endothelial lining was partially destroyed and detached into the sinusoidal space, with enlargement of the Disse’s spaces (Figure 3C, arrowheads). CD31 immunostaining showed significant destruction of the endothelial lining (Figure 3E). In contrast, in the diversion group, there was no intraparenchymal hemorrhage (Figure 3B), the sinusoidal endothelial cells and hepatocytes were well-preserved, and the structure of the endothelial lining was visible (Figure 3D, arrows). Furthermore, CD31 immunostaining showed a mild sinusoidal microarchitecture injury in the diversion group (Figure 3F).
Portal hyper-reperfusion following subtotal hepatectomy, in which the vascular bed of the liver remnant experiences a drastic decrease, has been demonstrated in animal experiments and clinical settings[3-5]. It has been reported to contribute to the high postoperative morbidity and mortality rates[3,6-10], via reduction in reticuloendothelial function[21], bacterial translocation[22], hepatic ischemia, and sepsis caused by small bowel infarction[23]. In many reports[24-26], to decompress portal hypertension in clinical or experimental settings, inflow modulations have been used, including a mesocaval or portacaval shunt (MCS/PCS)[8,24,27] and splenectomy or splenic-artery ligation[26,28,29]. These methods for portal decompression improved the prognosis of small grafts or liver remnants. Interestingly, there seems to be a reverse trend from the use of right-liver grafts to the use of left-liver grafts because of these inflow modifications. However, these techniques have many shortcomings, including surgical procedure-related complications[26,28-30] and the potential risk of excessively diverting the portal flow to systemic circulation, which could retard liver regeneration[8,26,28].
In fact, it has been demonstrated in our previous study, also in the clinical setting, that it is difficult to define the size of the PCS or MCS[18]. When a small-diameter shunt was selected, thrombus of the orifice often occurred and the shunt closed. In such cases, the pigs with extended hepatectomy did not benefit from the portal decompression. In cases where a large PCS was placed, the PVP decreased and the liver remnant failed to regenerate because of poor portal flow, due to “functional competition” between the portal vein and systemic circulation. Thus, an excessive decrease in PVP was as harmful to liver regeneration as high portal-vein pressure. In order to attain an optimum PVF with dynamic regulation following an extended hepatectomy, a partial, transient diversion of portal flow (that is, ECPD) might be beneficial to adjust the portal flow to affect “functional competition”. In this study, ECPD was used to optimize portal inflow by regulating the conversion flow. It has been reported that approximately triple the baseline portal venous inflow following a 70% hepatectomy[7] optimally promotes liver regeneration. In this study, the portal inflow in the diversion group was easily adjusted to 3.0-3.5 times the baseline inflow, and liver regeneration parameters were similar to the control group without portal decompression (Figure 4). However, in the diversion group, vascular shear stress in the portal vein (a determining factor in regeneration) was significantly lower than that in the control group. Moreover, the damage to hepatic parenchyma (Figure 2) and sinusoidal endothelium (Figure 3) with portal hyperperfusion, and the AI in the diversion group were significantly reduced compared with the control group (Figure 5).
However, after diverting the portal flow to the systemic circulation for 48 h, the liver volume rose to more than twice the postoperative residual volume (Figure 4A), and the PVP approached its baseline value (Table 2). When the ECPD was stopped, the PVP rose only slightly (Table 1). This indicated that the hyperperfusion could be relieved in a short time, which can be attributed to rapid regeneration of the liver remnant after massive hepatectomy (Figure 6). Therefore, with regard to portal hyperperfusion in massive hepatotectomy, ECPD sometimes only needs to be adopted for a short time.
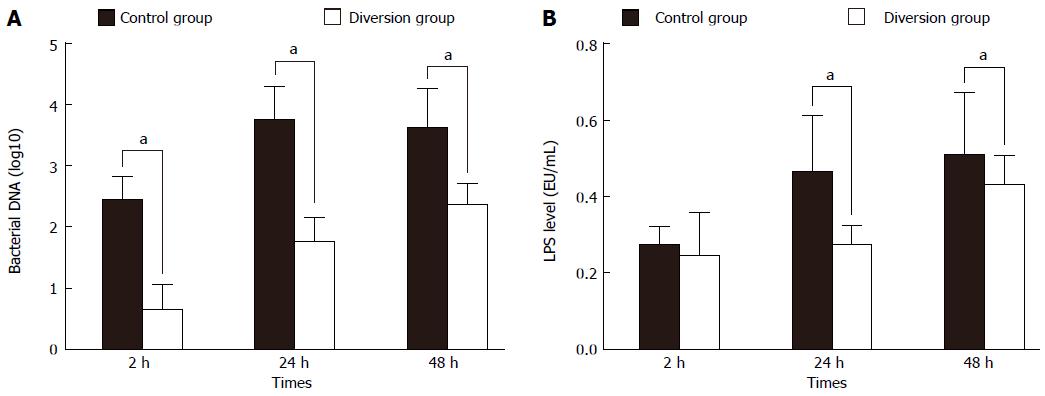
It was shown previously that innate immunity is significantly impaired after liver surgery; RES function greatly decreases after major hepatectomy[6,7]. However, portal hypertension may also cause endotoxin absorption and an increase in bacterial translocation, causing a rise in the serum endotoxin level and severe inflammation[31,32]. In this study, the serum endotoxin level and bacterial DNA in the diversion group with ECPD were significantly depressed compared to the control group without portal decompression, indicating that portal hypertension may aggravate endotoxin absorption or bacterial translocation (Figure 4). Therefore, ECPD provides an important strategy to improve liver regeneration by reducing LPS/bacterial translocation.
Continuous hemofiltration/hemodiafiltration is a routine clinical treatment used as renal replacement in critically ill patients[33]. The placement of a catheter in the portal vein makes ECPD feasible and safe. In clinical major hepatectomy or living donor liver transplantation, the catheter may be inserted via the gastroepiploic vein into the portal vein, using omental wrapping of the catheter tube to prevent bleeding while removing the catheter. Alternatively, the catheter can be inserted percutaneously. According to the present study, a catheter placed into a portal vein in major hepatectomy with a high risk of PLF may permit the dynamic monitoring or relief of portal hyperperfusion. Therefore, once high portal pressure is detected, ECPD is a viable alternative method to prevent or treat portal hyperperfusion in PLF or SFSS; moreover, this could be personalized and extracorporeal blood flow can be changed depending on the parameters measured in vivo.
Overall, ECPD can effectively relieve hyperperfusion in SFSS or PLF, and can dynamically adjust portal flow to the functional competition between the portal vein and systemic circulation, positioning it as an alternative to SFSS in clinical settings. In the future, this experimental model could still be improved, so that it could be personalized and extracorporeal blood flow can be changed depending on the parameters measured in vivo in each animal, which could aid its adoption towards clinical use.
Portal hypertension and splanchnic pooling following major hepatectomy or a small graft have been reported to contribute to high postoperative morbidity and mortality rates associated with these procedures. The placement of a portal-systemic shunt or splenic artery ligation improves survival following subtotal hepatectomy or a small graft, probably as a result of decompression of portal blood flow. However, excessive diversion or hypoperfusion by a portal-systemic shunt retards regeneration of the liver remnant or small graft, and meso/porta-caval shunts are permanent, having no benefit or harm to liver regeneration in late stages.
A portal-systemic shunt is adopted to relieve portal hyperperfusion following subtotal hepatectomy or a small graft, however, it can not avoid “functional competition” between the portal vein and systemic circulation.
Meso/porta-caval shunts cannot dynamically adjust portal inflow to affect “functional competition”. In this study, extracorporeal continuous porta-caval diversion (ECPD) was temporarily adopted to relieve hyperperfusion, dynamically adjusted portal inflow to affect functional competition, and preserved optimal portal inflow, which could perhaps replace permanent meso/porta-caval shunts that have no benefit or harm towards liver regeneration in late stages.
Continuous hemofiltration/hemodiafiltration is a routine clinical treatment used as a renal replacement. The placement of a catheter in the portal vein also makes ECPD feasible and safe, and could be personalized so that extracorporeal blood flow can be dynamically changed depending on parameters measured in real time.
This article is very interesting and useful. The methodology is explained clearly and the techniques used for recording and measuring the results are right. Temporary extracorporeal continuous portal diversion is a real solution for “small-for-size” syndrome in this experimental model.
P- Reviewer: Delgado JS, Mihaila RG, Pellicano R S- Editor: Ma YJ L- Editor: Webster JR E- Editor: Zhang DN
| 1. | Lo CM, Liu CL, Fan ST. Portal hyperperfusion injury as the cause of primary nonfunction in a small-for-size liver graft-successful treatment with splenic artery ligation. Liver Transpl. 2003;9:626-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 119] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 2. | Wang H, Ohkohchi N, Enomoto Y, Usuda M, Miyagi S, Masuoka H, Sekiguchi S, Kawagishi N, Fujimori K, Sato A. Effect of portocaval shunt on residual extreme small liver after extended hepatectomy in porcine. World J Surg. 2006;30:2014-2022; discussion 2023-2024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Oliver RH, Sutton PM. The effects of partial hepatectomy on portal pressure in rats. Br J Surg. 1966;53:138-141. [PubMed] |
| 4. | Lee SS, Hadengue A, Girod C, Braillon A, Lebrec D. Reduction of intrahepatic vascular space in the pathogenesis of portal hypertension. In vitro and in vivo studies in the rat. Gastroenterology. 1987;93:157-161. [PubMed] |
| 5. | Campos BD, Botha JF. Strategies to optimize donor safety with smaller grafts for adult-to-adult living donor liver transplantation. Curr Opin Organ Transplant. 2012;17:230-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Schindl MJ, Redhead DN, Fearon KC, Garden OJ, Wigmore SJ. The value of residual liver volume as a predictor of hepatic dysfunction and infection after major liver resection. Gut. 2005;54:289-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 416] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 7. | Sirinek KR, Thomford NR. The effect of vasopressin on portal hypertension following hepatectomy. Surg Gynecol Obstet. 1974;139:573-577. [PubMed] |
| 8. | Boillot O, Delafosse B, Méchet I, Boucaud C, Pouyet M. Small-for-size partial liver graft in an adult recipient; a new transplant technique. Lancet. 2002;359:406-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 187] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 9. | Fukuchi T, Hirose H, Onitsuka A, Hayashi M, Senga S, Imai N, Shibata M, Yamauchi K, Futamura N, Sumi Y. Effects of portal-systemic shunt following 90% partial hepatectomy in rats. J Surg Res. 2000;89:126-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Szawlowski AW, Saint-Aubert B, Gouttebel MC, Astre C, Joyeux H. Experimental model of extended repeated partial hepatectomy in the dog. Eur Surg Res. 1987;19:375-380. [PubMed] |
| 11. | Fondevila C, Hessheimer AJ, Taurá P, Sánchez O, Calatayud D, de Riva N, Muñoz J, Fuster J, Rimola A, García-Valdecasas JC. Portal hyperperfusion: mechanism of injury and stimulus for regeneration in porcine small-for-size transplantation. Liver Transpl. 2010;16:364-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 83] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 12. | Zhang XK, Gauthier T, Burczynski FJ, Wang GQ, Gong YW, Minuk GY. Changes in liver membrane potentials after partial hepatectomy in rats. Hepatology. 1996;23:549-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Itasaka H, Suehiro T, Wakiyama S, Yanaga K, Shimada M, Sugimachi K. Significance of hyaluronic acid for evaluation of hepatic endothelial cell damage after cold preservation/reperfusion. J Surg Res. 1995;59:589-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Eriksson S, Fraser JR, Laurent TC, Pertoft H, Smedsrød B. Endothelial cells are a site of uptake and degradation of hyaluronic acid in the liver. Exp Cell Res. 1983;144:223-228. [PubMed] |
| 15. | McGowan JA, Fausto N. Ornithine decarboxylase activity and the onset of deoxyribonucleic acid synthesis in regenerating liver. Biochem J. 1978;170:123-127. [PubMed] |
| 16. | Kahn D, Stadler J, Terblanche J, van Hoorn-Hickman R. Thymidine kinase: an inexpensive index of liver regeneration in a large animal model. Gastroenterology. 1980;79:907-911. [PubMed] |
| 17. | Couvelard A, Scoazec JY, Feldmann G. Expression of cell-cell and cell-matrix adhesion proteins by sinusoidal endothelial cells in the normal and cirrhotic human liver. Am J Pathol. 1993;143:738-752. [PubMed] |
| 18. | Castillo-Suescun F, Oniscu GC, Hidalgo E. Hemodynamic consequences of spontaneous splenorenal shunts in deceased donor liver transplantation. Liver Transpl. 2011;17:891-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Iwanaga S, Morita T, Harada T, Nakamura S, Niwa M, Takada K, Kimura T, Sakakibara S. Chromogenic substrates for horseshoe crab clotting enzyme. Its application for the assay of bacterial endotoxins. Haemostasis. 1978;7:183-188. [PubMed] |
| 20. | Lane DJ. 16S/23S rRNA sequencing. Nucleic acid techniques in bacterial systematics. New York: John Wiley and Sons 1991; 115-175. |
| 21. | Such J, Francés R, Muñoz C, Zapater P, Casellas JA, Cifuentes A, Rodríguez-Valera F, Pascual S, Sola-Vera J, Carnicer F. Detection and identification of bacterial DNA in patients with cirrhosis and culture-negative, nonneutrocytic ascites. Hepatology. 2002;36:135-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 200] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 22. | Amann RI, Ludwig W, Schleifer KH. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol Rev. 1995;59:143-169. [PubMed] |
| 23. | Ito T, Kiuchi T, Yamamoto H, Oike F, Ogura Y, Fujimoto Y, Hirohashi K, Tanaka AK. Changes in portal venous pressure in the early phase after living donor liver transplantation: pathogenesis and clinical implications. Transplantation. 2003;75:1313-1317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 209] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 24. | Taniguchi M, Shimamura T, Suzuki T, Yamashita K, Oura T, Watanabe M, Kamiyama T, Matsushita M, Furukawa H, Todo S. Transient portacaval shunt for a small-for-size graft in living donor liver transplantation. Liver Transpl. 2007;13:932-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Troisi R, Ricciardi S, Smeets P, Petrovic M, Van Maele G, Colle I, Van Vlierberghe H, de Hemptinne B. Effects of hemi-portocaval shunts for inflow modulation on the outcome of small-for-size grafts in living donor liver transplantation. Am J Transplant. 2005;5:1397-1404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 206] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 26. | Ishizaki Y, Kawasaki S, Sugo H, Yoshimoto J, Fujiwara N, Imamura H. Left lobe adult-to-adult living donor liver transplantation: Should portal inflow modulation be added? Liver Transpl. 2012;18:305-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 27. | Takada Y, Ueda M, Ishikawa Y, Fujimoto Y, Miyauchi H, Ogura Y, Ochiai T, Tanaka K. End-to-side portocaval shunting for a small-for-size graft in living donor liver transplantation. Liver Transpl. 2004;10:807-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 90] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 28. | Shimada M, Ijichi H, Yonemura Y, Harada N, Shiotani S, Ninomiya M, Terashi T, Yoshizumi T, Soejima Y, Suehiro T. The impact of splenectomy or splenic artery ligation on the outcome of a living donor adult liver transplantation using a left lobe graft. Hepatogastroenterology. 2004;51:625-629. [PubMed] |
| 29. | Kishi Y, Sugawara Y, Akamatsu N, Kaneko J, Tamura S, Kokudo N, Makuuchi M. Splenectomy and preemptive interferon therapy for hepatitis C patients after living-donor liver transplantation. Clin Transplant. 2005;19:769-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 30. | Konishi N, Ishizaki Y, Sugo H, Yoshimoto J, Miwa K, Kawasaki S. Impact of a left-lobe graft without modulation of portal flow in adult-to-adult living donor liver transplantation. Am J Transplant. 2008;8:170-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | Lin RS, Lee FY, Lee SD, Tsai YT, Lin HC, Lu RH, Hsu WC, Huang CC, Wang SS, Lo KJ. Endotoxemia in patients with chronic liver diseases: relationship to severity of liver diseases, presence of esophageal varices, and hyperdynamic circulation. J Hepatol. 1995;22:165-172. [PubMed] |
| 32. | Nakatani Y, Fukui H, Kitano H, Nagamoto I, Tsujimoto T, Kuriyama S, Kikuchi E, Hoppou K, Tsujii T. Endotoxin clearance and its relation to hepatic and renal disturbances in rats with liver cirrhosis. Liver. 2001;21:64-70. [PubMed] |
| 33. | Rimmelé T, Kellum JA. High-volume hemofiltration in the intensive care unit: a blood purification therapy. Anesthesiology. 2012;116:1377-1387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |