Published online Jul 28, 2015. doi: 10.3748/wjg.v21.i28.8687
Peer-review started: December 21, 2014
First decision: January 22, 2015
Revised: March 23, 2015
Accepted: April 16, 2015
Article in press: April 17, 2015
Published online: July 28, 2015
Processing time: 222 Days and 20.6 Hours
AIM: To investigate the correlation between human epidermal growth factor receptor (HER-2) protein expression and colorectal cancer (CRC) using a case-control study and meta-analysis.
METHODS: Tumor tissue specimens from 162 CRC patients were selected for the case group. Fifty cases were randomly selected, and normal CRC tissue at least 10 cm away from the tumor margins of these cases was used to generate the control group. The expression of the HER-2 protein in the 162 CRC tissue samples and the 50 adjacent normal mucosa tissue samples was detected via immunohistochemistry. The experimental data were analyzed using SPSS 18.0 software, and R software version 3.1.0 was utilized for further verification.
RESULTS: The expression of HER-2 protein in the 162 CRC tissue samples was significantly higher than in the normal tissue specimens. The data showed that the expression of HER-2 in CRC was related to the Dukes’ stage, the depth of invasion and lymph node metastasis. The HER-2-positive patients had lower 3- and 5-year OS rates than the HER-2-negative patients, but there was no significant difference. However, there was a statistically significant difference in the 3- and 5-year disease-free survival (DFS) rates of HER-2-positive and HER-2-negative patients. The results of the meta-analysis showed that the expression of HER-2 in CRC patients was statistically significantly increased over that of healthy people. The 3-year DFS rate in HER-2-positive patients was markedly lower than that in HER-2-negative patients.
CONCLUSION: Down-regulation of HER-2 expression might be a dependable strategy for CRC therapy.
Core tip: The first meta-analysis to evaluate the role of human epidermal growth factor receptor (HER-2) expression in colorectal cancer (CRC); HER-2 expression level is associated with the clinic-pathological features of CRC; HER-2 expression level is associated with the prognostic factors of CRC; HER-2 expression level is a biomarker for diagnosis of CRC; HER-2 expression level is a biomarker for determining the prognosis of CRC.
- Citation: Yang WJ, Shen XJ, Ma XX, Tan ZG, Song Y, Guo YT, Yuan M. Correlation of human epidermal growth factor receptor protein expression and colorectal cancer. World J Gastroenterol 2015; 21(28): 8687-8696
- URL: https://www.wjgnet.com/1007-9327/full/v21/i28/8687.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i28.8687
Colorectal cancer (CRC) is one of the most common cancers worldwide and is the fourth leading cause of cancer-related death in China[1]. There are more than 1 million CRC cases and 600000 deaths every year, and survival is strongly related to its stage at diagnosis[2]. The TNM staging system and the Dukes’ staging system have greatly improved the rational stratification of CRC patients and the design of therapeutic strategies[3]. Early diagnosis results in a highly favorable prognosis; stage 1 and stage 2 CRC have an 80%-90% five-year survival rate, whereas stage 3 and stage 4 metastatic diseases are associated with five-year survival rates of 60% and 8%, respectively[4,5]. Nearly 20% of patients are diagnosed at an advanced metastatic stage of the disease, and over half will ultimately develop metastases[6]. Recently, biomarkers have begun to play an increasingly important role in the detection and management of patients with gastrointestinal cancers[7]. The human epidermal growth factor receptor (HER-2) protein regulates cancer cell proliferation and apoptosis and has been validated as a relevant therapeutic target in several human cancers, including CRC[8].
HER-2 protein, a transmembrane tyrosine kinase growth factor receptor is found on normal and malignant epithelial cells and is involved in the regulation of cell proliferation and differentiation[9]. HER-2 amplification is observed in 2.5% of 1439 CRC cases, and HER-2 overexpression is observed in an additional 2.7% of cases; CRC amplification is strongly related to protein overexpression[9]. In CRC, HER-2 overexpression has been correlated with advanced tumor stage[10]. HER-2 is a clinically validated anticancer molecular target that is expressed in the majority of CRCs[11,12]. Previous studies have demonstrated that overexpression of HER-2 can lead to cell proliferation, increased motility and protection against apoptosis, whereas inhibition of the HER-2 pathway induces apoptosis and cell cycle arrest and inhibits angiogenesis, tumor invasion and metastasis[13-15]. One study suggested that overexpressed HER-2 protein is the therapeutic target for trastuzumab, a humanized monoclonal antibody[9]. However, some studies have suggested that HER-2 overexpression is not an independent prognostic marker of colorectal cancer[16,17]. To address the role of HER-2, we conducted an experiment to investigate the specific correlation between HER-2 protein expression and CRC, which was further supported by meta-analysis.
This study was carried out with the permission of the Institutional Review Board of the First People’s Hospital of Jining. Written informed consent was obtained from all participants. Ethical approval for this study conformed to the standards of the Declaration of Helsinki[18].
From January 2008 to December 2009, 162 patients who were pathologically diagnosed with CRC and had complete clinical data in the First People’s Hospital of Jining were randomly selected as part of the case group. None of the patients in the case group had received radiotherapy, chemotherapy or immunosuppressive therapy before surgery. Clinical indexes (gender, age, tumor location and size, and Dukes’ stage, among others) and pathology indexes (degree of tumor differentiation and depth of infiltration, among others) were collected from each CRC patient. Of the 162 CRC patients, 97 were male and 65 were female, and the median age was 54.7 ± 8.2 years. There were 76 cases of colon cancer and 86 of rectal cancer. In 99 cases, the tumor diameter was ≥ 5 cm, and in 63 cases, it was < 5 cm. There were 26 cases in Dukes’ A stage, 58 cases in Dukes’ B stage, 47 cases in Dukes’ C stage and 21 cases in Dukes’ D stage. The tumors were well differentiated in 30 cases, moderately differentiated in 54 cases, and poorly differentiated in 78 cases. The tumors had infiltrated into the muscular layer in 61 cases and into the serosa in 101 cases. Additionally, after surgery, there were 86 cases with lymph node metastasis (LNM) and 76 cases without LNM. The control group comprised samples of normal tissue located at least 10 cm away from the tumor margins of 50 randomly selected cases.
The expression of HER-2 protein in the 162 CRC tissue samples and 50 adjacent normal mucosa tissue samples was detected by immunohistochemistry (IHC). All tissue specimens were processed for hematoxylin and eosin (HE) staining and IHC staining using the following procedures: the samples were fixed in 10% formaldehyde solution, dehydrated in an ethanol gradient made transparent with dimethylbenzene, embedded in paraffin, cut into slices, dewaxed with dimethylbenzene and hydrated with an ethanol gradient. HER-2 positive CRC tissue slices (Beijing Zhongshan Jinqiao Biotechnology Co., Ltd., Beijing, China) were selected as positive controls. IHC staining was carried out with phosphate buffered saline instead of primary antibody as a negative control.
According to Broders’ method, the histological subtypes of CRC include well differentiated, moderately differentiated, poorly differentiated and undifferentiated. After routine HE staining, the characteristics of the CRC tissue could be observed: tumor cells of varying shape and size, bulky nuclear chromatin, clearly visible nucleoli, and an increase in the number, scant cytoplasm and basophilia.
IHC results were analyzed using HercepTest and scored according to the revised scoring system. After staining, HER-2-positive proteins were yellow and tan and were primarily localized in the cell membrane. At least 3 visual fields were randomly selected for counting under a microscope (× 100). Scoring was performed using two methods. In method 1, scoring was based on staining intensity: 0 (colorless), 1 (faint yellow or light red), 2 (tan or brownish red) and 3 (yellowish-brown or reddish-brown). In method 2, scoring was based on the percentage of positive cells. For each slide, 5 fields were observed under high power magnification (× 100), 100 tumor cells in each field were randomly chosen, and the positive cells were counted. If the number of positive cells was ≤ 10%, it was scored as 0; 10%-25% positive cells were scored as 1; 25%-50%positive cells were scored as 2; and > 50% positive cells were scored as 3. The scores from methods 1 and 2 were multiplied, and the results were classified into grades as follows: 0 indicated negative (-), 1-2 indicated weakly positive (+), 3-4 indicated positive (+ +) and ≥ 5 indicated strongly positive (+ + +). Two professional pathologists read the films and judged the outcomes based on the scoring criteria.
One hundred and sixty-two patients received follow-up through clinical examinations and regular telephone and mail contact. By March 2014, 7 patients had been lost.
PubMed, the Wanfang database, the China National Knowledge Infrastructure database and the VIP database were searched for relevant published studies using the following combination of keywords and free words: HER2 (erbB-2), human epidermal growth factor receptor-2, ErbB2-2 (c-erbb-2 proto-oncogenes), colorectal neoplasms, colon cancer, colorectal tumors, colorectal carcinoma, and colorectal cancer, among others. Studies included in the meta-analysis met all of the following criteria: (1) research type: case-control; (2) research subjects: patients with CRC (the case group) and healthy controls (the control group); and (3) the chosen studies provided complete data such as the number of cases, country, race, age, gender, ethnicity, pathological type, detection method, and the expression of HER-2 protein. Two investigators independently extracted the data from the selected articles. Disagreements were resolved by discussion until a consensus was reached. R version 3.1.0 (Robert Gentleman and Ross Ihaka, Auckland University, New Zealand) was utilized for statistical analysis. Comparisons of HER-2 protein expression, overall survival (OS) rates and disease-free survival (DFS) rates between the CRC patients and healthy subjects were assessed using an OR with a 95%CI under a fixed-effects or a random effects model. A Z-test was utilized to determine the significance of the pooled ORs[19]. Heterogeneity among the studies was assessed with Cochran’s Q test[20] as well as the I2 statistic[21]. A fixed-effects model was applied to calculate the parameters when heterogeneity was not an issue (P > 0.05 or I2 test results < 50%). By contrast, if there was substantial heterogeneity (P < 0.05 or I2 test results > 50%), a random-effects model was utilized to pool the data[22].
The experimental data were analyzed using SPSS 18 software. Survival curves were plotted based on the follow-up data. The measurements are presented as the mean ± SD. To compare between groups, t-tests were utilized. Count-based data are represented with percentages or ratios and are compared using χ2 tests. There was a significant difference if P was less than 0.05.
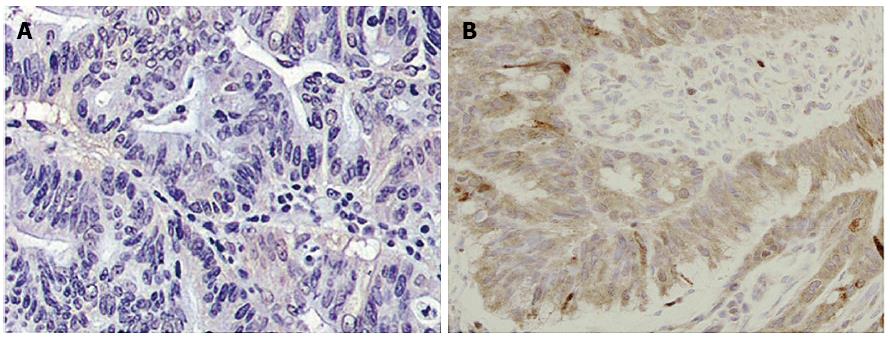
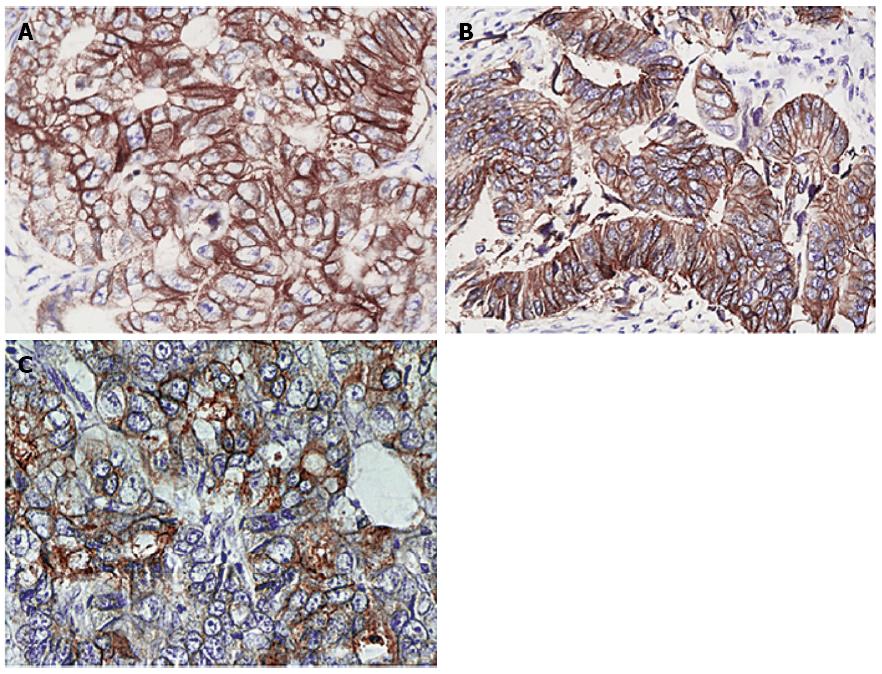
There was no expression of HER-2 protein in the normal mucosal samples as shown via IHC (Figure 1A); IHC-positive signals demonstrated the high expression of HER-2 protein in CRC tissue samples (Figure 1B). The expression of HER-2 protein in the 162 CRC tissue samples was significantly higher than in the control group (19.14% vs 0.00%, P = 0.001). Of these samples, 8 cases were weakly positive, with an expression rate of 4.94% (Figure 2A); 17 cases were positive, with an expression rate of 10.49% (Figure 2B); and 6 cases were strongly positive, with an expression rate of 3.70% (Figure 2C).
As shown in Table 1, among the CRC patients, the expression of HER-2 protein in Dukes’ stages A and B was significantly lower than that in Dukes’ stages C and D (14.29% vs 27.94%, P = 0.009); the expression of HER-2 protein in CRC patients with LNM was higher than that in CRC patients without LNM (25.58% vs 11.84%, P = 0.027); and the expression of HER-2 protein in CRC patients with tumors that had infiltrated into the muscular layer was higher than that of patients whose tumors had infiltrated into the serosa (27.87% vs 13.86%, P = 0.028). These data show that the expression of HER-2 protein in CRC is related to the Dukes’ stage, the depth of invasion and LNM, but not to gender, age, tumor location and size, or the degree of differentiation (P > 0.05).
| Clinicopathological parameters | Number of cases | HER-2 positive rate, n (%) | χ2value | P value | |
| Gender | Male | 97 | 17 (17.53) | 0.405 | 0.525 |
| Female | 65 | 14 (21.54) | |||
| Age | ≥ 60 | 74 | 15 (20.27) | 0.113 | 0.736 |
| < 60 | 88 | 16 (18.19) | |||
| Tumor location | Colon | 76 | 17 (22.37) | 0.967 | 0.326 |
| Rectum | 86 | 14 (16.28) | |||
| Tumor size/cm | ≥ 5 | 99 | 20 (20.20) | 0.187 | 0.665 |
| < 5 | 63 | 11 (17.46) | |||
| Dukes staging | |||||
| A + B | 84 | 12 (14.29) | 6.861 | 0.009 | |
| C + D | 68 | 19 (27.94) | |||
| Degree of differentiation | Well-differentiated | 30 | 6 (20.00) | 4.631 | 0.099 |
| Moderately differentiated | 54 | 15 (27.78) | |||
| Poorly differentiated | 78 | 10 (12.82) | |||
| Lymph node metastasis | With | 86 | 22 (25.58) | 4.922 | 0.027 |
| Without | 76 | 9 (11.84) | |||
| Depth of invasion | Serosa | 101 | 14 (13.86) | 4.822 | 0.028 |
| Muscular layer | 61 | 17 (27.87) | |||
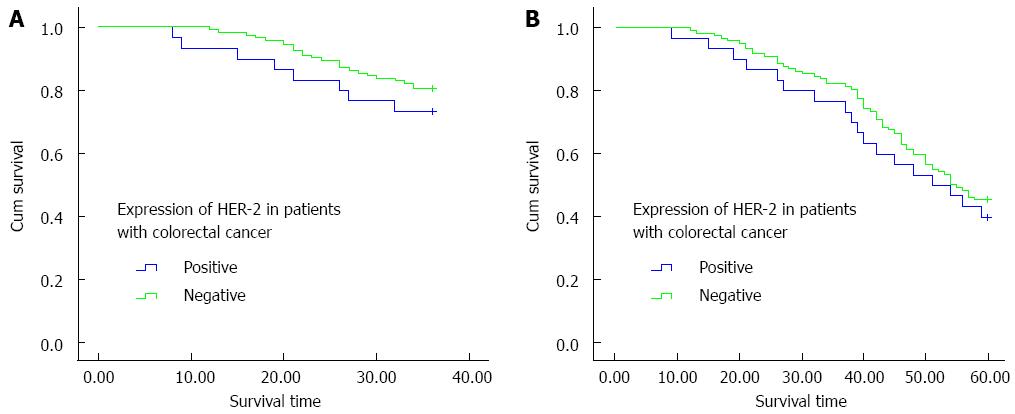
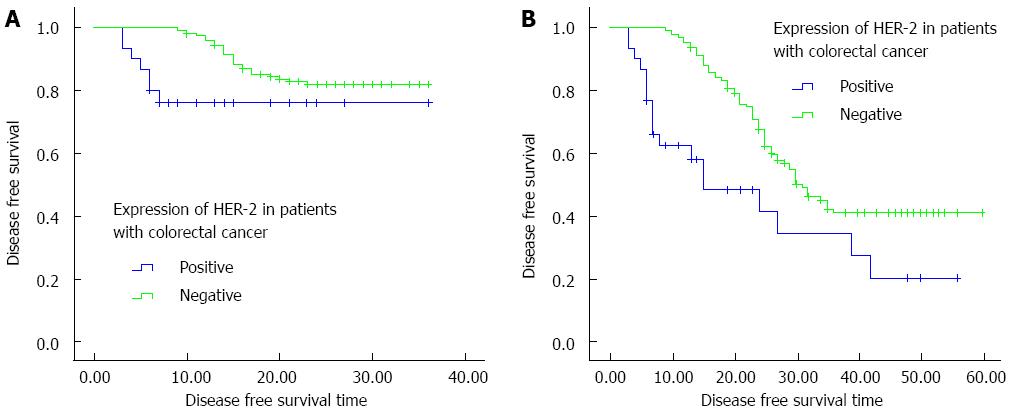
In this study, over a follow-up period of 5 years, detailed data were obtained from a total of 155 patients, including 30 HER-2-positive cases. Kaplan-Meier curves of HER-2-positive and HER-2-negative patients were drawn based on the follow-up data, and the 3-year OS, 3-year DFS, 5-year OS, and 5-year DFS rates were compared between the two groups. Compared with the HER-2-negative patients, the HER-2-positive patients had lower 3-year and 5-year OS rates but the difference was not significant (76.7% vs 82.4%, χ2 = 0.625, P = 0.429; 40.0% vs 45.6%, χ2 = 0.275, P = 0.600) (Figure 3). However, there was a statistically significant difference between the 3-year and 5-year DFS rates of HER-2-positive and HER-2-negative patients (χ2 = 4.40, P = 0.036; χ2 = 12.14, P < 0.01) (Figure 4).
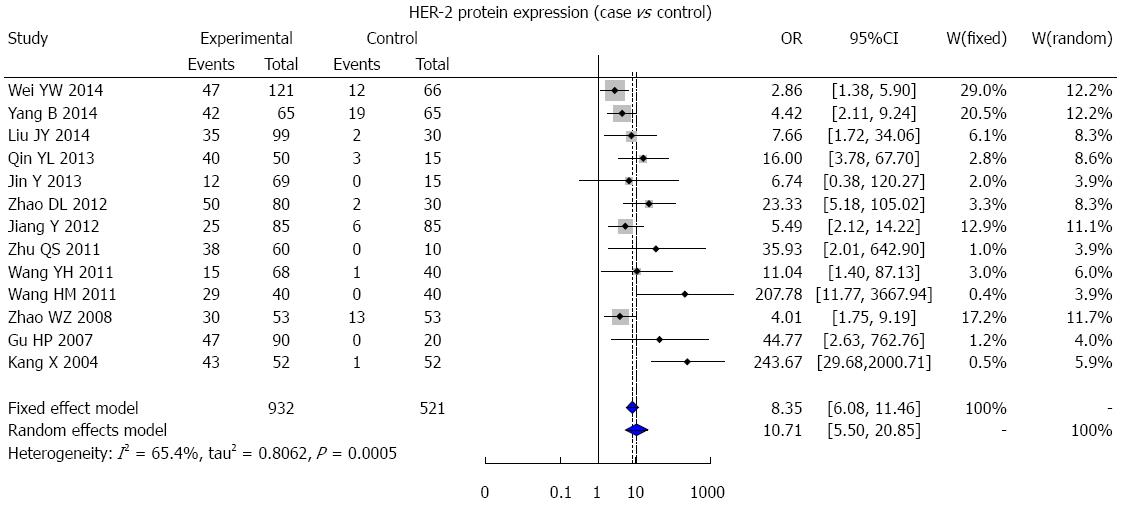
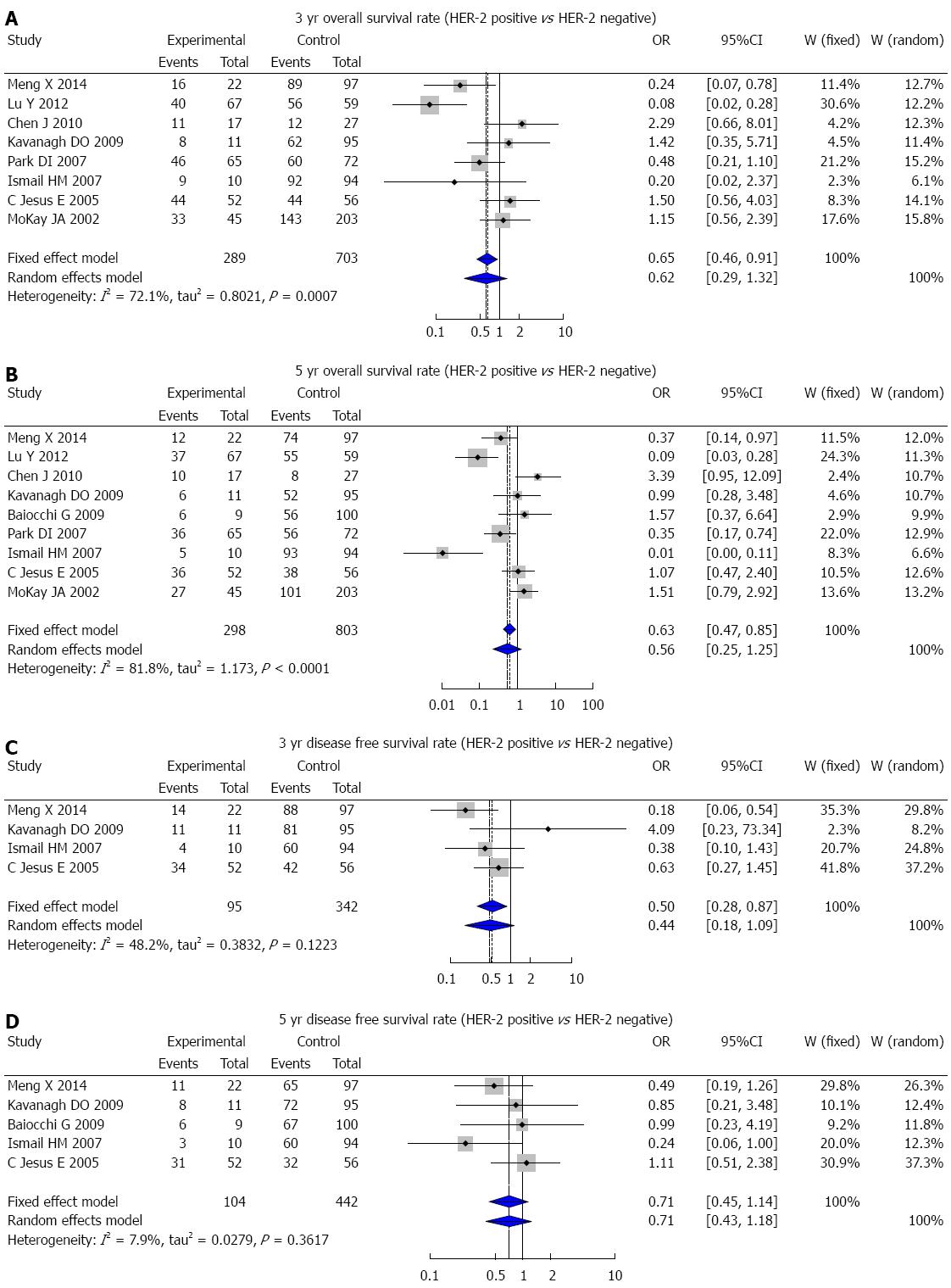
A total of 684 studies (227 in Chinese and 457 in English) were initially retrieved, and based on the selection criteria, 30 case-control studies (13 in Chinese and 17 in English) were ultimately used for the meta-analysis. There were 4942 CRC patients and 521 healthy controls in total. The results of the meta-analysis showed that HER-2 protein expression was statistically significantly higher in CRC patients than in healthy people (OR = 10.71, 95%CI: 5.50-20.85; P < 0.001) (Figure 5). The 3-year DFS rate in HER-2-positive patients was markedly lower than that of HER-2-negative patients (OR = 0.50, 95%CI: 0.28-0.87; P = 0.005) (Figure 6C), but there was no statistically significant difference in the 3-year OS, 5-year OS, and 5-year DFS rates between HER-2-positive and HER-2-negative patients (3-year OS: OR = 0.62, 95%CI: 0.29-1.32, P = 0.211; 5-year OS: OR = 0.56, 95%CI: 0.25-1.25, P = 0.155; 5-year DFS: OR = 0.71, 95%CI: 0.45-1.14, P = 0.178) (Figure 6A, B and D).
Overexpression and amplification of HER-2 has been reported in 25%-35% of breast cancers and the incidence of HER-2 overexpression in CRC varies (ranging from 0% to 83%)[23]. These discrepancies might be caused by differences in detection techniques, the antibodies used, and the tissue fixation methods, as well as the criteria used to assess the results[24]. To assess the correlation between HER-2 expression and CRC, we conducted a case-control study using clinicopathological and prognostic features, and we further verified our results using meta-analysis. Our study showed a significant difference in HER-2 expression between normal colon tissue (0%) and CRC lesions (19.14%). Moreover, fewer CRC patients staged as Dukes’ A and/or B (14.29%) were HER-2-positive compared with those staged as Dukes’ C and/or D (27.94%); CRC patients with lymph node metastases were more likely to be HER-2-positive; and HER-2-positive tissue was also more likely with increasing invasive depth. Furthermore, follow-up results showed that HER-2 expression was inversely correlated with the 3-year and/or 5-year OS and DFS rates. Thus, we suggest that HER-2 expression is correlated with the Dukes’ stage, lymph node metastasis and prognosis of CRC patients, indicating that HER-2 could be a practical tumor marker. This suggestion was further supported by our meta-analysis.
Overexpression of HER-2 has a documented association with disease and with a poor prognosis in various human cancers including ovarian, breast, and lung cancers[1,23,25]. HER-2, which encodes a transmembrane protein with tyrosine kinase activity, is reported to be vital for DNA repair, tumorigenesis, metastasis and drug resistance[26,27]. When its intracellular domain is phosphorylated, the HER-2 protein can both homo- and heterodimerize, promoting cell proliferation and repressing cell death via the Ras/MAPK pathway, as well as the PI3K/AKT pathway[28,29]. HER-2 can activate the Ras/MAP kinase cascade and up-regulate k-ras expression, promoting cancer cell growth and advancing the malignant phenotype. Furthermore, overexpression of HER-2 can down-regulate the p53 protein via the PI3K/AKT pathway, thereby increasing cell proliferation and decreasing adriamycin sensitivity[30,31]. In our research, HER-2 is overexpressed in CRC lesions and is positively correlated with Dukes’ staging and lymph node metastasis, suggesting that the expression of HER-2 may be closely related to the disease progression of CRC. Moreover, HER-2 expression correlates with a poor prognosis for CRC patients. Consequently, the HER-2 protein is a crucial biomarker and target for tumor biology and therapeutic intervention, as well as for cancer gene therapy; down-regulation of HER-2 expression might be an effective strategy for CRC therapy. Recently, herceptin, a humanized monoclonal antibody against HER-2, has been shown to have clear therapeutic effects in strongly HER-2/neu-positive breast carcinoma patients, especially when combined with other chemotherapeutic drugs[32,33]. Thus, further studies on the effect of HER-2 on CRC will demonstrate if herceptin can also be used as a target for CRC therapy.
There are also some limitations that should be noted. First, the number of samples in the present study is comparatively small, but our meta-analysis makes up for this limitation. Second, the HER-2 protein was detected using IHC, whose results could be influenced by various detection approaches and subjective criteria. However, we could not find a more suitable technique that is currently in use in China. In this regard, further study with more accurate detection methods could be warranted.
In brief, HER-2 is overexpressed in CRC lesions, and this overexpression is positively correlated with the Dukes’ stage and lymph node metastasis of CRC, indicating that the expression of HER-2 may be closely related to the progression of CRC and could be an effective biomarker for its diagnosis and treatment. Moreover, HER-2 expression is correlated with a poor prognosis for CRC patients, suggesting that it could be a valid prognostic marker. Thus, a new adjuvant therapy involving herceptin might be a dependable strategy for CRC therapy.
We would like to thank our instructors for their valuable advice. We also appreciate the reviewers’ valuable comments on this article.
Colorectal cancer (CRC) is one of the most common cancers worldwide and is the fourth leading cause of cancer-related death in China. Early diagnosis results in a highly favorable prognosis, while nearly 20% of patients are diagnosed at an advanced, metastatic stage of the disease, and over half will ultimately develop metastases. Human epidermal growth factor receptor (HER-2) is a clinically validated anticancer molecular target that is expressed in the majority of CRCs. HER2 might be an effective biomarker for the diagnosis and treatment of CRC.
HER-2 amplification is observed in 2.5% of 1439 CRC cases, and HER-2 overexpression is observed in an additional 2.7% of cases; CRC amplification is strongly related to protein overexpression. Previous studies have demonstrated that overexpression of HER-2 can lead to cell proliferation, increased motility and protection against apoptosis. In CRC, HER-2 overexpression has been correlated with advanced tumor stage.
To assess the correlation between HER-2 expression and CRC, the authors conducted a case-control study using clinicopathological and prognostic features, and they further verified our results using meta-analysis. This study showed a significant difference in HER-2 expression between normal colon tissue (0%) and CRC lesions (19.14%). Moreover, fewer CRC patients staged as Dukes’ A and/or B (14.29%) were HER-2-positive compared with those staged as Dukes’ C and/or D (27.94%); CRC patients with lymph node metastases were more likely to be HER-2-positive; and HER-2-positive tissue was also more likely with increasing invasive depth. Furthermore, follow-up results showed that HER-2 expression was inversely correlated with the 3-year and/or 5-year OS and DFS rates. Thus, we suggest that HER-2 expression is correlated with the Dukes’ stage, lymph node metastasis and prognosis of CRC patients, indicating that HER-2 could be a practical tumor marker. This suggestion was further supported by our meta-analysis.
The study results suggest that HER-2 expression may be closely related to the progression of CRC and could be an effective biomarker for the diagnosis and treatment of CRC. Moreover, HER-2 expression is correlated with a poor prognosis. Thus, the down-regulation of HER-2 expression might be a dependable strategy for CRC therapy.
CRC arises due to uncontrolled cell growth in the rectum or colon, both of which are part of the large intestine. It is one of the most common cancers worldwide and is the fourth leading cause of cancer-related death in China. HER-2 protein, a transmembrane tyrosine kinase growth factor receptor is found on normal and malignant epithelial cells and is involved in the regulation of cell proliferation and differentiation.
This is a good study in which the authors analyzed the correlation between HER-2 protein expression and CRC using a case-control study and meta-analysis. The results are interesting and suggest that HER-2 could be an effective biomarker for the diagnosis and treatment of CRC.
P- Reviewer: Ferguson HJM S- Editor: Qi Y L- Editor: O’Neill M E- Editor: Ma S
| 1. | Li Q, Wang D, Li J, Chen P. Clinicopathological and prognostic significance of HER-2/neu and VEGF expression in colon carcinomas. BMC Cancer. 2011;11:277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 2. | Atkin WS, Edwards R, Kralj-Hans I, Wooldrage K, Hart AR, Northover JM, Parkin DM, Wardle J, Duffy SW, Cuzick J. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet. 2010;375:1624-1633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1242] [Cited by in RCA: 1139] [Article Influence: 75.9] [Reference Citation Analysis (0)] |
| 3. | Li MX, Liu XM, Zhang XF, Zhang JF, Wang WL, Zhu Y, Dong J, Cheng JW, Liu ZW, Ma L. Prognostic role of neutrophil-to-lymphocyte ratio in colorectal cancer: a systematic review and meta-analysis. Int J Cancer. 2014;134:2403-2413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 339] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 4. | Mathur A, Ware C, Davis L, Gazdar A, Pan BS, Lutterbach B. FGFR2 is amplified in the NCI-H716 colorectal cancer cell line and is required for growth and survival. PLoS One. 2014;9:e98515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 5. | Kavanagh DO, Chambers G, O’Grady L, Barry KM, Waldron RP, Bennani F, Eustace PW, Tobbia I. Is overexpression of HER-2 a predictor of prognosis in colorectal cancer? BMC Cancer. 2009;9:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 112] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 6. | Zimonin FS. [Reliable protection of chicks against pullorum disease]. Veterinariia. 1973;8:69-70. [PubMed] |
| 7. | Duffy MJ, Lamerz R, Haglund C, Nicolini A, Kalousová M, Holubec L, Sturgeon C. Tumor markers in colorectal cancer, gastric cancer and gastrointestinal stromal cancers: European group on tumor markers 2014 guidelines update. Int J Cancer. 2014;134:2513-2522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 253] [Cited by in RCA: 248] [Article Influence: 22.5] [Reference Citation Analysis (1)] |
| 8. | Normanno N, Tejpar S, Morgillo F, De Luca A, Van Cutsem E, Ciardiello F. Implications for KRAS status and EGFR-targeted therapies in metastatic CRC. Nat Rev Clin Oncol. 2009;6:519-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 349] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 9. | Marx AH, Burandt EC, Choschzick M, Simon R, Yekebas E, Kaifi JT, Mirlacher M, Atanackovic D, Bokemeyer C, Fiedler W. Heterogenous high-level HER-2 amplification in a small subset of colorectal cancers. Hum Pathol. 2010;41:1577-1585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 10. | Antonacopoulou AG, Tsamandas AC, Petsas T, Liava A, Scopa CD, Papavassiliou AG, Kalofonos HP. EGFR, HER-2 and COX-2 levels in colorectal cancer. Histopathology. 2008;53:698-706. [PubMed] |
| 11. | Bokemeyer C, Bondarenko I, Makhson A, Hartmann JT, Aparicio J, de Braud F, Donea S, Ludwig H, Schuch G, Stroh C. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2009;27:663-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1218] [Cited by in RCA: 1241] [Article Influence: 73.0] [Reference Citation Analysis (0)] |
| 12. | Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol. 2010;28:4697-4705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1296] [Cited by in RCA: 1394] [Article Influence: 92.9] [Reference Citation Analysis (0)] |
| 13. | Misale S, Yaeger R, Hobor S, Scala E, Janakiraman M, Liska D, Valtorta E, Schiavo R, Buscarino M, Siravegna G. Emergence of KRAS mutations and acquired resistance to anti-EGFR therapy in colorectal cancer. Nature. 2012;486:532-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1313] [Cited by in RCA: 1475] [Article Influence: 113.5] [Reference Citation Analysis (0)] |
| 14. | Tol J, Dijkstra JR, Klomp M, Teerenstra S, Dommerholt M, Vink-Börger ME, van Cleef PH, van Krieken JH, Punt CJ, Nagtegaal ID. Markers for EGFR pathway activation as predictor of outcome in metastatic colorectal cancer patients treated with or without cetuximab. Eur J Cancer. 2010;46:1997-2009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 178] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 15. | Diaz LA, Williams RT, Wu J, Kinde I, Hecht JR, Berlin J, Allen B, Bozic I, Reiter JG, Nowak MA. The molecular evolution of acquired resistance to targeted EGFR blockade in colorectal cancers. Nature. 2012;486:537-540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1259] [Cited by in RCA: 1354] [Article Influence: 104.2] [Reference Citation Analysis (0)] |
| 16. | Pappas A, Lagoudianakis E, Seretis C, Tsiambas E, Koronakis N, Toutouzas K, Katergiannakis V, Manouras A. Clinical role of HER-2/neu expression in colorectal cancer. J BUON. 2013;18:98-104. [PubMed] |
| 17. | Zong L, Chen P, Wang DX. Death decoy receptor overexpression and increased malignancy risk in colorectal cancer. World J Gastroenterol. 2014;20:4440-4445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Glas J, Seiderer J, Bues S, Stallhofer J, Fries C, Olszak T, Tsekeri E, Wetzke M, Beigel F, Steib C. IRGM variants and susceptibility to inflammatory bowel disease in the German population. PLoS One. 2013;8:e54338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Chen H, Manning AK, Dupuis J. A method of moments estimator for random effect multivariate meta-analysis. Biometrics. 2012;68:1278-1284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 145] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 20. | Jackson D, White IR, Riley RD. Quantifying the impact of between-study heterogeneity in multivariate meta-analyses. Stat Med. 2012;31:3805-3820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 393] [Cited by in RCA: 449] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 21. | Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Comparison of two methods to detect publication bias in meta-analysis. JAMA. 2006;295:676-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1326] [Cited by in RCA: 1560] [Article Influence: 82.1] [Reference Citation Analysis (1)] |
| 22. | Zintzaras E, Ioannidis JP. Heterogeneity testing in meta-analysis of genome searches. Genet Epidemiol. 2005;28:123-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 558] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 23. | Schuell B, Gruenberger T, Scheithauer W, Zielinski Ch, Wrba F. HER 2/neu protein expression in colorectal cancer. BMC Cancer. 2006;6:123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 82] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 24. | Ooi A, Takehana T, Li X, Suzuki S, Kunitomo K, Iino H, Fujii H, Takeda Y, Dobashi Y. Protein overexpression and gene amplification of HER-2 and EGFR in colorectal cancers: an immunohistochemical and fluorescent in situ hybridization study. Mod Pathol. 2004;17:895-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 148] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 25. | Wu SW, Ma CC, Yang Y. The prognostic value of HER-2/neu overexpression in colorectal cancer: evidence from 16 studies. Tumour Biol. 2014;35:10799-10804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Steffen AC, Göstring L, Tolmachev V, Palm S, Stenerlöw B, Carlsson J. Differences in radiosensitivity between three HER2 overexpressing cell lines. Eur J Nucl Med Mol Imaging. 2008;35:1179-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Kyndi M, Sørensen FB, Knudsen H, Overgaard M, Nielsen HM, Overgaard J. Estrogen receptor, progesterone receptor, HER-2, and response to postmastectomy radiotherapy in high-risk breast cancer: the Danish Breast Cancer Cooperative Group. J Clin Oncol. 2008;26:1419-1426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 436] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 28. | Siddiqa A, Long LM, Li L, Marciniak RA, Kazhdan I. Expression of HER-2 in MCF-7 breast cancer cells modulates anti-apoptotic proteins Survivin and Bcl-2 via the extracellular signal-related kinase (ERK) and phosphoinositide-3 kinase (PI3K) signalling pathways. BMC Cancer. 2008;8:129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 88] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 29. | Wang SE, Narasanna A, Perez-Torres M, Xiang B, Wu FY, Yang S, Carpenter G, Gazdar AF, Muthuswamy SK, Arteaga CL. HER2 kinase domain mutation results in constitutive phosphorylation and activation of HER2 and EGFR and resistance to EGFR tyrosine kinase inhibitors. Cancer Cell. 2006;10:25-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 371] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 30. | He P, Zhu D, Hu JJ, Peng J, Chen LS, Lu GX. pcDNA3.1(-)-mediated ribozyme targeting of HER-2 suppresses breast cancer tumor growth. Mol Biol Rep. 2010;37:1597-1604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 31. | Jeong JH, An JY, Kwon YT, Li LY, Lee YJ. Quercetin-induced ubiquitination and down-regulation of Her-2/neu. J Cell Biochem. 2008;105:585-595. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 32. | Bellone S, Palmieri M, Gokden M, Joshua J, Roman JJ, Pecorelli S, Cannon MJ, Santin AD. Selection of HER-2/neu-positive tumor cells in early stage cervical cancer: implications for Herceptin-mediated therapy. Gynecol Oncol. 2003;91:231-240. [PubMed] |
| 33. | Vlahopoulos S, Gritzapis AD, Perez SA, Cacoullos N, Papamichail M, Baxevanis CN. Mannose addition by yeast Pichia Pastoris on recombinant HER-2 protein inhibits recognition by the monoclonal antibody herceptin. Vaccine. 2009;27:4704-4708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |