Published online Jul 28, 2015. doi: 10.3748/wjg.v21.i28.8697
Peer-review started: November 3, 2014
First decision: March 10, 2015
Revised: March 27, 2015
Accepted: April 28, 2015
Article in press: April 28, 2015
Published online: July 28, 2015
Processing time: 270 Days and 2.7 Hours
AIM: To evaluate the efficacy of autologous bone marrow mononuclear cell transplantation in decompensated liver disease.
METHODS: Medline, EMBASE, PubMed, Science Direct, and the Cochrane Library were searched for relevant studies. Retrospective case-control studies were included along with randomized clinical trials. Meta-analysis was performed in line with recommendations from the Cochrane Collaboration software review manager. Heterogeneity was assessed using a random-effects model.
RESULTS: Four randomized controlled trials and four retrospective studies were included. Cell transplantation increased serum albumin level by 1.96 g/L (95%CI: 0.74-3.17; P = 0.002], 2.55 g/L (95%CI: 0.32-4.79; P = 0.03), and 3.65 g/L (95%CI: 0.76-6.54; P = 0.01) after 1, 3, and 6 mo, respectively. Patients who had undergone cell transplantation also had a lower level of total bilirubin [mean difference (MD): -1.37 mg/dL; 95%CI: -2.68-(-0.06); P = 0.04] after 6 mo. This decreased after 1 year when compared to standard treatment (MD: -1.26; 95%CI: -2.48-(-0.03); P = 0.04]. A temporary decrease in alanine transaminase and aspartate transaminase were significant in the cell transplantation group. However, after 6 mo treatment, patients who had undergone cell transplantation had a slightly longer prothrombin time (MD: 5.66 s, 95%CI: 0.04-11.28; P = 0.05). Changes in the model for end-stage liver disease score and Child-Pugh score were not statistically significant.
CONCLUSION: Autologous bone marrow transplantation showed some benefits in patients with decompensated liver disease. However, further studies are still needed to verify its role in clinical treatment for end-stage liver disease.
Core tip: Autologous bone marrow mononuclear cells prevent immune rejection. In this systematic review and meta-analysis, we attempted to gather evidence for the therapeutic use of autologous bone marrow mononuclear cell transplantation for decompensated liver disease and cirrhosis. Although we found that autologous bone marrow transplantation is satisfactory in patients with decompensated liver disease, there are important issues that require verification by large-volume centers.
- Citation: Pankaj P, Zhang Q, Bai XL, Liang TB. Autologous bone marrow transplantation in decompensated liver: Systematic review and meta-analysis. World J Gastroenterol 2015; 21(28): 8697-8710
- URL: https://www.wjgnet.com/1007-9327/full/v21/i28/8697.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i28.8697
Liver disease is a global health problem, with hepatitis B and C being two of the most epidemic and serious types[1]. As the disease advances, liver function may be decompensated, leading to decompensated liver diseases (mostly cirrhosis). There are currently few therapeutic options for decompensated liver disease, although new strategies have been explored for several decades. Regeneration of functional hepatocytes is believed to be vital for maintaining liver function in these patients. Autologous bone marrow mononuclear cells (BM-MNCs), including mesenchymal stem cells, hematopoietic stem cells, endothelial progenitor cells, and stromal cells, are beneficial for patients with decompensated liver disease[2]. Due to the contribution of non-hematopoietic stem cells, BM-MNCs can generate various types of cells in different tissues[3-5]. Other cell components of BM-MNCs may facilitate stem cell differentiation by secreting cytokines, modeling microenvironments, or interacting with stem cells.
In autologous transplantation, cells are derived from the patients themselves, which theoretically can prevent the possibility of immune rejection. At present, autologous BM-MNCs have been widely applied in the treatment of liver diseases[6-9]. It is proposed that pluripotent non-hematopoietic stem cells from BM might participate in the repopulation of a damaged liver, and may even improve its function[10]. The combination of mobilization, isolation, and direct infusion of BM stem cells into the liver via the hepatic artery or portal vein, or autologous human BM stem cell transplantation, showed inconsistent improvement in liver function[10-12]. However, the evidence for autologous BM-MNCs transplantation for decompensated liver disease is controversial. This systematic review aimed to summarize the currently available literature on this topic.
Five electronic databases (Medline, EMBASE, PubMed, Science Direct, and the Cochrane Library) were searched from their respective dates of inception up to October 2014. The following key words were used: “bone marrow”, “autologous”, “transfusion”, “liver OR hepatic”, and “systematic review”; similar headings were also searched, such as “treatment of decompensated liver disease”, “treatment option for cirrhosis”, and “meta-analysis”. Further articles were identified by a manual search of reference lists from the retrieved publications. The databases were used again to retrieve abstracts and, if favorable, the full text was downloaded for the final review. All articles included in this study were published in English.
Two reviewers independently screened the title and abstract of each identified publication. Citations with suspected compliance with our eligibility criteria underwent a full review. If either of the two reviewers identified a citation to be potentially relevant, we obtained the full text for a full review. Any disagreements were resolved through discussions with a third reviewer.
This study compared autologous BM stem cell therapy in decompensated liver disease, regardless of blinding and concealment of allocation. Retrospective studies were included along with randomized clinical trials. Eligibility criteria were as follows: (1) explicitly reporting the indications for BM-MNCs; (2) comparing at least one of the following outcomes: albumin, total bilirubin, coagulation function tests (prothrombin time and activity), Child-Pugh score, model for end-stage liver disease (MELD) score, alanine transaminase (ALT), and aspartate transaminase (AST); and (3) when two studies were published by the same institution or authors and data were repeated, the higher quality or the most recent choice was included.
Studies were excluded if: (1) it was impossible to extract or reasonably estimate the data from the published articles; and (2) there was considerable overlap among patients in the studies published.
Data presented as changes were preferred. When changes were unavailable but could be calculated from the data before and after treatment, they were calculated using appropriate methods[13]. In brief, we calculated a correlation coefficient from a comparable study with considerable detail using the formula suggested by the Cochrane Collaboration[13]. The desired SD of a certain study could then be calculated using a modification of the same formula. When no correlation coefficient acquired from other comparable studies could be found, meaning that calculation was impossible for the missing data, we evaluated the SD using the most conservative method (assuming that the correlation coefficient was -1). The influence of the evaluation was then discussed.
Meta-analysis was performed using Review Manager (version 5.3) and followed the recommendations from the Cochrane Collaboration. Heterogeneity was assessed using a random-effects model and P < 0.10 with I2 > 50% was considered statistically significant. Statistical analysis of continuous variables was carried out using the mean difference (MD) as the summary statistic by the Inverse-Variance method, and was reported with a 95% confidential interval (CI). The MD was considered to be statistically significant at P < 0.05.
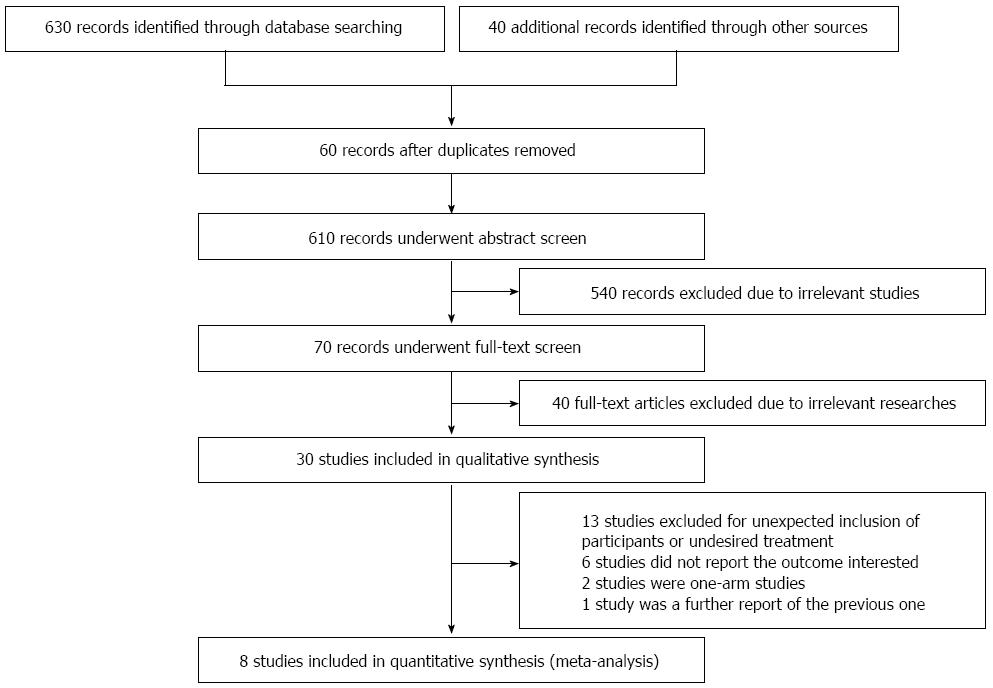
A total of 610 non-duplicated publications were identified and underwent abstract screening. For the 110 publications that underwent full-text screening, 40 were excluded because they were irrelevant to our topic, 13 studies were not eligible due to the unexpected inclusion of participants or undesired treatment, 6 studies did not report the interested outcomes, 2 studies were one-arm studies, and 1 study was a further report of a previous one (Figure 1). Eight studies were included for quantitative analysis[2,14-21], with four being randomized clinical trials[2,14,19,20]. The characteristics of the included studies are shown in Table 1.
| Trials | Region | Design | Duration | No. of patients | Etiology of liver disease | Intervention | Follow-up |
| Lyra 2010 | Brazil | RCT | 2006.1-2006.4 | 15 vs 15 | Chronic liver disease waiting for liver transplantation, mainly alcoholic and/or hepatitis C | About 3.78 × 108 BM-MNCs, through hepatic artery | Up to 12 mo |
| Salama 2010 | Egypt | RCT | 2008.6-2009.5 | 90 vs 50 | Post-HCV liver cirrhosis | Autologous BM-derived CD34+ and CD133+ stem cell infusion in the portal vein | Up to 6 mo |
| Spahr 2012 | Switzerland | RCT | 2008.2-2011.3 | 28 vs 30 | Decompensated alcoholic liver disease | About 4.7 × 107/kg BM-MNCs, including CD34+ cells and MSCs | Up to 3 mo |
| Mohanmadnejad 2013 | Iran | RCT | 2007.7-2010.8 | 15 vs 12 | 2 PBC, 2 HBV, 1 HCV, 9 AIH, 11 unknown | About 1.95 × 108 MSCs through cubital vein | Up to 12 mo |
| Bai 2004 | China | Case-control | 2009.3-2011.3 | 32 vs 15 | Decompensated liver cirrhosis, 91.5% with HBV infection | BM-MNCs through hepatic artery | Up to 24 mo |
| Peng 2011 | China | Case-control | 2005.5-2009.6 | 53 vs 105 | Chronic hepatitis B induced liver disease, 73% with cirrhosis | BM-derived MSCs through proper hepatic artery | Up to 192 wk |
| Saito 2011 | Japan | Case-control | NA | 5 vs 5 | Alcoholic liver cirrhosis | About 8 × 109 BM-MNCs through cubital vein | Up to 48 wk |
| El-Ansary 2012 | Egypt | Prospective cohort study | NA | 15 vs 10 | HCV induced liver cirrhosis | About 1 × 106 MSCs/kg, intravenously | Up to 6 mo |
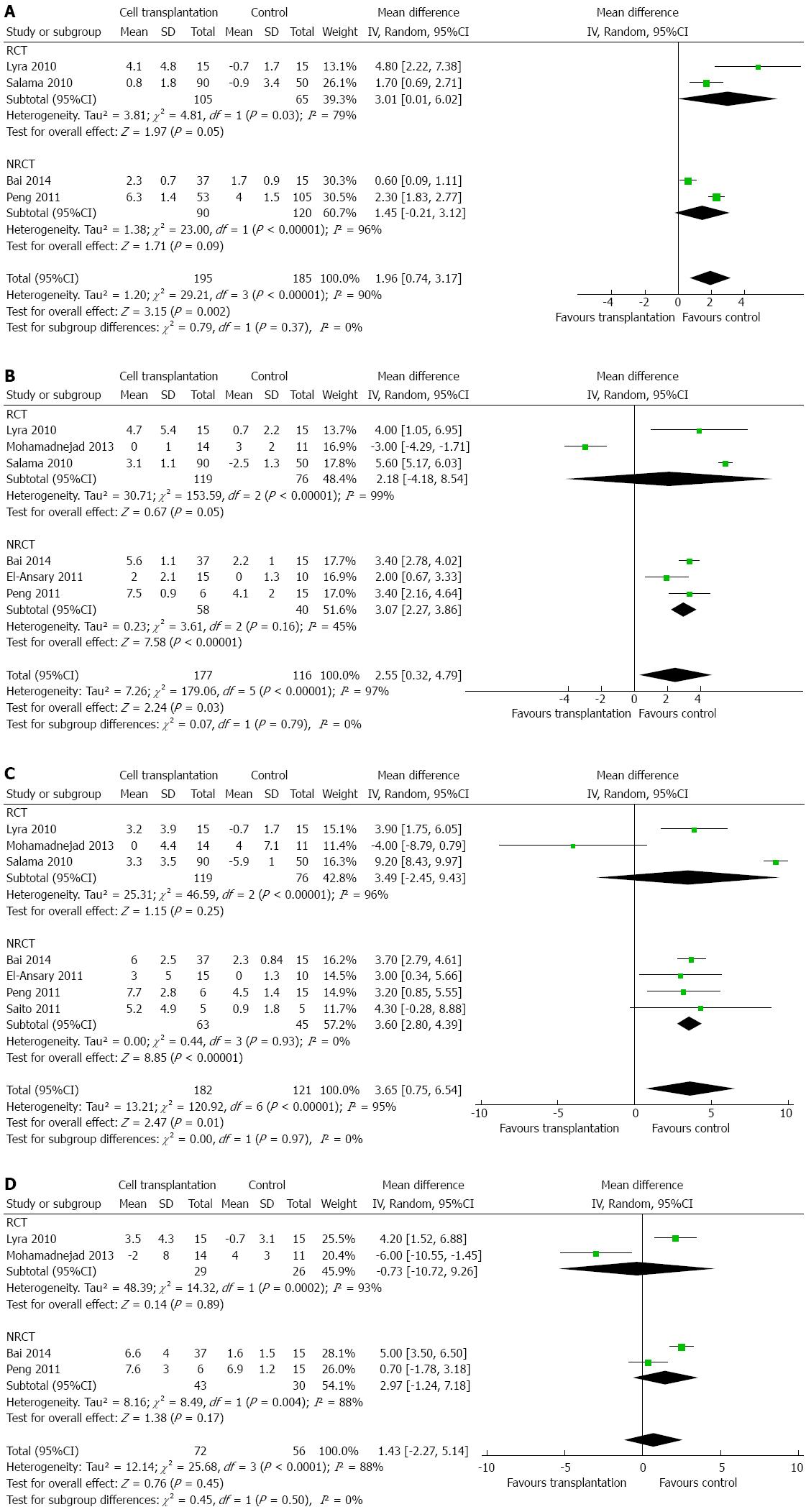
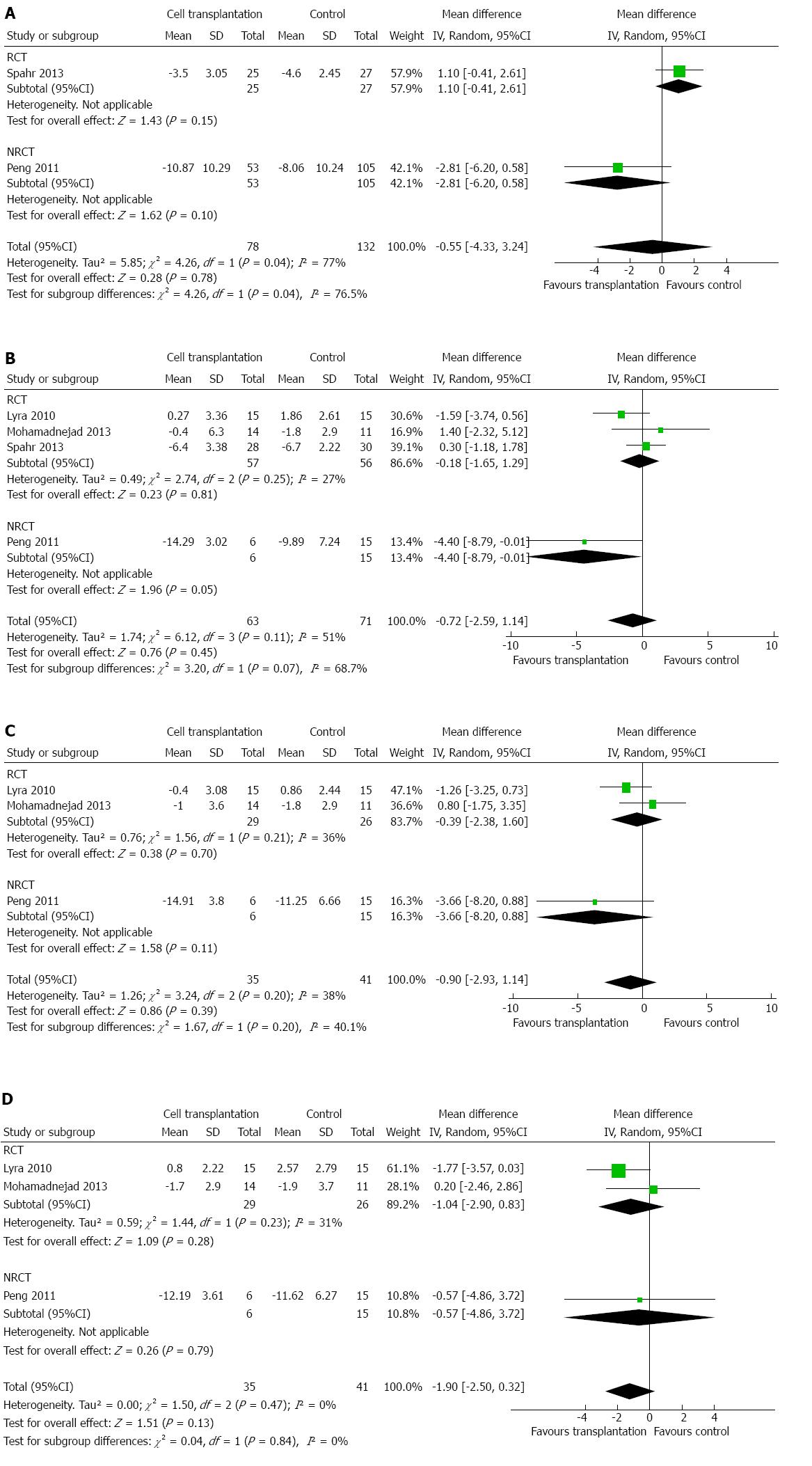
Indicators for liver function (such as serum albumin, total bilirubin, ALT, and AST) were assessed by most studies. MELD score[2,15,19,20] and Child-Pugh score[2,19] were used in some articles to evaluate the effects of cell transplantation. Meta-analysis showed that, compared to the control group, patients who had undergone cell transplantation had a higher level of serum albumin within 6 mo after treatment. The additional increase in serum albumin by cell transplantation was 1.96 g/L (95%CI: 0.74-3.17; P = 0.002), 2.55 g/L (95%CI: 0.32-4.79; P = 0.03), and 3.65 g/L (95%CI: 0.75-6.54; P = 0.01) g/L after 1, 3, and 6 mo[2,14-17,19,21], respectively (Figure 2A-C). However, this effect disappeared after 1 year (Figure 2D), by which time there was no difference regarding serum albumin between the experimental and control groups (MD: 1.43 g/L; 95%CI: -2.27-5.14; P = 0.45). However, heterogeneity was high among these studies (I2: 88%-97%), and could not be explained by only one or two studies.
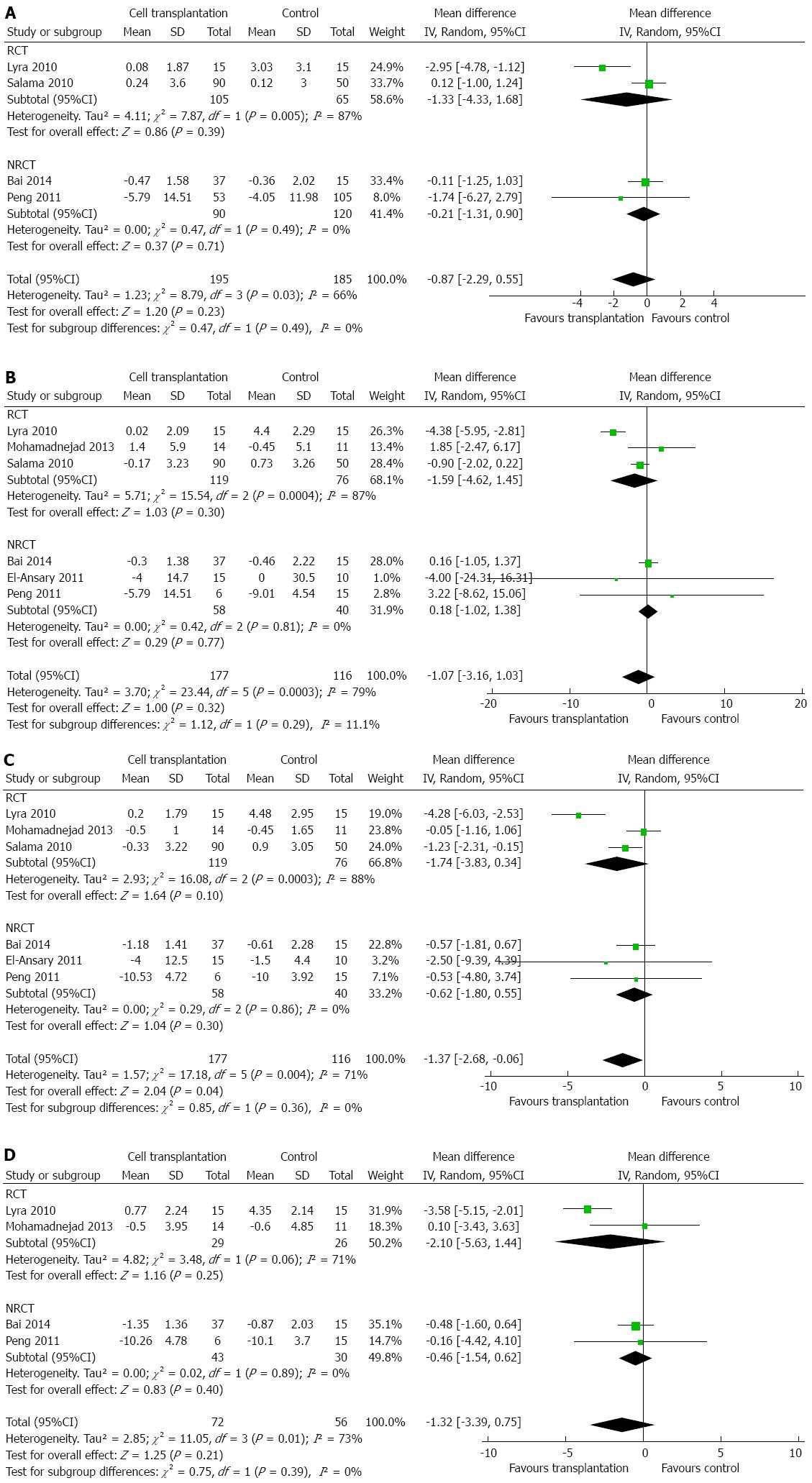
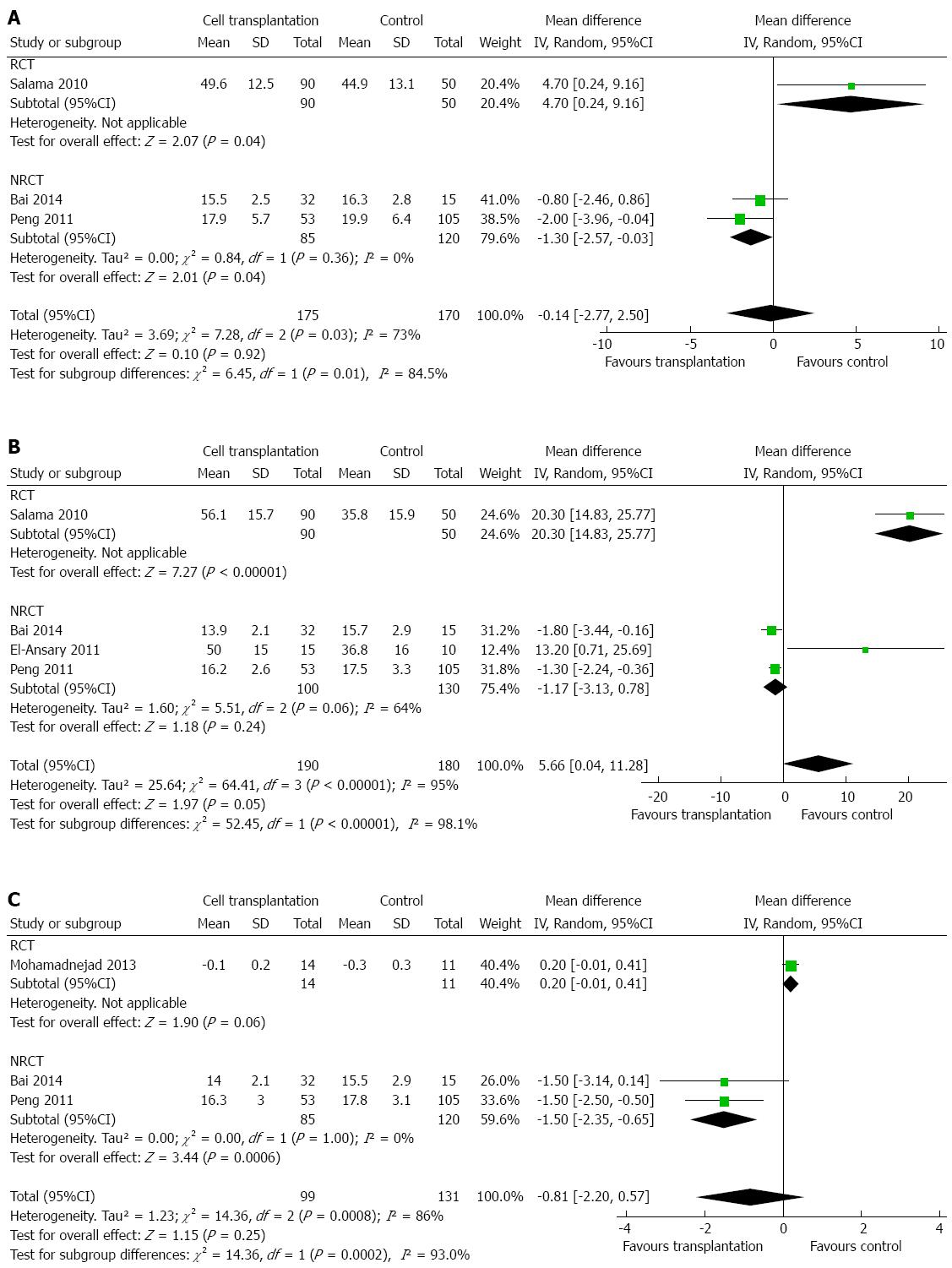
With regard to total bilirubin, a meta-analysis of six studies[2,14,15,17,19,21] did not show any significant difference between the two groups, except at 6 mo (Figure 3A-D). At 6 mo after cell transplantation, patients had a lower level of total bilirubin (MD: -1.37 mg/dL; 95%CI: -2.68-(-0.06); P = 0.04). Heterogeneity was high (I2: 66%-79%) and mainly contributed by the study by Lyra et al[2]. Exclusion of this study did not change the conclusions.
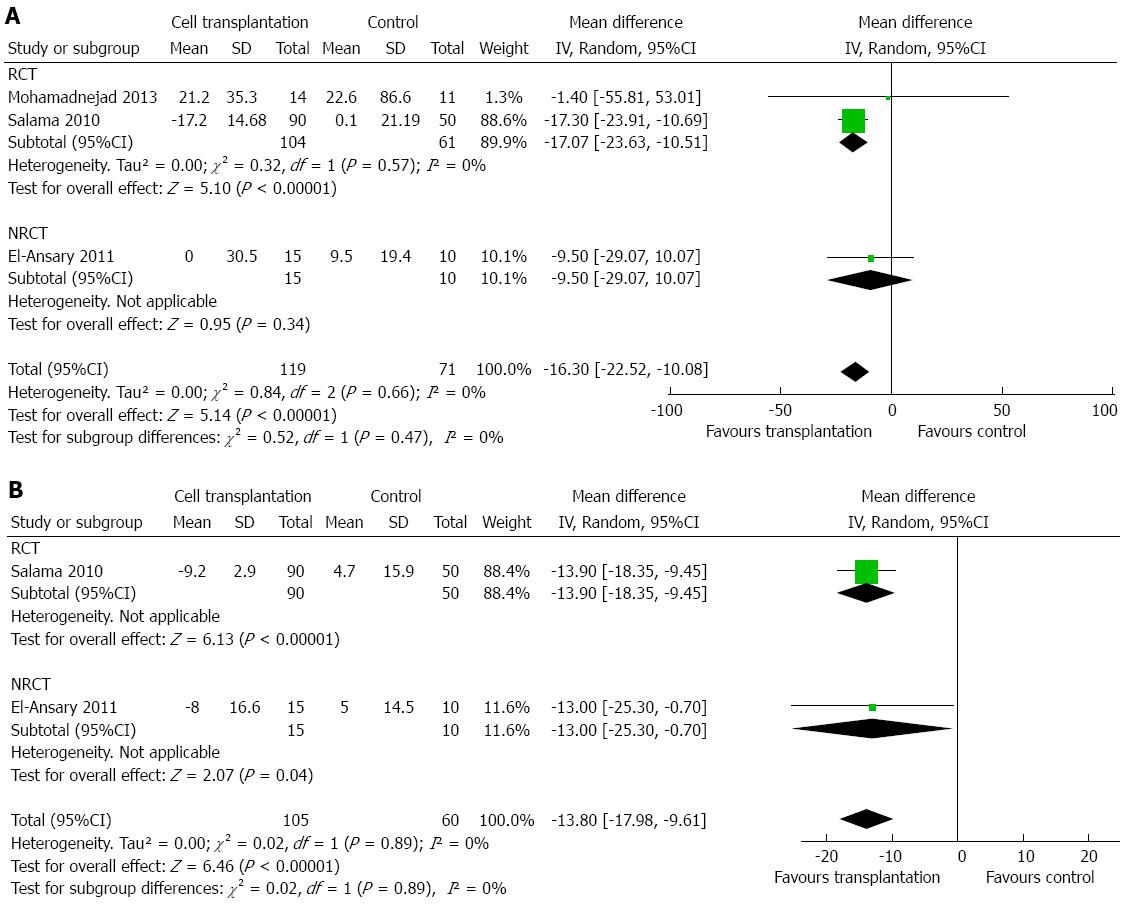
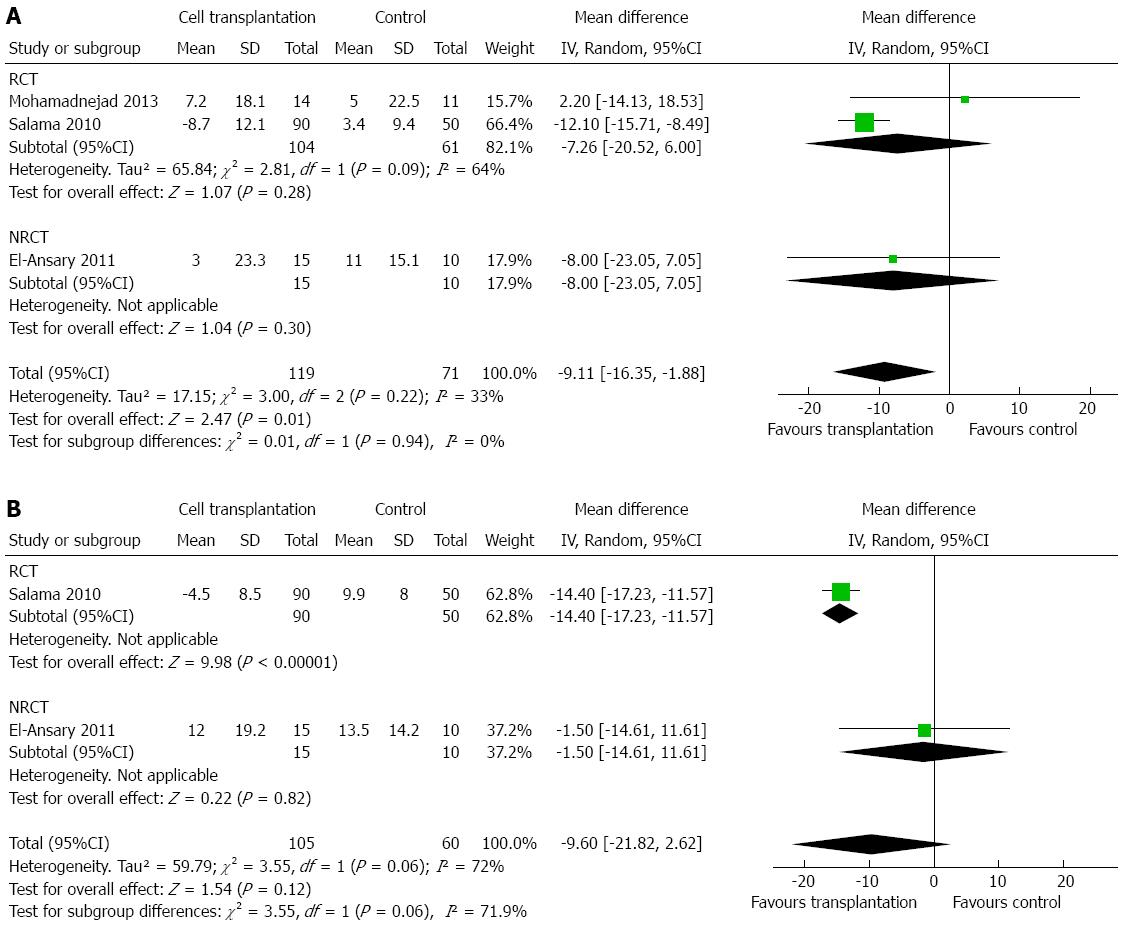
Two studies reported changes in AST after treatment[14,17]. There was a decrease in AST level at 3 mo after cell transplantation (MD: -16.30 U/L; 95%CI: -22.52-(-10.08); P < 0.00001; Figure 4A) and after 6 mo (MD: -13.80 U/L; 95%CI: -17.98-(-9.61); P < 0.00001; Figure 4B). No heterogeneity was detected (P > 0.10, with I2 = 0). Three studies reported changes in ALT after cell transplantation[14,17,19]. Similarly, cell transplantation showed a lower ALT level at 3 mo (MD: -9.11 U/L; 95%CI: -16.35-(-1.88); P = 0.01; Figure 5A), but failed to show a consistent effect at 6 mo (MD: -9.60 U/L; 95%CI: -21.82-2.62; P = 0.12; Figure 5B). No heterogeneity was detected regarding ALT level at 3 mo (P = 0.22), but heterogeneity should be taken into consideration regarding ALT level at 6 mo (P = 0.06, with I2 =72%).
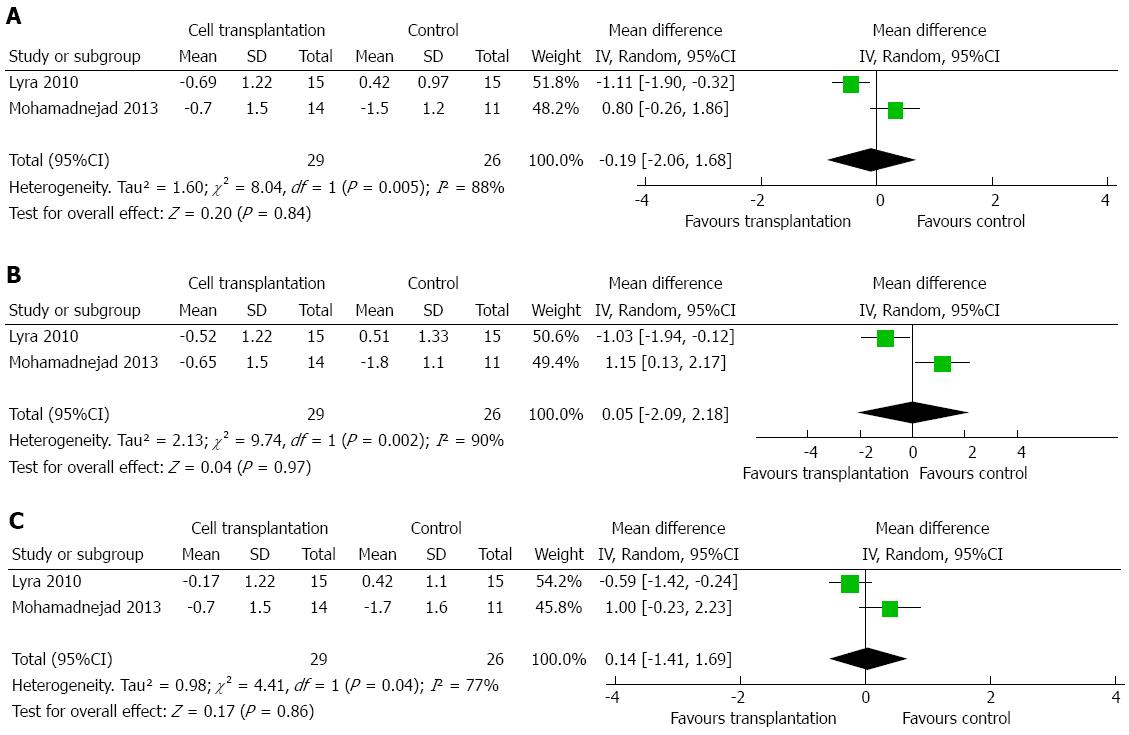
MELD score was reported by two to four studies, depending on the time point assessed[2,15,19,20]. Meta-analysis did not show any difference within 12 mo of treatment (Figure 6A-D). Two studies reported that Child-Pugh score could be incorporated into the meta-analysis[2,19]. No significant difference was detected at up to 1 year after treatment (Figure 7A-C).
Four studies reported changes in prothrombin time[14,15,17,21]. Changes in prothrombin time were noted only at 6 mo after therapy, when patients with cell transplantation showed a slight increase (MD: 5.66 s, 95%CI: 0.04-11.28; P = 0.05; Figure 8A-C). However, heterogeneity was high (P < 0.00001, with I2 = 95%). Only one study followed changes in prothrombin time up to 24 mo[21], and suggested that there was no statistical difference (MD: -1.3 s, 95%CI: -2.67-0.07; P = 0.06).
In this systematic review and meta-analysis, we attempted to collect evidence for BM-MNC, particularly with regards to bone marrow stem cell (BMSC) transplantation as a potential therapeutic approach for patients with decompensated liver disease. It is suggested that pluripotent non-hematopoietic stem cells derived from the BM may be able to repopulate the impaired liver and improve its function[10]. Genomic plasticity is the intrinsic foundation of trans-differentiation of BMSCs[22]. BM-MNCs may facilitate differentiation of BM non-hematopoietic stem cells into hepatoblasts and hepatocytes by remodeling the liver microenvironment[23]. Many growth factors, such as granulocyte colony-stimulating factor and hepatic growth factor, were also found to facilitate the effectiveness of BM-MNC transplantation by stimulating cell proliferation or suppressing liver inflammation and fibrosis[24,25]. In addition, transplanted BM-MNCs may have different roles according to the etiology of liver disease, which may in turn influence the clinical effects of BM transplantation. Most patients in the included studies have hepatitis virus (either HBV or HCV) infection and/or high alcohol consumption, and suffered from decompensate liver cirrhosis (see Table 1). Limited evidence has shown that BM-MNC transplantation might help remove the aforementioned viruses. In the study by Salama et al, there was a negative correlation between HCV titer changes and changes in AST at 3 and 6 mo after cell transplantation, and a moderate negative correlation between HCV titer changes and changes in ALT at 2 mo after cell transplantation[13]. However, another study demonstrated that the severity of liver disease is independent of serum levels of HCV[26]. To date, no direct antivirus effect of BM-MNCs has been clearly demonstrated, and this possible role, if it exists, could be due to the enhancement of hepatic local immune function induced by BM-MNCs. In general, autologous BM-MNC transplantation may restore liver function from two aspects: assisting with the repair of the damaged liver and a beneficial effect for hepatitis virus clearance. However, the exact process whereby BM-MNCs promote hepatocyte regeneration or liver restoration remains to be determined.
There was usually a gradual improvement in the hepatic functional reserve in patients who underwent BM-MNC transplantation. For instance, the maximum improvement in Child-Pugh score occurred after 6 mo in the largest cohort of HCV-associated end-stage liver disease patients[14]. On the other hand, the long-term benefits of BM-MNC transplantation were not as convincible as the short-term ones. Peng et al[15] documented the positive results of short-term analysis; however, a longer investigation revealed these beneficial effects failed to last more than 2 years. Indeed, most relevant studies only reported short-term benefits, and for the studies containing long-term results, beneficial effects failed to last for a long period of time. There were many reasons for the ambiguous results in long-term follow-up. For instance, the parameters used to assess clinical improvement are different. These parameters have different clinical implications, and some are more sensible than others. Another reason is that the details of autologous BM-MNC transplantation are actually distinct among the studies, causing different long-term results. Thirdly, the activity of transplanted cells and whether they induced the process of liver repopulation are critical to preserving the long-term effects of this therapy. Accordingly, two strategies are needed to verify and improve the long-term effects. On one hand, basic researchers need to understand the biological process of BMSC-induced liver repopulation and find appropriate methods to boost it. On the other hand, clinical investigators should use more parameters to comprehensively evaluate clinical improvement.
Given the undetermined role of BM-MNC transplantation in long-term follow-up, some investigators have tried to assess whether repetitive transplantation of autologous BM-MNCs at regular intervals could be a strategy for improving the conditions of decompensated cirrhosis[21]. Repeated autologous BMSC infusions or the combination of cell therapy with granulocyte colony-stimulating factor might be a promising treatment option for patients with advanced chronic liver disease[2]. It has been shown that transfusion through peripheral veins might result in promising outcomes[6,8]. However, Mohamadnejad et al[19] recommended that infusion of BMSCs through peripheral veins was probably not beneficial in decompensated cirrhosis by concluding that “liver transplantation is still considered the standard treatment for decompensated cirrhosis”. It should be remembered that the natural history of cirrhosis is often varying. Methods to improve the infusion method and optimize the cell transplant type and transplantation strategy require further study[21]. Long-term studies are needed to improve BM-MNC transplantation for treatment of cirrhosis.
Due to the considerable clinical and statistical heterogeneity among these studies, the results of the meta-analysis should be cautiously interpreted. Several reasons contributed to the heterogeneity. Firstly, only half of the included studies were RCTs, with the inclusion of non-RCTs reducing the quality of the evidence. Since BM transplantation is an invasive procedure that requires general anesthesia, patients who underwent BM transplantation were prone to relatively better liver function in non-RCTs; this could introduce bias. Although most results were similar with or without the inclusion of non-RCTs, some results became insignificant after the exclusion of non-RCTs; this was mostly because of the low number of included studies. Secondly, the exact type (BM-MNCs or BMSCs) and number of cells transplanted were different among studies (see Table 1), as well as the heterogeneity of etiology and the severity of the diseases. Even among the RCTs, contradictory results were reported. For instance, the study by Mohamadnejad et al[19] showed a relatively poor prognosis in the experimental group compared to that of the control group with regard to albumin and Child-Pugh score. Thirdly, there was a lack of clinical follow-up details to ascertain the specific results at certain time points in some studies, and we had to calculate or estimate these data using appropriate means. Although we carefully performed the estimation, this could make the results deviate from the actual values. Additionally, some parameters were not comparable among studies, which led to painstaking work to evaluate the particular connection in view of the numerous outcomes. To be conventional, we simulated that the correlation coefficient equaled -1 when mandatory. This assumption resulted in a large SD and a wide range of 95%CI, which made the meta-analysis less sensitive with the minimized type II error, and thus could have underestimated the efficacy of this treatment.
In conclusion, our study implies that the short-term outcomes of autologous BM transplantation were satisfactory in patients with decompensated liver disease. However, many concerns need to be addressed by further basic research and clinical trials in large-volume centers. For instance, the type and number of cell transplanted, the route of transplantation (e.g., portal vein, or hepatic artery), pretreatment of cells, repeat cell transplantation, and concomitant adjuvant therapy are important to authenticate autologous BM-MNC transplantation. In addition, well-designed randomized controlled trials are required to verify and enhance effectiveness, especially for long-term outcomes, by addressing these issues. Cell transplantation is an innovative intervention for end-stage liver disease, although caution is advised when advocating its effectiveness in clinical practice with the currently available evidence.
Bone marrow mononuclear cells (BM-MNCs), including non-hematopoietic stem cells, were found to help liver regeneration. BM-MNC transplantation is a potential treatment for decompensated liver disease. However, some contradictory results exist.
The cells used for treating decompensated liver disease varied from mesenchymal stem cells to BM stem cells and BM-MNCs. These types of cells can acquired from BM, however they have different components. In addition, the purity, routes, and repeated cycles were distinct in different studies. Most studies demonstrated positive short-term results, although long-term efficacy was inconclusive.
BM-MNC transplantation, if proved beneficial for liver regeneration, will be a promising strategy for improving the prognosis of patients with decompensated liver disease.
BM-MNCs, including mesenchymal stem cells, hematopoietic stem cells, endothelial progenitor cells, and stromal cells, are beneficial for patients with decompensated liver disease. Bone marrow stem cell transplantation is a potential therapeutic approach for patients with decompensated liver disease.
This systematic review and meta-analysis describes the effectiveness of autologous bone marrow transplantation for the treatment of decompensated liver disease. The data show that autologous bone marrow transplantation is an effective treatment for restoring liver function in patients with advanced liver cirrhosis, albeit with limited duration. The data are well-analyzed and well-written.
P- Reviewer: Akbar SMF, Kanda T, Larrubia JR, Pompili M, Shimizu Y, Wang K S- Editor: Ma YJ L- Editor: Rutherford A E- Editor: Zhang DN
| 1. | Lee WM. Hepatitis B virus infection. N Engl J Med. 1997;337:1733-1745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1728] [Cited by in RCA: 1712] [Article Influence: 61.1] [Reference Citation Analysis (0)] |
| 2. | Lyra AC, Soares MB, da Silva LF, Braga EL, Oliveira SA, Fortes MF, Silva AG, Brustolim D, Genser B, Dos Santos RR. Infusion of autologous bone marrow mononuclear cells through hepatic artery results in a short-term improvement of liver function in patients with chronic liver disease: a pilot randomized controlled study. Eur J Gastroenterol Hepatol. 2010;22:33-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 3. | Khurana S, Mukhopadhyay A. In vitro transdifferentiation of adult hematopoietic stem cells: an alternative source of engraftable hepatocytes. J Hepatol. 2008;49:998-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Banas A, Teratani T, Yamamoto Y, Tokuhara M, Takeshita F, Osaki M, Kato T, Okochi H, Ochiya T. Rapid hepatic fate specification of adipose-derived stem cells and their therapeutic potential for liver failure. J Gastroenterol Hepatol. 2009;24:70-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 196] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 5. | Yan Y, Xu W, Qian H, Si Y, Zhu W, Cao H, Zhou H, Mao F. Mesenchymal stem cells from human umbilical cords ameliorate mouse hepatic injury in vivo. Liver Int. 2009;29:356-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 116] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 6. | Terai S, Ishikawa T, Omori K, Aoyama K, Marumoto Y, Urata Y, Yokoyama Y, Uchida K, Yamasaki T, Fujii Y. Improved liver function in patients with liver cirrhosis after autologous bone marrow cell infusion therapy. Stem Cells. 2006;24:2292-2298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 349] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 7. | Pai M, Zacharoulis D, Milicevic MN, Helmy S, Jiao LR, Levicar N, Tait P, Scott M, Marley SB, Jestice K. Autologous infusion of expanded mobilized adult bone marrow-derived CD34+ cells into patients with alcoholic liver cirrhosis. Am J Gastroenterol. 2008;103:1952-1958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 153] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 8. | Kharaziha P, Hellström PM, Noorinayer B, Farzaneh F, Aghajani K, Jafari F, Telkabadi M, Atashi A, Honardoost M, Zali MR. Improvement of liver function in liver cirrhosis patients after autologous mesenchymal stem cell injection: a phase I-II clinical trial. Eur J Gastroenterol Hepatol. 2009;21:1199-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 322] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 9. | Gilchrist ES, Plevris JN. Bone marrow-derived stem cells in liver repair: 10 years down the line. Liver Transpl. 2010;16:118-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 10. | Fausto N. Liver regeneration and repair: hepatocytes, progenitor cells, and stem cells. Hepatology. 2004;39:1477-1487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 537] [Cited by in RCA: 517] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 11. | Houlihan DD, Newsome PN. Critical review of clinical trials of bone marrow stem cells in liver disease. Gastroenterology. 2008;135:438-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 131] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 12. | Almeida-Porada G, Zanjani ED, Porada CD. Bone marrow stem cells and liver regeneration. Exp Hematol. 2010;38:574-580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 13. | Higgins JPT, Green S, editors . Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. 2011; Available from: http://www.cochrane-handbook.org. |
| 14. | Salama H, Zekri AR, Bahnassy AA, Medhat E, Halim HA, Ahmed OS, Mohamed G, Al Alim SA, Sherif GM. Autologous CD34+ and CD133+ stem cells transplantation in patients with end stage liver disease. World J Gastroenterol. 2010;16:5297-5305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 60] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Peng L, Xie DY, Lin BL, Liu J, Zhu HP, Xie C, Zheng YB, Gao ZL. Autologous bone marrow mesenchymal stem cell transplantation in liver failure patients caused by hepatitis B: short-term and long-term outcomes. Hepatology. 2011;54:820-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 273] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 16. | Saito T, Okumoto K, Haga H, Nishise Y, Ishii R, Sato C, Watanabe H, Okada A, Ikeda M, Togashi H. Potential therapeutic application of intravenous autologous bone marrow infusion in patients with alcoholic liver cirrhosis. Stem Cells Dev. 2011;20:1503-1510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | El-Ansary M, Abdel-Aziz I, Mogawer S, Abdel-Hamid S, Hammam O, Teaema S, Wahdan M. Phase II trial: undifferentiated versus differentiated autologous mesenchymal stem cells transplantation in Egyptian patients with HCV induced liver cirrhosis. Stem Cell Rev. 2012;8:972-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 126] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 18. | Amin MA, Sabry D, Rashed LA, Aref WM, el-Ghobary MA, Farhan MS, Fouad HA, Youssef YA. Short-term evaluation of autologous transplantation of bone marrow-derived mesenchymal stem cells in patients with cirrhosis: Egyptian study. Clin Transplant. 2013;27:607-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 19. | Mohamadnejad M, Alimoghaddam K, Bagheri M, Ashrafi M, Abdollahzadeh L, Akhlaghpoor S, Bashtar M, Ghavamzadeh A, Malekzadeh R. Randomized placebo-controlled trial of mesenchymal stem cell transplantation in decompensated cirrhosis. Liver Int. 2013;33:1490-1496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 124] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 20. | Spahr L, Chalandon Y, Terraz S, Kindler V, Rubbia-Brandt L, Frossard JL, Breguet R, Lanthier N, Farina A, Passweg J. Autologous bone marrow mononuclear cell transplantation in patients with decompensated alcoholic liver disease: a randomized controlled trial. PLoS One. 2013;8:e53719. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 21. | Bai YQ, Yang YX, Yang YG, Ding SZ, Jin FL, Cao MB, Zhang YR, Zhang BY. Outcomes of autologous bone marrow mononuclear cell transplantation in decompensated liver cirrhosis. World J Gastroenterol. 2014;20:8660-8666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Cho KA, Lim GW, Joo SY, Woo SY, Seoh JY, Cho SJ, Han HS, Ryu KH. Transplantation of bone marrow cells reduces CCl4 -induced liver fibrosis in mice. Liver Int. 2011;31:932-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Terai S, Sakaida I, Yamamoto N, Omori K, Watanabe T, Ohata S, Katada T, Miyamoto K, Shinoda K, Nishina H. An in vivo model for monitoring trans-differentiation of bone marrow cells into functional hepatocytes. J Biochem. 2003;134:551-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 122] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 24. | Piscaglia AC, Shupe TD, Oh SH, Gasbarrini A, Petersen BE. Granulocyte-colony stimulating factor promotes liver repair and induces oval cell migration and proliferation in rats. Gastroenterology. 2007;133:619-631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 98] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 25. | Oyagi S, Hirose M, Kojima M, Okuyama M, Kawase M, Nakamura T, Ohgushi H, Yagi K. Therapeutic effect of transplanting HGF-treated bone marrow mesenchymal cells into CCl4-injured rats. J Hepatol. 2006;44:742-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 229] [Article Influence: 12.1] [Reference Citation Analysis (0)] |