Published online Jul 28, 2015. doi: 10.3748/wjg.v21.i28.8730
Peer-review started: December 17, 2014
First decision: January 22, 2015
Revised: February 22, 2015
Accepted: March 30, 2015
Article in press: March 31, 2015
Published online: July 28, 2015
Processing time: 225 Days and 4.1 Hours
Hepatic inflammatory pseudotumors are uncommon benign lesions. Accurately diagnosing hepatic inflammatory pseudotumor can be very challenging because the clinical presentation and radiological appearances are nonspecific and cannot be certainly distinguished from malignant neoplastic processes. Herein, we present a case of hepatic IPT in an 8-year-old boy who presented to clinic with a 3-mo history of a tender hepatic mass, fever of unknown origin, and 9-kg weight loss. The physical examination was notable for tender hepatomegaly. Laboratory investigations were notable for a normal hepatic profile and elevated erythrocyte sedimentation rate and C-reactive protein. A T2-attenuated magnetic resonance imaging scan of the abdomen showed a 4.7 cm × 4.7 cm × 6.6 cm, contrast-enhancing, hyper-intense, well-defined lesion involving the right hepatic lobe. In view of the unremitting symptoms, tender hepatomegaly, thrombosed right hepatic vein, nonspecific radiological findings, and high suspicion of a deep-seated underlying infection or malignancy, a right hepatic lobectomy was recommended. Microscopically, the hepatic lesion exhibited a mixture of inflammatory cells (histiocytes, plasma cells, mature lymphocytes, and occasional multinucleated giant cells) in a background of dense fibrous tissue. Immunohistochemically, the cells stained negative for SMA, ALK-1, CD-21 and CD-23, diffusely positive for CD-68, and focally positive for IgG4. The final histopathological diagnosis was consistent with hepatic IPT. At the postoperative 4-mo follow-up, the patient was asymptomatic without radiological evidence of recurrence.
Core tip: Because hepatic inflammatory pseudotumors (IPTs) are considered benign lesions and their natural clinical course advances toward regression, it is extremely critical to establish the most accurate diagnosis and not to regard them as malignant neoplasms that may lead to needless surgical excisions. However, accurately diagnosing hepatic IPTs can be very challenging, likely attributable to the fact that the clinical presentation and radiological appearances are nonspecific and cannot be certainly distinguished from malignant neoplastic processes. Management options include conservative treatment, and if failed, surgical resection. Histopathological and immunohistochemical analysis of the hepatic lesion yields the definitive diagnosis.
- Citation: Al-Hussaini H, Azouz H, Abu-Zaid A. Hepatic inflammatory pseudotumor presenting in an 8-year-old boy: A case report and review of literature. World J Gastroenterol 2015; 21(28): 8730-8738
- URL: https://www.wjgnet.com/1007-9327/full/v21/i28/8730.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i28.8730
Inflammatory pseudotumors (IPTs) are rare benign lesions characterized histologically by proliferation of inflammatory cells (for example, neutrophils, eosinophils, lymphocytes, plasma cells, histiocytes, and multinucleated giant cells), myofibroblasts and spindle-shaped cells[1]. The most frequent site of origin is the lungs[2]. Infrequently, extra-pulmonary sites include the orbit, spleen, mesentery, intestines and other organs[3]. As of 2011, fewer than 300 cases of hepatic IPTs have been reported in the medical literature[4]. More specifically, as of 2008, only 35 cases of pediatric (childhood) hepatic IPTs have been reported so far[5]. Herein, we present the unusual case of hepatic IPT in an 8-year-old boy who presented to the clinic with a 3-mo history of a hepatic mass and fever of unknown origin and who was treated successfully with right hepatic lobectomy.
An 8-year-old boy presented to the clinic with a 3-mo history of a tender hepatic mass and fever of unknown origin for which he underwent extensive workups with no definitive diagnosis. The past medical history was significant for one episode of bilateral hip pain, which was diagnosed as septic arthritis and treated with intravenous and oral antibiotics 4 mo prior to the clinical presentation. The past surgical history was remarkable for unilateral orchidopexy at the age of 6 years and status post adenoidectomy and tonsillectomy at the age of 2 years. The family history was negative for consanguinity, chronic illnesses, or malignancies. The review of systems was remarkable for night sweats, decreased appetite, and 9-kg weight loss over the past 3 mo.
The physical examination was notable for tender hepatomegaly, palpable 2 cm below the costal margin.
Laboratory investigations revealed a normal white blood cell count (no leukocytosis) and normal renal, bone, hepatic, and coagulation profiles. The peripheral blood smear displayed a microcytic hypochromic picture and few reactive lymphocytes with hyper-segmented neutrophils and thrombocytosis.
Other laboratory tests showed a high erythrocyte sedimentation rate (ESR) of 140 mm/h, a C-reactive protein (CRP) level of 246 mg/L, and a CD-25 level of 3125 μ/mL. Serological tests for hepatitis-A, hepatitis-B, hepatitis-C, echinococcus, brucella, cytomegalovirus, Epstein-Barr virus (EBV), and human immunodeficiency virus were negative. Tumor markers such as alpha-fetoprotein (AFP), carcinoembryonic antigen (CEA), and cancer antigen (CA) 19-9 were negative. The hemophagocytic lymphohistiocytosis workup profile was negative. Flow cytometric assays for phagocyte oxidative burst, blastogenesis, and lymphocyte markers were within normal limits.
An abdominal Doppler ultrasound revealed a 4.6-cm, well-defined, heterogeneous lesion in the right hepatic lobe (with a nonspecific appearance) and complete thrombosis of the right hepatic vein.
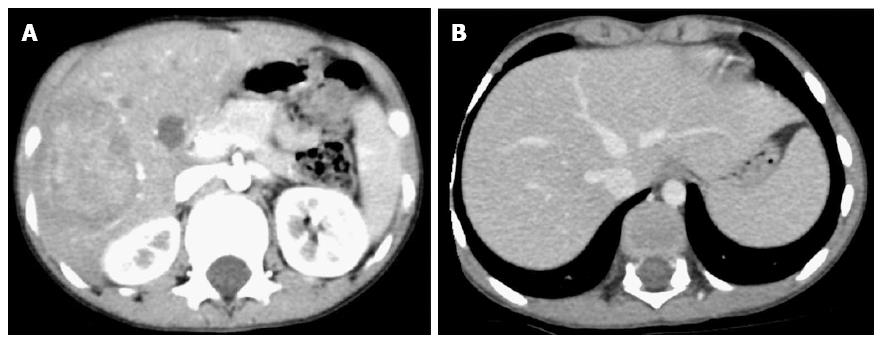
Contrast-enhanced computed tomography (CT) of the abdomen showed a 6.3 cm × 5.1 cm × 5.5 cm, relatively well-defined, hypodense lesion with internal enhancement involving the right hepatic lobe (segments V, IV, VII) (Figure 1A). No additional similar lesions were identified elsewhere. In the delayed venous phase, the right hepatic vein was thrombosed, whereas the middle and left hepatic veins were patent (Figure 1B). The portal vein and hepatic artery were unremarkable.
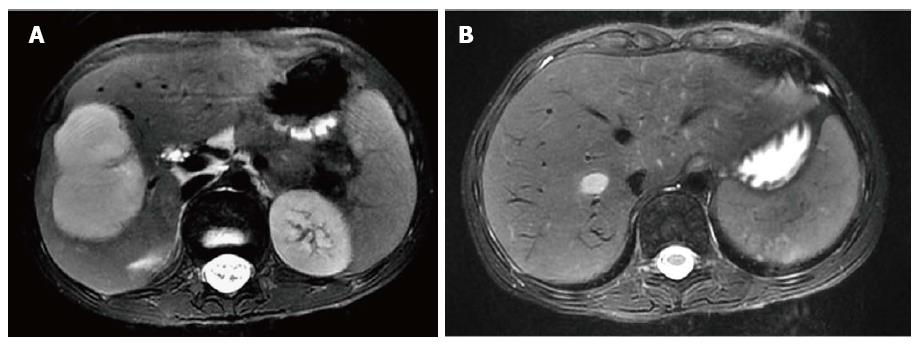
Magnetic resonance imaging (MRI) of the abdomen revealed a 4.7 cm × 4.7 cm × 6.6 cm, contrast-enhancing, well-defined, moderate- to large-sized lesion involving the right hepatic lobe, particularly segments V, VI and VII (Figure 2A). The right hepatic vein was completely thrombosed up to the inferior vena cava (Figure 2B).
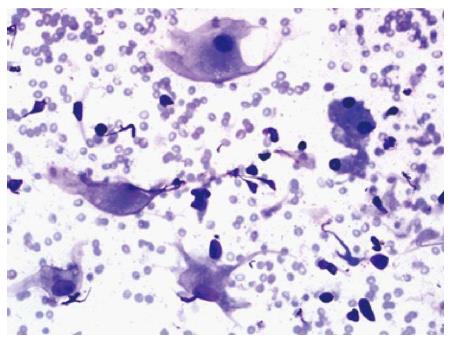
Fine-needle aspiration (FNA) was performed. The aspirate was stained with Diff-Quik and displayed a small amount of cellular yield composed of benign liver cells mixed with benign-appearing histiocytic cells (Figure 3).
In view of the unremitting fever of unknown origin (FUO), tender hepatomegaly, thrombosed right hepatic vein, nonspecific radiological findings, and high suspicion of a deep-seated underlying infection or malignancy, a right hepatic lobectomy was recommended.
The patient underwent resection of the right hepatic lobe, gall bladder and falciform ligament. Histopathological examination of the gall bladder revealed mild inflammation, whereas the histopathological examination of the falciform ligament showed fibro-fatty tissue without significant pathology. The resected right hepatic lobe was sent for further macroscopic, microscopic, and immunohistochemical analysis.
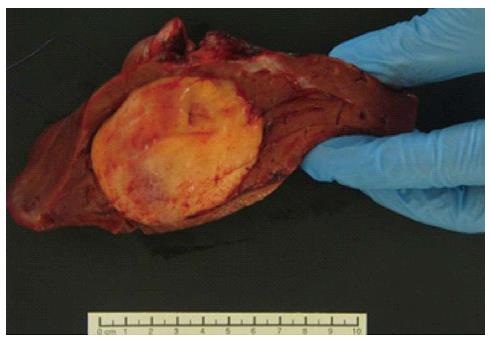
Macroscopically, the resected right hepatic lobe measured 18 cm × 14 cm × 4 cm and weighted 595 g. There was a focal, soft, fleshy, yellow-white, tanned nodule measuring 8 cm × 6 cm × 5 cm (Figure 4).
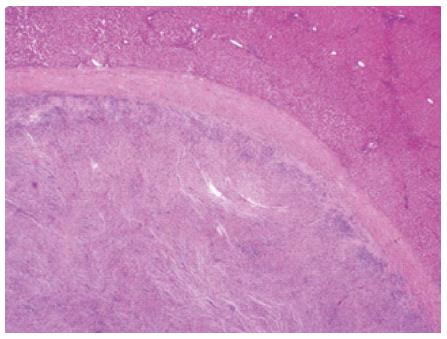
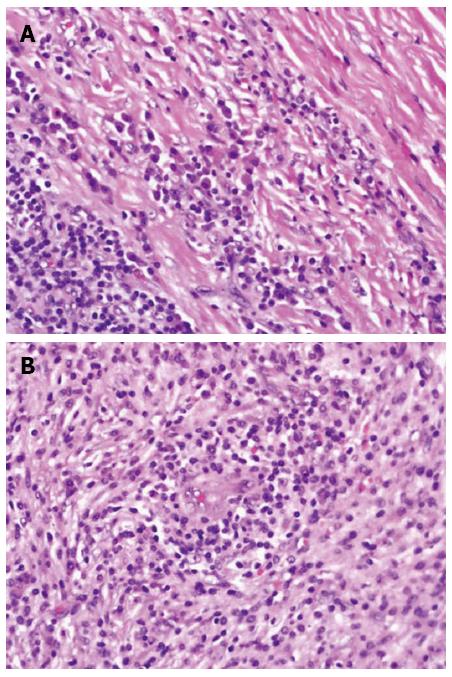
Microscopically, the hepatic lesion was surrounded by a well-demarcated thin capsule (Figure 5). The hepatic lesion exhibited a mixture of inflammatory cells in a background of dense fibrous tissue (Figure 6 A, B). The inflammatory cells were mostly composed of histiocytes with abundant cytoplasm, plasma cells, mature lymphocytes, and occasional multinucleated giant cells. The inflammatory cells were concentrated mainly in the sub-capsular area at the periphery (Figure 6A) and around the blood vessels (Figure 6B). There were no granulomas or necrotic areas.
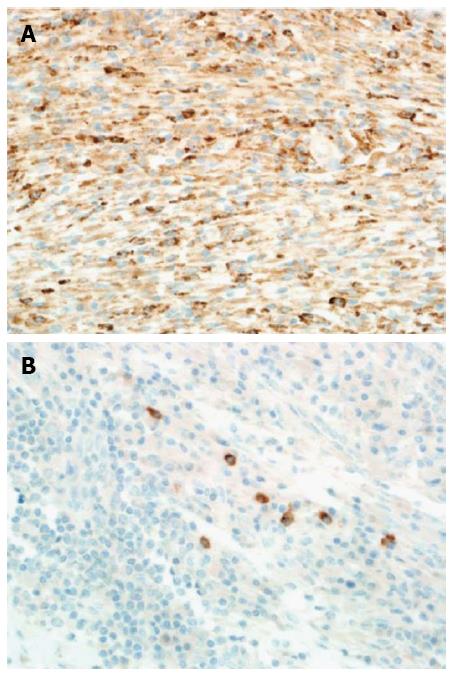
Immunohistochemically, special stains for acid-fast bacilli and fungi were negative. Moreover, the hepatic lesion stained negative for SMA, ALK-1, CD-21 and CD-23. Conversely, it stained diffusely positive for CD-68 (Figure 7A). Furthermore, the hepatic lesion displayed focal positivity for IgG4 (7 plasma cells/high-power field), which was inadequate for classifying it as an IgG4-related disease (> 10 plasma cells/high-power field is required for the diagnosis) (Figure 7B). In situ hybridization ruled out EBV infection.
Based on the histopathological and immunohistochemical examinations (along with the clinical history), a final diagnosis of hepatic inflammatory pseudotumor (fibro-histiocytic subtype) was established.
The patient had an uneventful postoperative course following surgery. At the postoperative 4-mo follow-up, the patient was afebrile and symptom-free, with no radiological evidence of hepatic recurrence.
The etiology of hepatic IPTs is largely unidentified[6]. Other probable etiologies include infections such as actinomyces, bacteroides, Escherichia coli, Klebsiella, enterococcus, gram-positive cocci and beta-hemolytic streptococci. These seeding gut infectious bacteria may gain entry to the liver through the portal circulation and subsequently provoke an inflammatory response, which sometimes decreases or completely resolves upon administration of antibiotics[4,7] or non-steroidal anti-inflammatory drugs[8]. Additional likely etiologies include autoimmunity, trauma and surgical-related infections[9-11]. Recently, an EBV-positive infection has been reported as a possible predisposing factor[12]. The autoimmune etiology is supported by the association with certain IgG4-related diseases[13].
Histologically, hepatic IPTs can be categorized into two variants: fibrohistiocytic and lymphoplasmacytic[13]. Fibrohistiocytic-variant hepatic IPTs are typically characterized by abundant xanthogranulomatous inflammation (foamy histiocytes or multinucleated giant cells); lymphocytic and neutrophilic infiltrations in a background of plentiful fibrous tissue; mass-forming lesions mostly located in the peripheral hepatic parenchyma (sub-capsular); venous obstruction with minimal inflammation; cholangitis without periductal fibrosis; and minimal IgG4 positivity of plasma cells[13]. Conversely, lymphoplasmacytic-variant hepatic IPTs are typically characterized by diffuse inflammatory cells mainly consisting of plasma cells and lymphocytes (minimal histiocytes); conspicuous eosinophilic infiltration in a background of reduced fibrous tissue; mass-forming lesions mostly located around the hepatic hilum; obliterative phlebitis; cholangitis with periductal fibrosis; and extremely marked IgG4 positivity of plasma cells[14].
Bearing in mind that hepatic IPTs are considered benign lesions and that their natural clinical course advances toward regression, it is critical to establish the most accurate diagnosis and not to regard them as malignant neoplasms that may lead to needless surgical excisions[14,15]. However, accurately diagnosing hepatic IPTs can be very challenging because the clinical presentation and radiological appearances are nonspecific and cannot be certainly distinguished from other malignant neoplastic processes[7,16,17]. Further research is needed.
Clinically, the most frequent presenting symptoms of hepatic IPTs are abdominal pain and subjective constitutional symptoms such as fever, malaise, fatigue and weight loss[5,6]. Other infrequent symptoms may include myalgia, jaundice and gastrointestinal upset. Despite the vast majority of patients being symptomatic (91%), a small proportion of patients are asymptomatic (9%)[6]. A study conducted by Torzilli et al[18] revealed that hepatic IPTs were incidentally found in approximately 1% of 403 patients undergoing resection of focal liver lesions.
From the laboratory testing viewpoint[18], more than one-third (37%) of hepatic IPT patients present with high white blood cell counts (WCCs) and elevations of acute-phase reactant inflammatory markers such as ESR and CRP. Deranged liver function tests are also present in approximately 15% of patients. Our patient had neither leukocytosis nor deranged LFTs; however, he had high ESR and CRP levels.
Measuring the soluble CD-25 serum level is not a routine practice in our hospital. In fact, globally, the routine use of soluble CD-25 is largely unavailable in the vast majority of laboratories, and its current use is limited to only experimental research purposes.
In our case, the reason for ordering the soluble CD-25 serum-level test was rationalized by (1) its availability at our advanced tertiary-care hospital and, more importantly; and (2) the possible differential diagnosis of macrophage activation syndrome (MAS). MAS is a fatal complication of pediatric rheumatic diseases. It occurs most often in children with systemic juvenile idiopathic arthritis (SJIA) and is characterized by the presence of neurologic symptoms, pancytopenia, coagulopathy mimicking disseminated intravascular coagulation and hepatic insufficiency. Its presumed pathogenesis is the life-threatening activation and uncontrolled expansion of T-lymphocytes and macrophagic histiocytes, causing excessive hemophagocytosis and cytokine overproduction[19,20].
Recently, soluble serum CD-25 (the alpha-chain of the IL-2 receptor) has been demonstrated to be a potentially useful marker for identifying early (subclinical) MAS in patients with SJIA[21,22]. Additionally, at the very onset of MAS, patients may experience a paradoxical improvement in the underlying inflammatory disease, with eventual resolution of arthritis signs and symptoms, as well as a rapid decrease in the ESR. The latter phenomenon may be attributed to hypofibrinogenemia due to fibrinogen consumption and hepatic dysfunction[23]. Despite the high level of soluble CD-25 in the serum, our patient did not adequately meet the other diagnostic criteria for MAS complicating SJRA[24-26].
In our case, the patient developed right hepatic vein thrombosis, and this event could be attributed to three main reasons. First, hepatic IPTs are characterized by extensive proliferation of multiple inflammatory cells[1] that jointly produce various thrombogenic cytokines, leading to pro-coagulant activity (that is, thrombosis) of the vascular endothelium[27]. Figure 6B shows that the inflammatory cells were concentrated around blood vessels. Second, as revealed by CT (Figure 1A) and MRI (Figure 2A) scans, the hepatic IPT lesion involved the right hepatic lobe (segments V, VI, and VII), which is known to be anatomically drained by the right hepatic vein. Accordingly, extension of the hepatic IPT lesion (thrombo-embolus) into the right hepatic vein lumen is a plausible reason for the right hepatic vein thrombosis/obstruction. Third, as opposed to the central (hilar) location, the peripheral location of the hepatic IPT (in our case-right lobe) and occurrence of hepatic venous thrombosis/occlusion is a well-documented phenomenon in the literature[13].
In our case, the portal vein was unremarkable. Local thrombosis of the portal vein is a well-recognized complication of IPT lesions involving the hepatic hilum rather than the peripheral hepatic parenchyma[13,28,29]. However, although this condition is rare, Fernandes et al[30] reported the extremely unusual case of extensive portal venous thrombosis and peripheral right hepatic lobe IPT in a 9-year-old boy who presented to an emergency department with a 20-d history of abdominal pain and fever of 2 days’ duration.
Despite the advances in imaging technologies, distinguishing between IPTs and other focal hepatic lesions continues to be a major challenge[4]. Radiologically, hepatic IPTs may largely mimic other granulomatous lesions (such as sarcoidosis and tuberculosis) or malignant lesions (such as metastatic tumors, hepatocellular carcinoma, lymphoma, and malignant fibrous histiocytoma)[4]. Hepatic IPTs can present as a single or multi-focal mass with a non-specific radiological appearance[5,7,16,17]. On ultrasound imaging, these lesions may appear hyper-echoic or hypo-echoic, with septations and increased through-transmission[31]. On contrast-enhanced CT imaging, variable patterns of enhancement exist, such as peripheral enhancement with delayed central filling, homogeneous enhancement, heterogeneous enhancement and enhancement of septa. Alternatively, there may be negative enhancement or central necrosis[9,31]. On MRI imaging, these lesions express hyper-intensity and hypo-intensity on T2-attenuated and T1-attenuated scans, respectively[32]. Moreover, the presence of small nodular extensions at the boundary of the hepatic IPT lesions during the hepatobiliary phase of gadoxetic acid (Gd-EOB)-MRI may represent a distinctive radiological finding of hepatic IPT. However, this finding is still investigational and needs to be validated and reproduced in other cases[33]. On fluorodeoxyglucose (FDG) positron imaging tomography/computed tomography (PET/CT) scan, the FDG uptake in hepatic IPTs range from low to high uptake, depending on several factors, such as (1) lesion cellularity; (2) biological behavior; (3) composition and number of inflammatory cells; and (4) degree of inflammatory cell activation[34]. Additionally, FDG PET/CT scans may be valuable for identifying new primary IPTs, loco-regional relapses, and distant metastatic foci[34].
Despite the nonspecific radiologic appearances[7,16,17], it can be concluded that the various imaging modalities may offer valuable clues for diagnosing hepatic IPT in patients with the following characteristics: (1) clinical signs and symptoms of inflammation; (2) laboratory results suggesting inflammation; (3) normal serum tumor markers such as CEA, CA 19-9 and AFP; and (4) evidence of mass-occupying hepatic lesions on imaging[35].
When radiological findings are inconclusive, FNA may be helpful in establishing the diagnosis[36]. However, on several occasions, the FNA findings may not be sufficient to establish the diagnosis; therefore, hepatic resection of suspicious lesions may be required with subsequent histopathological and immunohistochemical analysis to establish the definitive diagnosis[37].
The management of hepatic IPTs remains a topic of dispute[6,14]. Hepatic IPTs are largely benign lesions[14,15]. The natural clinical course is spontaneous regression without intervention[7,14,38,39]. However, therapeutic interventions are sometimes necessary. Management options include conservative or surgical approaches. Conservative management with antibiotics[4,7], non-steroidal anti-inflammatory drugs[8], or steroids[40,41] yields complete resolution of hepatic IPTs, although subsets of patients develop subsequent recurrences[42]. More-aggressive radical treatment (that is, surgical resection) is advocated by many authors[43-45]. Malignant transformation of IPTs (into hepatic sarcomas and non-Hodgkin lymphomas) and recurrent distant metastases a few years following surgical resection have been described in the literature[46,47].
All cases of pediatric hepatic IPTs from 1971 to 2008 (n = 35) were reviewed by Nagarajan et al[5].
Some recent reports of hepatic IPTs in childhood from 2009 to 2014 (n = 9) are summarized in Table 1[5,6,30,48-52].
| Ref. | Age | Sex | Clinical presentation | Tumor markers | Important notes | No. of lesions | Site of the lesions | Treatment | Outcome |
| Kim et al[48] | 5-mo-old | Male | Hepatomegaly | Single | Right lobe | Surgery | Recovery | ||
| Fernandes et al[30] | 9-mo-old | Male | Abdominal pain and fever | Normal | Extensive portal venous thrombosis | Multiple | Right lobe | Conservative (antibiotics) | Recovery |
| Xiao et al[49] | 4-mo-old | Male | Abdominal pain | Normal | Single | Right lobe | Conservative (antibiotics) | Recovery | |
| Nagarajan et al[5] | 15-mo-old | Male | Hepatomegaly | Normal | Pre-term birth and disrupted iron profile | Single | Left lobe | Surgery | Recovery |
| Fragoso et al[50] | 16-mo-old | Male | Thoracic wall mass | Single | Right lobe | Conservative (antibiotics) | Recovery | ||
| Manolaki et al[51] | 9-mo-old | Female | Epigastric pain, fever and anorexia (1.5-kg weight loss) | Normal | Mycobacterium tuberculosis infection in an immunocompetent girl | Single | Left lobe | Surgery | Recovery |
| Goldsmith et al[6] | 7-mo-old | Male | Multiple | Hilum with extension to segments IV and VIII | Surgery | Postoperative Portal vein thrombosis - underwent liver transplant. Alive 5 years post-transplant | |||
| Geramizadeh et al[52] | 14-mo-old | Male | Fever, chills, and anorexia (8-kg weight loss) | Single | Left lobe | Surgery | Recovery | ||
| Geramizadeh et al[52] | 15-mo-old | Male | Anorexia and weight loss | HBS-positive | Surgery | Recovery |
In conclusion, hepatic IPTs are uncommon, benign lesions. Accurately diagnosing hepatic IPTs can be very challenging because the clinical presentation and radiological appearance are nonspecific and cannot be certainly distinguished from malignant neoplastic processes. Hepatic IPTs should be considered in the differential diagnosis of all pediatric patients presenting with FUO. The consensus regarding management includes conservative treatment, and if failed, surgical resection. Histopathological and immunohistochemical analysis of the hepatic lesion yields the definitive diagnosis.
An 8-year-old boy presented to the clinic with a 3-mo history of a tender hepatic mass (hepatomegaly) and fever of unknown origin (FUO).
The physical examination revealed tender hepatomegaly; the liver was palpable 2 cm below the costal margin.
Underlying deep-seated infection, abscess, granulomatous lesions (such as sarcoidosis and tuberculosis), malignant lesions (such as metastatic tumors and malignant fibrous histiocytoma) and macrophage activation syndrome.
High erythrocyte sedimentation rate of 140 mm/h and C-reactive protein level of 246 mg/L; normal tumor markers [alpha-fetoprotein (AFP), carcinoembryonic antigen (CEA), and cancer antigen (CA) 19-9]; and negative workup for hepatitis, echinococcus, brucella, cytomegalovirus, Epstein-Barr virus, human immunodeficiency virus and hemophagocytic lymphohistiocytosis.
Contrast-enhanced computed tomography revealed a 6.3 cm × 5.1 cm × 5.5 cm, relatively well-defined, hypodense lesion with internal enhancement involving the right hepatic lobe (segments V, VI and VII), as well as a thrombosed right hepatic vein; magnetic resonance imaging yielded similar yet nonspecific findings.
Microscopically, the right hepatectomy tissue exhibited a mixture of inflammatory cells (histiocytes, plasma cells, mature lymphocytes, and occasional multinucleated giant cells) in a background of dense fibrous tissue without granulomas, whereas immunohistochemical staining showed negativity for SMA, ALK-1, CD-21 and CD-23 but diffuse positivity for CD-68 and focal positivity for IgG-4.
The patient underwent a right hemi-hepatectomy.
Pediatric (childhood) hepatic inflammatory pseudotumors (IPTs) are exceedingly uncommon, and fever of unknown origin is a rare presenting clinical symptom.
Inflammatory pseudotumors, also known as inflammatory myofibroblastic tumors, are rare, benign lesions characterized histologically by the proliferation of inflammatory cells (for example, neutrophils, eosinophils, lymphocytes, plasma cells, histiocytes, and multinucleated giant cells), myofibroblasts and spindle-shaped cells.
Although rare, hepatic IPTs should be suspected in pediatric patients with the following characteristics: (1) clinical and laboratory findings of inflammation/infection; (2) normal serum tumor markers such as AFP, CEA, and CA 19-9; and (3) evidence of liver mass-occupying lesions on imaging.
This article presents a rare yet challenging case of pediatric hepatic IPT. This case report highlights that-in view of FUO, tender hepatomegaly, a thrombosed right hepatic vein, nonspecific radiological findings, and a high suspicion of deep-seated underlying infection or malignancy-surgical resection is the recommended management strategy.
P- Reviewer: Jia NY, Mihaila RG, Matsuo Y S- Editor: Qi Y L- Editor: A E- Editor: Zhang DN
| 1. | Souid AK, Ziemba MC, Dubansky AS, Mazur M, Oliphant M, Thomas FD, Ratner M, Sadowitz PD. Inflammatory myofibroblastic tumor in children. Cancer. 1993;72:2042-2048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Melloni G, Carretta A, Ciriaco P, Arrigoni G, Fieschi S, Rizzo N, Bonacina E, Augello G, Belloni PA, Zannini P. Inflammatory pseudotumor of the lung in adults. Ann Thorac Surg. 2005;79:426-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 85] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 3. | Coffin CM, Humphrey PA, Dehner LP. Extrapulmonary inflammatory myofibroblastic tumor: a clinical and pathological survey. Semin Diagn Pathol. 1998;15:85-101. [PubMed] |
| 4. | Ntinas A, Kardassis D, Miliaras D, Tsinoglou K, Dimitriades A, Vrochides D. Inflammatory pseudotumor of the liver: a case report and review of the literature. J Med Case Rep. 2011;5:196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Nagarajan S, Jayabose S, McBride W, Prasadh I, Tanjavur V, Marvin MR, Rodriguez-Davalos MI. Inflammatory myofibroblastic tumor of the liver in children. J Pediatr Gastroenterol Nutr. 2013;57:277-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Goldsmith PJ, Loganathan A, Jacob M, Ahmad N, Toogood GJ, Lodge JP, Prasad KR. Inflammatory pseudotumours of the liver: a spectrum of presentation and management options. Eur J Surg Oncol. 2009;35:1295-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Ishida H, Tatsuta M, Furukawa H, Ohta H, Hashimoto K, Hayashi N, Morimoto O, Ikeda M, Miya A, Masutani S. Multiple inflammatory pseudotumors mimicking liver metastasis from colon cancer: report of a case. Surg Today. 2000;30:530-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Vassiliadis T, Vougiouklis N, Patsiaoura K, Mpoumponaris A, Nikolaidis N, Giouleme O, Evgenidis N. Inflammatory pseudotumor of the liver successfully treated with nonsteroidal anti-inflammatory drugs: a challenge diagnosis for one not so rare entity. Eur J Gastroenterol Hepatol. 2007;19:1016-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Narla LD, Newman B, Spottswood SS, Narla S, Kolli R. Inflammatory pseudotumor. Radiographics. 2003;23:719-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 299] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 10. | Maves CK, Johnson JF, Bove K, Malott RL. Gastric inflammatory pseudotumor in children. Radiology. 1989;173:381-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 46] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Sanders BM, West KW, Gingalewski C, Engum S, Davis M, Grosfeld JL. Inflammatory pseudotumor of the alimentary tract: clinical and surgical experience. J Pediatr Surg. 2001;36:169-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 106] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 12. | You Y, Shao H, Bui K, Bui M, Klapman J, Cui Q, Coppola D. Epstein-Barr virus positive inflammatory pseudotumor of the liver: report of a challenging case and review of the literature. Ann Clin Lab Sci. 2014;44:489-498. [PubMed] |
| 13. | Zen Y, Fujii T, Sato Y, Masuda S, Nakanuma Y. Pathological classification of hepatic inflammatory pseudotumor with respect to IgG4-related disease. Mod Pathol. 2007;20:884-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 177] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 14. | Koea JB, Broadhurst GW, Rodgers MS, McCall JL. Inflammatory pseudotumor of the liver: demographics, diagnosis, and the case for nonoperative management. J Am Coll Surg. 2003;196:226-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 86] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 15. | Jerraya H, Jarboui S, Daghmoura H, Zaouche A. A new case of spontaneous regression of inflammatory hepatic pseudotumor. Case Rep Med. 2011;2011:139125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Kim SR, Hayashi Y, Kudo M, Matsuoka T, Imoto S, Sasaki K, Shintani S, Song KB, Park SY, Kim JH. Inflammatory pseudotumor of the liver in a patient with chronic hepatitis C: difficulty in differentiating it from hepatocellular carcinoma. Pathol Int. 1999;49:726-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Kitajima K, Shiba H, Nojiri T, Uwagawa T, Ishida Y, Ichiba N, Yanaga K. Intrahepatic cholangiocarcinoma mimicking hepatic inflammatory pseudotumor. J Gastrointest Surg. 2007;11:398-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Torzilli G, Inoue K, Midorikawa Y, Hui AM, Takayama T, Makuuchi M. Inflammatory pseudotumors of the liver: prevalence and clinical impact in surgical patients. Hepatogastroenterology. 2001;48:1118-1123. [PubMed] |
| 19. | Sawhney S, Woo P, Murray KJ. Macrophage activation syndrome: a potentially fatal complication of rheumatic disorders. Arch Dis Child. 2001;85:421-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 295] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 20. | Ravelli A. Macrophage activation syndrome. Curr Opin Rheumatol. 2002;14:548-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 195] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 21. | Bleesing J, Prada A, Siegel DM, Villanueva J, Olson J, Ilowite NT, Brunner HI, Griffin T, Graham TB, Sherry DD. The diagnostic significance of soluble CD163 and soluble interleukin-2 receptor alpha-chain in macrophage activation syndrome and untreated new-onset systemic juvenile idiopathic arthritis. Arthritis Rheum. 2007;56:965-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 236] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 22. | Reddy VV, Myles A, Cheekatla SS, Singh S, Aggarwal A. Soluble CD25 in serum: a potential marker for subclinical macrophage activation syndrome in patients with active systemic onset juvenile idiopathic arthritis. Int J Rheum Dis. 2014;17:261-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Minoia F, Davì S, Horne A, Demirkaya E, Bovis F, Li C, Lehmberg K, Weitzman S, Insalaco A, Wouters C. Clinical features, treatment, and outcome of macrophage activation syndrome complicating systemic juvenile idiopathic arthritis: a multinational, multicenter study of 362 patients. Arthritis Rheumatol. 2014;66:3160-3169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 302] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 24. | Ravelli A, Magni-Manzoni S, Pistorio A, Besana C, Foti T, Ruperto N, Viola S, Martini A. Preliminary diagnostic guidelines for macrophage activation syndrome complicating systemic juvenile idiopathic arthritis. J Pediatr. 2005;146:598-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 278] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 25. | Davì S, Consolaro A, Guseinova D, Pistorio A, Ruperto N, Martini A, Cron RQ, Ravelli A. An international consensus survey of diagnostic criteria for macrophage activation syndrome in systemic juvenile idiopathic arthritis. J Rheumatol. 2011;38:764-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 105] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 26. | Davì S, Minoia F, Pistorio A, Horne A, Consolaro A, Rosina S, Bovis F, Cimaz R, Gamir ML, Ilowite NT. Performance of current guidelines for diagnosis of macrophage activation syndrome complicating systemic juvenile idiopathic arthritis. Arthritis Rheumatol. 2014;66:2871-2880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 27. | Das Narla L, Siddiqi NH, Hingsbergen EA. Inflammatory pseudotumor of the right atrium. Pediatr Radiol. 2001;31:351-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Tsou YK, Lin CJ, Liu NJ, Lin CC, Lin CH, Lin SM. Inflammatory pseudotumor of the liver: report of eight cases, including three unusual cases, and a literature review. J Gastroenterol Hepatol. 2007;22:2143-2147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | Ueda M, Yukihide Y, Ogawa K, Haga H, Ogura Y, Ito T, Tanaka K. A case of inflammatory pseudotumor of the liver hilum successfully treated with aggressive hepatectomy. J Pediatr Surg. 2003;38:E9-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Fernandes T, Viamonte B, Cunha R, Trindade E, Jesus JP. Peripheral inflammatory pseudotumor of the liver and extensive thrombosis of the portal venous system in a child. Pediatr Radiol. 2013;43:1041-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 31. | Nam KJ, Kang HK, Lim JH. Inflammatory pseudotumor of the liver: CT and sonographic findings. AJR Am J Roentgenol. 1996;167:485-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 67] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 32. | Yan FH, Zhou KR, Jiang YP, Shi WB. Inflammatory pseudotumor of the liver: 13 cases of MRI findings. World J Gastroenterol. 2001;7:422-424. [PubMed] |
| 33. | Durmus T, Kamphues C, Blaeker H, Grieser C, Denecke T. Inflammatory myofibroblastic tumor of the liver mimicking an infiltrative malignancy in computed tomography and magnetic resonance imaging with Gd-EOB. Acta Radiol Short Rep. 2014;3:2047981614544404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 34. | Dong A, Wang Y, Dong H, Gong J, Cheng C, Zuo C, Lu J. Inflammatory myofibroblastic tumor: FDG PET/CT findings with pathologic correlation. Clin Nucl Med. 2014;39:113-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 35. | Park JY, Choi MS, Lim YS, Park JW, Kim SU, Min YW, Gwak GY, Paik YH, Lee JH, Koh KC. Clinical features, image findings, and prognosis of inflammatory pseudotumor of the liver: a multicenter experience of 45 cases. Gut Liver. 2014;8:58-63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 36. | Ahn KS, Kang KJ, Kim YH, Lim TJ, Jung HR, Kang YN, Kwon JH. Inflammatory pseudotumors mimicking intrahepatic cholangiocarcinoma of the liver; IgG4-positivity and its clinical significance. J Hepatobiliary Pancreat Sci. 2012;19:405-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 37. | Patnana M, Sevrukov AB, Elsayes KM, Viswanathan C, Lubner M, Menias CO. Inflammatory pseudotumor: the great mimicker. AJR Am J Roentgenol. 2012;198:W217-W227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 230] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 38. | Horiuchi R, Uchida T, Kojima T, Shikata T. Inflammatory pseudotumor of the liver. Clinicopathologic study and review of the literature. Cancer. 1990;65:1583-1590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 39. | Toda K, Yasuda I, Nishigaki Y, Enya M, Yamada T, Nagura K, Sugihara J, Wakahara T, Tomita E, Moriwaki H. Inflammatory pseudotumor of the liver with primary sclerosing cholangitis. J Gastroenterol. 2000;35:304-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 40. | Maze GL, Lee M, Schenker S. Inflammatory pseudotumor of the liver and pregnancy. Am J Gastroenterol. 1999;94:529-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 41. | Zamir D, Jarchowsky J, Singer C, Abumoch S, Groisman G, Ammar M, Weiner P. Inflammatory pseudotumor of the liver--a rare entity and a diagnostic challenge. Am J Gastroenterol. 1998;93:1538-1540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 42. | Jaïs P, Berger JF, Vissuzaine C, Paramelle O, Clays-Schouman E, Potet F, Mignon M. Regression of inflammatory pseudotumor of the liver under conservative therapy. Dig Dis Sci. 1995;40:752-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 43. | Materne R, Van Beers BE, Gigot JF, Horsmans Y, Lacrosse M, Pringot J. Inflammatory pseudotumor of the liver: MRI with mangafodipir trisodium. J Comput Assist Tomogr. 1998;22:82-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 44. | Li GH, Li JQ, Lin YZ. Inflammatory pseudotumor of the liver. J Surg Oncol. 1989;42:244-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 45. | Kaneko K, Ando H, Watanabe Y, Seo T, Nagino M, Kamiya J, Nimura Y. Aggressive preoperative management and extended surgery for inflammatory pseudotumor involving the hepatic hilum in a child. Surgery. 2001;129:757-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 46. | Pecorella I, Ciardi A, Memeo L, Trombetta G, de Quarto A, de Simone P, di Tondo U. Inflammatory pseudotumour of the liver--evidence for malignant transformation. Pathol Res Pract. 1999;195:115-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 47. | Zavaglia C, Barberis M, Gelosa F, Cimino G, Minola E, Mondazzi L, Bottelli R, Ideo G. Inflammatory pseudotumour of the liver with malignant transformation. Report of two cases. Ital J Gastroenterol. 1996;28:152-159. [PubMed] |
| 48. | Kim SH, Cho YH, Kim HY. Two Cases of Infantile Intra-abdominal Inflammatory Myofibroblastic Tumor. Pediatr Gastroenterol Hepatol Nutr. 2014;17:116-120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 49. | Xiao Y, Zhou S, Ma C, Luo J, Zhu H, Tang F. Radiological and histopathological features of hepatic inflammatory myofibroblastic tumour: analysis of 10 cases. Clin Radiol. 2013;68:1114-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 50. | Fragoso AC, Eloy C, Estevão-Costa J, Campos M, Farinha N, Lopes JM. Abdominal inflammatory myofibroblastic tumor a clinicopathologic study with reappraisal of biologic behavior. J Pediatr Surg. 2011;46:2076-2082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 51. | Manolaki N, Vaos G, Zavras N, Sbokou D, Michael C, Syriopoulou V. Inflammatory myofibroblastic tumor of the liver due to Mycobacterium tuberculosis in an immunocompetent girl. Pediatr Surg Int. 2009;25:451-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 52. | Geramizadeh B, Tahamtan MR, Bahador A, Sefidbakht S, Modjalal M, Nabai S, Hosseini SA. Inflammatory pseudotumor of the liver: two case reports and a review of the literature. Indian J Pathol Microbiol. 2009;52:210-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |