Published online Aug 7, 2015. doi: 10.3748/wjg.v21.i29.8894
Peer-review started: January 17, 2015
First decision: April 13, 2015
Revised: April 27, 2015
Accepted: June 26, 2015
Article in press: June 26, 2015
Published online: August 7, 2015
Processing time: 203 Days and 21 Hours
AIM: To elucidate the natural history and the longitudinal outcomes in cirrhotic patients with non-forward portal flow (NFPF).
METHODS: The present retrospective study consisted of 222 cirrhotic patients (120 males and 102 females; age, 61.7 ± 11.1 years). The portal hemodynamics were evaluated at baseline and during the observation period using both pulsed and color Doppler ultrasonography. The diameter (mm), flow direction, mean flow velocity (cm/s), and mean flow volume (mL/min) were assessed at the portal trunk, the splenic vein, the superior mesenteric vein, and the collateral vessels. The average values from 2 to 4 measurements were used for the data analysis. The portal flow direction was defined as follows: forward portal flow (FPF) for continuous hepatopetal flow; bidirectional flow for to-and-fro flow; and reversed flow for continuous hepatofugal flow. The bidirectional flow and the reversed flow were classified as NFPF in this study. The clinical findings and prognosis were compared between the patients with FPF and those with NFPF. The median follow-up period was 40.9 mo (range, 0.3-156.5 mo).
RESULTS: Twenty-four patients (10.8%) demonstrated NFPF, accompanied by lower albumin level, worse Child-Pugh scores, and model for end-stage liver disease scores. The portal hemodynamic features in the patients with NFPF were smaller diameter of the portal trunk; presence of short gastric vein, splenorenal shunt, or inferior mesenteric vein; and advanced collateral vessels (diameter > 8.7 mm, flow velocity > 10.2 cm/s, and flow volume > 310 mL/min). The cumulative incidence rates of NFPF were 6.5% at 1 year, 14.5% at 3 years, and 23.1% at 5 years. The collateral vessels characterized by flow velocity > 9.5 cm/s and those located at the splenic hilum were significant predictive factors for developing NFPF. The cumulative survival rate was significantly lower in the patients with NFPF (72.2% at 1 year, 38.5% at 3 years, 38.5% at 5 years) than in those with forward portal flow (84.0% at 1 year, 67.8% at 3 years, 54.3% at 5 years, P = 0.0123) using the Child-Pugh B and C classifications.
CONCLUSION: NFPF has a significant negative effect on the prognosis of patients with worse liver function reserve, suggesting the need for careful management.
Core tip: The influence of non-forward portal flow (NFPF) in cirrhosis has not been determined. The present study examined the effect of NFPF on the natural history of cirrhosis in 222 patients (median follow-up period 40.9 mo). The cumulative incidences of NFPF was as follows: 6.5% at 1 year, 14.5% at 3 years, and 23.1% at 5 years. The cumulative survival rate was significantly lower in patients with NFPF (72.2% at 1 year, 38.5% at 3 years, and 38.5% at 5 years) than in those with forward portal flow (84.0% at 1 year, 67.8% at 3 years, and 54.3% at 5 years, P = 0.0123) using Child-Pugh B and C classifications, suggesting the need for careful management of these patients.
- Citation: Kondo T, Maruyama H, Sekimoto T, Shimada T, Takahashi M, Yokosuka O. Reversed portal flow: Clinical influence on the long-term outcomes in cirrhosis. World J Gastroenterol 2015; 21(29): 8894-8902
- URL: https://www.wjgnet.com/1007-9327/full/v21/i29/8894.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i29.8894
The prevalence of chronic liver disease is increasing worldwide with an extensive range of etiologies[1-3]. Cirrhosis, which is the end stage of liver disease, continues one of the leading causes of death because of the increased risk for developing variceal bleeding, hepatic failure, and hepatocellular carcinoma (HCC)[3-7]. Managing cirrhosis should be based on the proper assessment of disease severity and the prediction of outcomes[8-10].
Portal hypertension, which represents the underlying pathophysiology of cirrhosis, is the basic mechanism responsible for several major complications. The impaired hemodynamics typified by the development of collateral vessels might explain the clinical presentations[3,11-13]. Doppler ultrasound (US), which is a frequently used diagnostic tool, offers real-time observation of hemodynamics with simple and reliable methodology[14-18]. It may also be the only method that can demonstrate the direction of blood flow in vessels under physiological conditions.
Non-forward portal flow (NFPF), i.e., reversed or bidirectional flow in the portal venous system, is an abnormal but not rare condition in cirrhosis. The unique and impaired hemodynamics associated with NFPF are based on the anatomical features of the portal venous system and may represent a sign of advanced portal hypertension[19-22]. However, the influence of NFPF on the long-term clinical outcomes in patients with portal hypertension remains undetermined.
We designed the current study to compare the clinical manifestations and the long-term outcomes between patients with forward flow in the portal venous system and those with NFPF. The aim of the study was to elucidate the effect of portal flow direction on the long-term clinical outcomes of patients with cirrhosis.
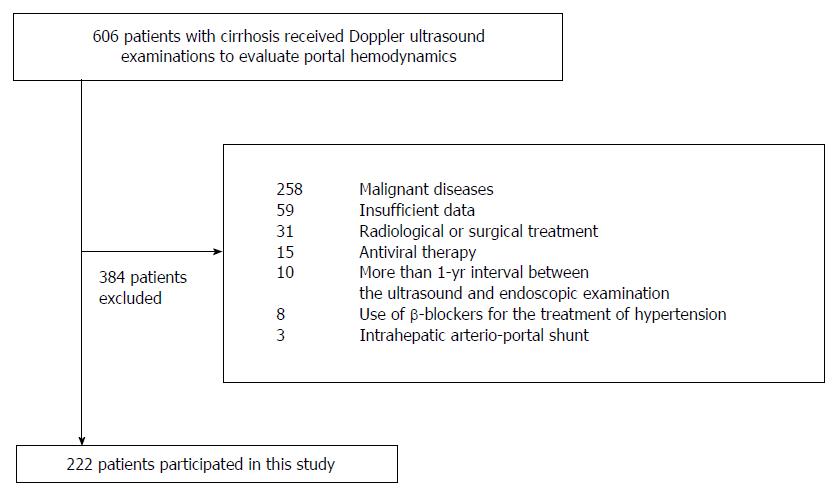
This retrospective study was based on the medical records in our department from June 2001 through November 2012. The information included the results of regular check-ups of patient’s physical status, blood tests, and findings from endoscopic and Doppler US examinations. The study enrolled patients with cirrhosis who received Doppler US examinations for evaluating their portal hemodynamics. The diagnosis of cirrhosis was based on a combination of biochemical findings and US examination. The exclusion criteria were as follows: (1) patients with a malignant disease (including a history of HCC diagnosis based on radiological findings and/or histology[23]); (2) patients with less than 1 year of follow-up; (3) patients with more than a 1-year interval between Doppler US and endoscopic examinations; (4) patients receiving radiological or surgical treatment, such as stomach surgery, transjugular intrahepatic portosystemic shunt, or shunt embolization that may affect the flow direction in the portal vein; (5) patients receiving antiviral therapy during the study period; (6) patients using vasoactive drugs, such as β-blockers, because these agents are not approved as treatment for portal hypertension in Japan; and (7) patients with vascular abnormalities, such as an intrahepatic arterio-portal shunt diagnosed using Doppler US examination.
Hepatic encephalopathy (HE) was assessed using the West-Haven grading system[24], and grade II or above was classified as overt HE. The degree of ascites was defined according to the following established guidelines[25]: mild for ascites that were only detectable by US examination, moderate for ascites that caused moderate symmetrical distention of the abdomen, and severe for ascites that caused marked abdominal distension. Moderate or severe ascites was classified as overt ascites. Portal vein thrombosis was defined as an echogenic structure that partially or completely occupied the lumen of the portal vein.
Decompensated cirrhosis was defined by the detection of at least one of the following presentations: variceal bleeding, overt ascites, overt HE, or jaundice (bilirubin level, > 3.0 mg/dL)[26,27]. The observation period was defined as the time between the initial US examination and the date of the last hospital visit, death, or liver transplantation.
Informed written consent for research use of medical records was obtained from all of the patients. This study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Chiba University Graduate School of Medicine.
Gastroesophageal varices were assessed according to the general guidelines of the Japan Research Society for Portal Hypertension[28]. Variceal bleeding was defined by both of the following criteria: (1) presence of bleeding history, such as hematemesis or melena; and (2) endoscopic evidence of active bleeding or a fibrin clot on the varices.
The study applied endoscopic band ligation for the patients with active bleeding from esophageal varices followed by sclerotherapy. Regarding the primary prophylaxis in patients with medium/large grade varices, endoscopic sclerotherapy was the treatment of choice. However, repeated band ligation was performed in patients with poor liver function (Child-Pugh C classification) or advanced liver cancer. Meanwhile, in patients with active bleeding from gastric varices, cyanoacrylate injection therapy (Histoacryl, B. Braun, Melsungen AG, Germany) was performed to achieve hemostasis.
There were 606 consecutive patients with cirrhosis who underwent Doppler US examinations to evaluate portal hemodynamics during the study period. Because 384 patients were considered to be ineligible for this study according to the exclusion criteria (Figure 1), the study included a total of 222 patients: age range, 20-89 years; mean age ± SD, 61.7 ± 11.1 years; and 120 males, 102 females. The routine blood tests for chronic liver disease, including serum albumin, bilirubin, prothrombin time, and platelet count, were performed within 5.5 mo (median, 0.1 mo; range, 0-5.5 mo) before and after the Doppler US examinations. The follow-up US and physical examinations were performed at least once per year in all of the patients. The median observation period was 40.9 mo (range, 0.3-156.5 mo).
The US equipment used was an SSA-390A or an SSA-770A (Toshiba, Tokyo, Japan) and a 3.75-MHz convex probe. The examination was performed with the patients placed in the supine position after fasting for more than 4 h.
The portal hemodynamics were evaluated using both pulsed and color Doppler US[13,18]. The diameter (mm), flow direction, mean flow velocity (cm/s), and mean flow volume (mL/min) were assessed at the portal trunk, splenic vein (SV), superior mesenteric vein (SMV), and collateral vessels. The blood flow was measured with a sampling width corresponding to the diameter of the vessel and an angle less than 60o between the US beam and the vessel. The average values from 2 to 4 measurements were used for the data analysis. The portal flow direction was defined as follows: forward portal flow (FPF) for continuous hepatopetal flow; bidirectional flow for to-and-fro flow; and reversed flow for continuous hepatofugal flow. The bidirectional flow and reversed flow were classified as NFPF in this study.
The US examinations were performed by Maruyama H or Takahashi M, who have more than 8 years of experience.
All of the data are expressed as the mean ± SD, median, or percentages. The comparisons were made between the patients with FPF and those with NFPF. The continuous variables were analyzed using Student’s t-test or Mann-Whitney U-test, as appropriate. The categorical variables were analyzed using the χ2 test. The best cut-off value was calculated based on the receiver operating characteristics (ROC) analysis. The cumulative survival rate was calculated using the Kaplan-Meier method, and multivariate analysis was performed using Cox regression analysis. A P value of less than 0.05 was considered to be statistically significant. Statistical analyses were performed using SAS version 9.4 software (SAS Institute, Cary, NC, United States).
The patterns of the portal flow direction were forward in 198 patients (89.2%), bidirectional in 5 patients (2.3%), and reversed in 19 patients (8.6%). Therefore, NFPF was found in 24 patients (10.8%), portal trunk in 6 patients (2.7%), SV in 22 patients (9.9%), and SMV in 2 patients (0.9%) (Table 1).
| Forward portal flow | Non-forward portal flow | ||
| Bidirectional flow | Reversed flow | ||
| Total | 198 (89.2) | 5 (2.3) | 19 (8.6) |
| In portal trunk | - | 2 (0.9) | 4 (1.8) |
| In splenic vein | - | 5 (2.3) | 17 (7.7) |
| In superior mesenteric vein | - | 2 (0.9) | 0 (0) |
The portal trunk diameter was smaller in the patients with NFPF than in those with FPF (P = 0.0013). The development of collateral vessels was closely associated with the portal flow direction (Table 2); the presence of the left gastric vein (LGV) (P = 0.0020) and the paraumbilical vein (P = 0.0482) was more frequent in the patients with FPF, whereas the presence of the short gastric vein (SGV) (P < 0.0001), the splenorenal shunt (SRS) (P = 0.0088), and the inferior mesenteric vein (P = 0.0169) was more frequent in those with NFPF.
| Forward portal flow | Non-forward portal flow | P value | |
| Number of subjects | 198 | 24 | |
| Portal vein thrombosis (-/+)1 | 181/17 | 23/1 | 0.4538 |
| Diameter of portal trunk (mm), mean ± SD (range) | 11.9 ± 2.4 (4.9-22.7) | 10.1 ± 2.0 (7.7-14.0) | 0.0013 |
| Collateral vessels (-/+)1 | 29/169 | 0/24 | 0.0443 |
| Left gastric vein (hepatofugal) | 137 (69.2) | 9 (37.5) | 0.0020 |
| Short gastric vein (hepatofugal) | 32 (16.2) | 12 (50.0) | < 0.0001 |
| Splenorenal shunt (hepatofugal) | 16 (8.1) | 6 (25.0) | 0.0088 |
| Paraumbilical vein (hepatofugal) | 53 (26.8) | 2 (8.3) | 0.0482 |
| Inferior mesenteric vein (hepatofugal) | 9 (4.5) | 4 (16.7) | 0.0169 |
| Characteristics of the largest collateral vessel | |||
| Diameter > 8.7 (mm)2 | 26 (13.1) | 17 (70.8) | < 0.0001 |
| Flow velocity > 10.2 (cm/s)2 | 87 (43.9) | 17 (70.8) | 0.0126 |
| Flow volume > 310 (mL/min)2 | 52 (26.3) | 16 (66.7) | < 0.0001 |
The best cut-off values of diameter, flow velocity, and flow volume in the largest collateral vessel selected from each individual were calculated using the ROC analysis between the patients with FPF and those with NFPF, 8.7 mm for diameter (P < 0.0001), 10.2 cm/s for flow velocity (P = 0.0126), and 310 mL/min for flow volume (P < 0.0001) (Table 2).
The presence of gastric varices was significantly more frequent in patients with NFPF than in those with FPF (P = 0.0093). The albumin level was lower in the patients with NFPF than in those with FPF (P = 0.0039). The Child-Pugh classification (P = 0.0015) and the model for end-stage liver disease (MELD) score (P = 0.0335) were worse in the patients with NFPF than in those with FPF (Table 3). The prevalence of decompensated cirrhosis did not differ between the patients with FPF and those with NFPF (P = 0.1074) (Table 3).
| Forward portal flow | Non-forward portal flow | P value | |
| Number of subjects | 198 | 24 | |
| Age (yr), mean ± SD (range) | 61.9 ± 10.9 (35-89) | 60.0 ± 12.6 (20-78) | 0.4070 |
| Sex (male/female) | 110/88 | 10/14 | 0.1973 |
| Etiology (Virus/Alcohol/NASH/PBC/AIH/others) | 93/33/9/20/11/32 | 8/4/1/4/0/7 | 0.4003 |
| Ascites (-/+/++)1 | 137/32/29 | 14/6/4 | 0.4949 |
| Esophageal varices (-/+)2 | 73/125 | 10/14 | 0.6464 |
| Gastric varices (-/+)2 | 155/43 | 13/11 | 0.0093 |
| History of variceal bleeding (-/+)2 | 140/58 | 18/6 | 0.6610 |
| History of variceal treatment (-/+)2 | 152/46 | 18/6 | 0.8469 |
| Spleen (cm2), mean ± SD (range) | 28.9 ± 12.9 (10.3-99.6) | 29.0 ± 16.4 (10.5-90.5) | 0.9900 |
| Hepatic encephalopathy (-/+)3 | 190/8 | 23/1 | 0.9764 |
| Child-Pugh (A/B/C) | 92/84/22 | 6/9/9 | 0.0015 |
| Decompensated cirrhosis (-/+)2 | 92/106 | 7/17 | 0.1074 |
| Model for end-stage liver disease score4, mean ± SD (range) | 11.0 ± 3.9 (6-24) | 13.1 ± 4.8 (6-24) | 0.0335 |
| Blood test | |||
| Bilirubin (mg/dL), mean ± SD (range) | 1.8 ± 2.0 (0.3-16.9) | 3.2 ± 3.3 (0.6-13.4) | 0.0578 |
| Albumin (g/dL), mean ± SD (range) | 3.4 ± 0.6 (1.5-4.7) | 3.1 ± 0.5 (1.8-3.9) | 0.0039 |
| Prothrombin time (%), mean ± SD (range) | 69.0 ± 17.9 (29-133) | 62.5 ± 21.4 (19-106) | 0.1026 |
| Platelet count (× 104/μL), mean ± SD (range) | 9.5 ± 5.9 (1.7-43.9) | 10.8 ± 6.0 (2.5-23.3) | 0.2976 |
Among the patients with compensated cirrhosis at baseline, the development of decompensated cirrhosis during the study period was found in 40.2% (37/92) (16 overt ascites, 9 variceal bleeding, 8 jaundice, and 4 HE) of the patients with FPF, and 42.9% (3/7) (2 HE, and 1 jaundice) of the patients with NFPF, and the results did not differ significantly between the groups (P = 0.8909). However, regarding the causes of decompensation, the development of HE was observed to be significantly more frequent in the patients with NFPF (2/7) than in those with FPF (4/92) (P = 0.0096).
Thirty-one patients developed NFPF from FPF during the study period, and the cumulative occurrence rates of NFPF were 6.5% at 1 year, 14.5% at 3 years, and 23.1% at 5 years. A univariate analysis showed that the diameters of the portal trunk < 11.2 mm (P = 0.0097) and the collateral vessels with flow velocity > 9.5 cm/s (P = 0.0136) were the best cut-off values using the ROC analysis and that the presence of SGV (P = 0.0061) and the presence of SRS (P = 0.0115) were significantly associated with the development of NFPF (Table 4). In the multivariate analysis, the collateral vessels with flow velocity > 9.5 cm/s (P = 0.0123), the presence of SGV (P = 0.0011), and the presence of SRS (P = 0.0279) were significant predictive factors for the development of NFPF (Table 4).
| Univariate hazard ratio (95%CI) | P value | Multivariate hazard ratio (95%CI) | P value | |
| Diameter of portal trunk (< 11.2 mm)1 | 2.677 (1.269-5.648) | 0.0097 | - | - |
| Collateral vessels (flow velocity > 9.5 cm/s)1 | 2.818 (1.238-6.416) | 0.0136 | 3.070 (1.276-7.389) | 0.0123 |
| Short gastric vein (hepatofugal) | 3.039 (1.374-6.720) | 0.0061 | 3.987 (1.736-9.158) | 0.0011 |
| Splenorenal shunt (hepatofugal) | 3.485 (1.323-9.180) | 0.0115 | 3.428 (1.143-10.278) | 0.0279 |
Among the patients with NFPF at baseline, the bidirectional flow changed to reversed flow in 1 patient, and the reversed flow changed to bidirectional flow in 3 patients during the study period.
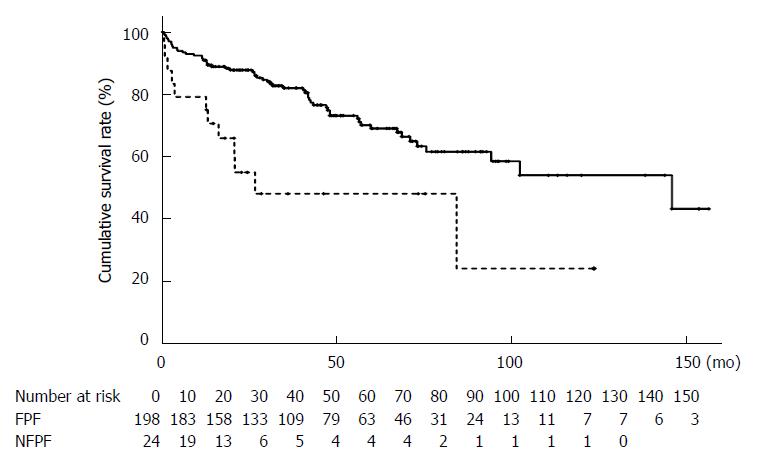
The cumulative overall survival rate was significantly worse in the patients with NFPF (79.2% at 1 year, 48.0% at 3 years, and 48.0% at 5 years) than those with FPF (90.9% at 1 year, 82.0% at 3 years, and 69.0% at 5 years; P = 0.0009) (Figure 2).
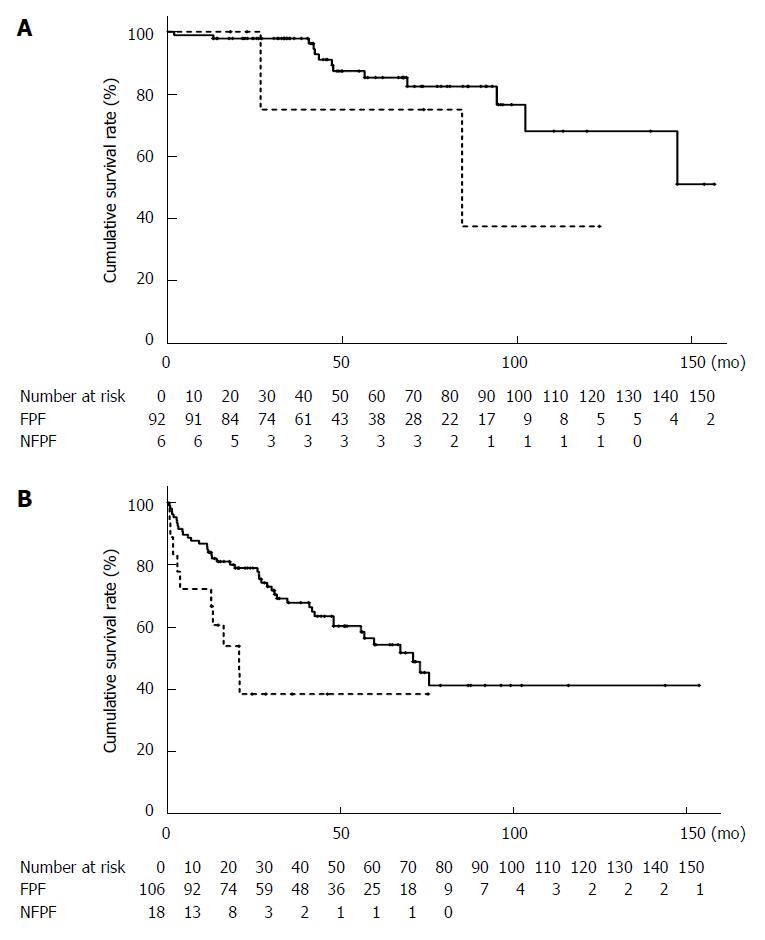
When stratified using Child-Pugh classification, there was no significant difference in the cumulative survival rate between the patients with FPF (98.9% at 1 year, 98.9% at 3 years, and 85.3% at 5 years) and those with NFPF (100% at 1 year, 75.0% at 3 years, and 75.0% at 5 years; P = 0.2314) using Child-Pugh A classification. However, the cumulative survival rate was significantly lower in the patients with NFPF (72.2% at 1 year, 38.5% at 3 years, and 38.5% at 5 years) than in those with FPF (84.0% at 1 year, 67.8% at 3 years, and 54.3% at 5 years; P = 0.0123) using Child-Pugh B and C classifications (Figure 3).
Twelve patients received liver transplantations, and 56 died during the study period: 39 died from hepatic failure, 8 died from HCC, 3 died from unknown cause, 2 died from variceal bleeding, 1 died from sepsis, 1 died from peritonitis of unknown origin, 1 died from a ruptured thoracic aortic aneurysm, and 1 died from heart failure.
The alternation in the blood flow direction is a unique phenomenon, that is observed in the portal venous system because of anatomical features and disease progression[19-21]. The present study focused initially on determining the frequency of this hemodynamic abnormality. NFPF was observed in 8.6% of the patients with reversed flow and 2.3% of the patients with bidirectional flow in the portal venous system in cirrhosis. This result may be comparable to the data in the literature, indicating that the overall incidence of reversed flow in the portal venous system in cirrhosis was 8.3% (3.1% in the portal vein, 3.1% in the SV, and 2.1% in the SMV)[20]. Other studies have reported frequency rates of 2.3% (3/132) in the portal trunk[29] and 9% in the intrahepatic portal vein[21]. However, presumably, the prevalence in chronic hepatitis is 1% in the SV, which is lower than the rate in cirrhosis[21].
Our study reported the cumulative incidence of NFPF, 6.5% at 1 year, 14.5% at 3 years, and 23.1% at 5 years, which have not been reported elsewhere. The presence of collateral vessels characterized by higher velocity (> 9.5 cm/s) or location at the splenic hilum was identified as significant hemodynamic factors associated with the future development of NFPF. In fact, there was no patient with NFPF who did not develop shunt vessels in our study. These data may suggest the influence of potentially advanced portal hypertension on the alternation of portal flow direction, and clearly encourage us to give these patients proper management.
Previous studies have shown a close relationship between flow direction in the portal venous system and liver function; hepatofugal flow was found to be more frequent in patients with Child-Pugh C (15.4%) and B (12.5%) classification than in those with Child-Pugh A (2.7%, P < 0.02) classification and was associated with a higher prevalence of HE (21% vs 7.2%; P < 0.05)[20]. Another study reported that reversed flow in the intrahepatic portal vein was significantly common in patients with Child-Pugh C (8/31, 25.8%) classification than in those with Child-Pugh A (0%)/B (5/104, 4.8%) classification[21]. One likely mechanism that could explain these results is the impaired nutritional metabolism with a poor hepatic clearance of toxic substance under the condition of NFPF[30]. Our study found that NFPF was closely associated with poor liver function, represented by serum albumin level, Child-Pugh classification, and MELD score. However, the portal flow direction was not a significant factor for the presence of liver decompensation at baseline in our study, which may be due to the definition of decompensation employed, i.e., one of the major presentations is bleeding from the esophageal varices that is related to the development of LGV. In the present study, however, the prevalence of LGV was higher in the patients with FPF and there was no difference in the bleeding history between the patients with FPF and those with NFPF. Collectively, our data indicate that there is little, if any, relationship between NFPF and clinical decompensation.
A major clinical interest regarding NFPF in cirrhosis is its influence on the patient’s prognosis. A previous study reported no significant difference in the survival rates between the patients with and without NFPF[20]. However, because the mean observation period in the study was only 13 mo (range, 12-18 mo), it may be difficult to draw a definite conclusion regarding the influence of NFPF on the long-term clinical course of patients with cirrhosis. The present study compared the long-term outcomes with a median observation time of 41 mo between the patients with FPF and those with NFPF. Although flow direction was not a significant survival factor in Child-Pugh A subjects, prognosis was significantly poorer in Child-Pugh B and C subjects with NFPF than in those with FPF. Thus, the influence of portal flow on the patient’s prognosis depends on the patient’s liver function, which is an important point to consider in clinical practice.
Our study had several limitations. First, the data were obtained from the analysis under a retrospective setting. Therefore, the data must be validated in a prospective study. Second, our study lacks portal pressure data. The hemodynamics in patients with reversed flow direction should be assessed from the perspective of portal pressure in the future.
In conclusion, this longitudinal study has demonstrated the underlying hemodynamic mechanism, natural course and clinical influence of bidirectional or reversed portal flow in patients with cirrhosis. The presence of collateral vessels with high velocity and those at the splenic hilum are predictive features of the alternation of portal flow direction. Moreover, NFPF has a significant negative effect on the prognosis of patients with impaired liver function reserve. These observations highlight the usefulness of hemodynamic assessment using Doppler US, and suggest a likely direction for improving the clinical management of patients with cirrhosis.
Non-forward portal flow, i.e., reversed or bidirectional flow in the portal venous system, is an abnormal but not rare condition in cirrhosis. However, the influence on the long-term clinical outcomes in patients with portal hypertension remains undetermined.
Portal hemodynamic abnormality, which is the key pathophysiology of cirrhosis patients, may account for the various manifestations. It may determine the long-term outcomes, which are informative for the clinical management of these patients.
Non-forward portal flow is associated with the development of collateral vessels and poor liver function. Furthermore, non-forward portal flow has a significant negative effect on the prognosis of patients with worse liver function reserve.
Careful management may be needed in the clinical practice of cirrhotic patients with non-forward portal flow.
Non-forward portal flow was defined for the bidirectional portal flow or reversed portal flow in this study.
This is a very solid study based on an interesting finding. The results of this study include important data.
P- Reviewer: Ganguly E, Gencdal G, Song YH S- Editor: Yu J L- Editor: A E- Editor: Zhang DN
| 1. | Younossi ZM, Stepanova M, Afendy M, Fang Y, Younossi Y, Mir H, Srishord M. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol. 2011;9:524-530.e1; quiz e60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 758] [Cited by in RCA: 790] [Article Influence: 56.4] [Reference Citation Analysis (0)] |
| 2. | Asrani SK, Larson JJ, Yawn B, Therneau TM, Kim WR. Underestimation of liver-related mortality in the United States. Gastroenterology. 2013;145:375-382.e1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 239] [Article Influence: 19.9] [Reference Citation Analysis (2)] |
| 3. | Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014;383:1749-1761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127:S35-S50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1691] [Cited by in RCA: 1790] [Article Influence: 85.2] [Reference Citation Analysis (2)] |
| 5. | D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1892] [Cited by in RCA: 2127] [Article Influence: 111.9] [Reference Citation Analysis (3)] |
| 6. | Adrogué HJ, Madias NE. Hypernatremia. N Engl J Med. 2000;342:1493-1499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 639] [Cited by in RCA: 509] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 7. | Rincón D, Lo Iacono O, Tejedor M, Hernando A, Ripoll C, Catalina MV, Salcedo M, Matilla A, Senosiain M, Clemente G. Prognostic value of hepatic venous pressure gradient in patients with compensated chronic hepatitis C-related cirrhosis. Scand J Gastroenterol. 2013;48:487-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D’Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3462] [Cited by in RCA: 3674] [Article Influence: 153.1] [Reference Citation Analysis (0)] |
| 9. | Kim WR, Biggins SW, Kremers WK, Wiesner RH, Kamath PS, Benson JT, Edwards E, Therneau TM. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med. 2008;359:1018-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Zipprich A, Garcia-Tsao G, Rogowski S, Fleig WE, Seufferlein T, Dollinger MM. Prognostic indicators of survival in patients with compensated and decompensated cirrhosis. Liver Int. 2012;32:1407-1414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 197] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 11. | Sanyal AJ, Bosch J, Blei A, Arroyo V. Portal hypertension and its complications. Gastroenterology. 2008;134:1715-1728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 238] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 12. | Ohnishi K, Sato S, Saito M, Terabayashi H, Nakayama T, Saito M, Chin N, Iida S, Nomura F, Okuda K. Clinical and portal hemodynamic features in cirrhotic patients having a large spontaneous splenorenal and/or gastrorenal shunt. Am J Gastroenterol. 1986;81:450-455. [PubMed] |
| 13. | Maruyama H, Kondo T, Kiyono S, Sekimoto T, Takahashi M, Yokosuka O. Influence of splenorenal shunt on long-term outcomes in cirrhosis. Scand J Gastroenterol. 2015;50:593-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Mitchell DG. Color Doppler imaging: principles, limitations, and artifacts. Radiology. 1990;177:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 125] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 15. | Harvey CJ, Pilcher JM, Eckersley RJ, Blomley MJ, Cosgrove DO. Advances in ultrasound. Clin Radiol. 2002;57:157-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 114] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 16. | Bolondi L, Li Bassi S, Gaiani S, Barbara L. Doppler flowmetry in portal hypertension. J Gastroenterol Hepatol. 1990;5:459-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Baik SK. Haemodynamic evaluation by Doppler ultrasonography in patients with portal hypertension: a review. Liver Int. 2010;30:1403-1413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 18. | Maruyama H, Kamezaki H, Kondo T, Sekimoto T, Shimada T, Takahashi M, Okugawa H, Yokosuka O. Effects of inferior mesenteric vein flow in patients with cirrhosis. Clin Gastroenterol Hepatol. 2013;11:1648-1654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Watanabe K, Kimura K, Matsutani S, Ohto M, Okuda K. Portal hemodynamics in patients with gastric varices. A study in 230 patients with esophageal and/or gastric varices using portal vein catheterization. Gastroenterology. 1988;95:434-440. [PubMed] |
| 20. | Gaiani S, Bolondi L, Li Bassi S, Zironi G, Siringo S, Barbara L. Prevalence of spontaneous hepatofugal portal flow in liver cirrhosis. Clinical and endoscopic correlation in 228 patients. Gastroenterology. 1991;100:160-167. [PubMed] |
| 21. | Tarantino L, Giorgio A, de Stefano G, Mariniello N, Perrotta A, Aloisio V V, Forestieri MC, Del Viscovo L, Borsellino G. Reverse flow in intrahepatic portal vessels and liver function impairment in cirrhosis. Eur J Ultrasound. 1997;6:171-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Kamezaki H, Maruyama H, Shimada T, Takahashi M, Okugawa H, Yokosuka O. Short- and long-term clinical outcome after balloon-occuluded retrograde transvenous obliteration: is pretreatment portal flow direction a predictive factor? Hepatol Int. 2013;7:241-247. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6566] [Article Influence: 469.0] [Reference Citation Analysis (1)] |
| 24. | Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT. Hepatic encephalopathy--definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35:716-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1594] [Cited by in RCA: 1408] [Article Influence: 61.2] [Reference Citation Analysis (0)] |
| 25. | Moore KP, Aithal GP. Guidelines on the management of ascites in cirrhosis. Gut. 2006;55 Suppl 6:vi1-vi12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 207] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 26. | Planas R, Ballesté B, Alvarez MA, Rivera M, Montoliu S, Galeras JA, Santos J, Coll S, Morillas RM, Solà R. Natural history of decompensated hepatitis C virus-related cirrhosis. A study of 200 patients. J Hepatol. 2004;40:823-830. [PubMed] |
| 27. | Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, Durand F, Gustot T, Saliba F, Domenicali M. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426-1437, 1437.e1-9. [PubMed] |
| 28. | The Japan Society for Portal Hypertension. The General Rules for Study of Portal Hypertension. 3rd ed. Tokyo: Kanehara 2013; . |
| 29. | Kawasaki T, Moriyasu F, Nishida O, Ban N, Nakamura T, Tamada T, Kimura T, Yamashita Y, Ono S, Uchino H. Analysis of hepatofugal flow in portal venous system using ultrasonic Doppler duplex system. Am J Gastroenterol. 1989;84:937-941. [PubMed] |
| 30. | Olde Damink SW, Deutz NE, Dejong CH, Soeters PB, Jalan R. Interorgan ammonia metabolism in liver failure. Neurochem Int. 2002;41:177-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 101] [Article Influence: 4.4] [Reference Citation Analysis (0)] |