Published online Jul 28, 2015. doi: 10.3748/wjg.v21.i28.8644
Peer-review started: February 28, 2015
First decision: March 26, 2015
Revised: April 1, 2015
Accepted: May 2, 2015
Article in press: May 4, 2015
Published online: July 28, 2015
Processing time: 152 Days and 11.6 Hours
AIM: To investigate the influence of nodal status on response and clarify the optimal treatment for operable esophageal squamous cell carcinoma (OSCC).
METHODS: We retrospectively analyzed 1490 OSCC patients who underwent transthoracic esophagectomy and lymphadenectomy between December 1996 and December 2009 at the Sun Yat-sen University Cancer Center. The surgical approach and the number of resected lymph nodes (LNs) were considered in the assessment of surgery. Patients were classified according to their nodal statuses (N0 vs N1 vs N2-3). Overall survival was defined as the time from the date of death or final follow-up. Survival analysis was performed using the Kaplan-Meier method and differences between curves were assessed by the log-rank test. Univariate and multivariate Cox regression analyses were used to identify factors associated with prognosis. Statistical significance was assumed at a P < 0.05.
RESULTS: With a median time from surgery to the last censoring date for the entire cohort of 72.2 mo, a total of 631 patients were still alive at the last follow-up and the median survival time was 35.5 mo. The surgical approach (left transthoracic vs Ivor-Lewis/tri-incisional) was verified as independent prognostic significance in patients with N0 or N1 status, but not in those with N2-3 status. Similar results were also observed with the number of resected LNs (≤ 14 vs≥ 15). Compared with surgery alone, combined therapy achieved better outcomes in patients with N1 or N2-3 status, but not in those with N0 status. For those with N2-3 status, neither the surgical approach nor the number of resected LNs reached significance by univariate analysis, with unadjusted HRs of 0.826 (95%CI: 0.644-1.058) and 0.849 (95%CI: 0.668-1.078), respectively, and aggressiveness of surgery did not influence the outcome; the longest survival was observed in those patients who received the combined therapy.
CONCLUSION: Combined therapy has a positive role in OSCC with LN metastasis, and aggressive surgical resection does not improve survival in patients with N2-3 status.
Core tip: Esophageal cancer is one of the most fatal malignant cancers worldwide, and survival is still unsatisfactory for locally advanced subjects. Until now, the optimal multimodality therapy has not yet been established. The assessment of nodal status might facilitate the selection of the most effective treatment for operable esophageal squamous cell carcinoma (OSCC). We retrospectively analyzed 1490 OSCC patients classified according to their nodal statuses (N0 vs N1 vs N2-3). Our results demonstrate the positive role of combined therapy in OSCC with lymph node metastasis, and further suggest that aggressive surgical resection does not improve survival in patients with N2-3 status.
- Citation: Zheng YZ, Zhao W, Hu Y, Ding-Lin XX, Wen J, Yang H, Liu QW, Luo KJ, Huang QY, Chen JY, Fu JH. Aggressive surgical resection does not improve survival in operable esophageal squamous cell carcinoma with N2-3 status. World J Gastroenterol 2015; 21(28): 8644-8652
- URL: https://www.wjgnet.com/1007-9327/full/v21/i28/8644.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i28.8644
Esophageal cancer is a frequent cause of death worldwide, and the traditional management has always been surgical resection[1-3]. Over the previous decades, great improvements in preoperative examination and surgical technology have led to significantly enhanced long-term survival[1-13]. However, survival is still unsatisfactory for locally advanced esophageal cancer, which has prompted an evaluation of neoadjuvant (preoperative) and adjuvant (postoperative) combined-modality therapy[14-25]. Until now, the optimal multimodality therapy has not yet been established.
Lymph node metastasis (LNM) is one of the worst prognostic factors for localized esophageal cancer. Furthermore, LNM decreases the curative effects of surgery[1,3-5,9-11] and enhances the necessity of multimodality treatment[14-20,22]. Therefore, we speculated that the assessment of nodal status might facilitate the selection of the most effective treatment.
In this study, we recruited esophageal squamous cell carcinoma (OSCC) patients who had undergone surgery at a Chinese institution over a 13-year period. The aim of this study was to investigate the influence of nodal status on therapeutic response and attempt to clarify the optimal treatment for operable OSCC.
This study was approved by the Medical Ethics Committee of Sun Yat-sen University Cancer Center. Informed written consent was obtained from all participants. Patients diagnosed with OSCC who underwent transthoracic esophagectomy and lymphadenectomy at the thoracic surgery department of Sun Yat-sen University Cancer Center from December 1996 to December 2009 were screened for study recruitment. Our institutional electronic medical record system was initiated in December 1996; therefore, this was considered the date after which highly reliable data could be obtained.
All patients with pathologically confirmed OSCC who fit the following inclusion criteria were included for analysis: (1) pathologic T status of T1, T2, T3, or T4a; (2) pathologic stage of I, II, or III; (3) microscopically complete resection (R0); and (4) patients who underwent surgery or surgery followed by cytotoxic chemotherapy/radiotherapy. Patients with T1N0 were excluded because most of them were treated with endoscopic resection. We did not include patients who received neoadjuvant treatment due to the limited power in predicting the number of LNMs prior to surgery that was observed during the study period.
Preoperative examination included physical examination, blood test, chest X-ray, barium esophagography, endoscopy, and CT scans of the chest, abdomen and cervical region. If tracheal invasion was suspected, a bronchoscope test was recommended. Endoscopic ultrasonography was widely applied beginning in 2003. Positron emission tomography/CT was initiated in 2005, but was used for few cases due to its high cost. Pathologic staging was performed based on the 7th American Joint Committee on Cancer staging system[26].
Surgical approaches applied in this study included left transthoracic, Ivor-Lewis, and tri-incisional procedures, which were mainly selected based on tumor location and preoperative inspection results. For patients with middle/lower thoracic OSCC with no evidence of superior mediastinum metastasis, we generally performed left transthoracic esophagectomies. For patients with middle/lower thoracic OSCC with evidence of superior mediastinum metastasis, we performed Ivor-Lewis/tri-incisional esophagectomies. In patients with upper thoracic OSCC, we only carried out tri-incisional procedures.
Lymphadenectomy extent was influenced by the surgical approach. For left transthoracic esophagectomy, we could not achieve upper mediastinal lymphadenectomy in most cases, and instead performed standard two-field lymphadenectomy, including subcarinal, lower mediastinal, and upper abdominal dissections. However, for Ivor-Lewis or tri-incisional esophagectomies, an additional upper mediastinal dissection was completed in most cases by so-called extended two-field lymphadenectomy. Cervical lymphadenectomy was not routinely carried out.
Because there were no standard guidelines for the adjuvant treatment of OSCC, treatment options were selected based on tumor stage, the doctor’s opinion, and the patient’s desires. In our hospital, adjuvant treatment was started at 4-6 wk after operation, mostly in patients with LNM. Chemotherapy was typically applied as platinum-based two-drug regimen for 4-6 cycles. Radiotherapy was mainly delivered to the position of the anastomosis, mediastinum, pericardium, and left epiploic lymphatic vessels, at a total dose of 50-60 Gy.
After completion of primary treatment, the patients were asked to participate in outpatient follow-up every 3 mo for the first 2 years, every 6 mo for years 3-5, and every 12 mo thereafter. Regular assessment included physical examination, blood test, endoscopy, chest X-ray, and ultrasound testing. CT scans of the chest, abdomen and cervical region were performed at least once a year. For those could not afford regular follow-up visits, a telephone follow-up was performed. Survival status was reclassified using the best available methods in March 2014. The median time from surgery to the last censoring date for the entire cohort was 72.2 mo.
Statistical analysis was performed using SPSS 19.0 software package (IBM Corp., Armonk, NY, United States). We defined death as the event and overall survival (OS) as the time from the date of surgery to the date of death or final follow-up. Survival analysis was performed using the Kaplan-Meier method and differences between curves were assessed by the log-rank test. Univariate and multivariate Cox regression analyses were used to identify factors associated with prognosis. Statistical significance was assumed at a P < 0.05. The statistical methods of this study were reviewed by Yin Guo from Sun Yat-sen University Cancer Center.
A total of 1490 patients were enrolled as the target population. There were 1150 male and 340 female patients with a median age of 58 years (range: 30-88 years). In this study, 4.1% (61/1490) of the patients were pathologically diagnosed as stage I, 48.6% (724/1490) as stage II, and 47.3% (705/1490) as stage III. The most frequently applied approach was left transthoracic esophagectomy (917/1490; 61.5%), and the median number of resected lymph nodes (LNs) was 14 (range: 1-91). Combined therapy was used for 319 patients, including 194 who received adjuvant chemotherapy, 91 who received adjuvant chemoradiotherapy, and 34 who received adjuvant radiotherapy. The patient characteristics are listed in Table 1.
| Variable | Case |
| Total | 1490 |
| Sex | |
| Male | 1150 (77.2) |
| Female | 340 (22.8) |
| Age (yr) | |
| ≤ 58 | 796 (53.4) |
| > 58 | 694 (46.6) |
| Tumor location | |
| Upper thoracic | 181 (12.1) |
| Middle thoracic | 831 (55.8) |
| Lower thoracic | 478 (32.1) |
| Pathologic T status | |
| T1 | 6 (0.4) |
| T2 | 373 (25.0) |
| T3 | 1039 (69.7) |
| T4a | 72 (4.8) |
| Pathologic N | |
| N0 | 727 (48.8) |
| N1 | 419 (28.1) |
| N2 | 267 (17.9) |
| N3 | 77 (5.2) |
| Tumor cell differentiation | |
| Well | 349 (23.4) |
| Moderate | 727 (48.8) |
| Poor | 414 (27.8) |
| Surgical approach | |
| Left thoracotomy | 917 (61.5) |
| Ivor-Lewis/tri-incisional | 573 (38.5) |
| Resected lymph nodes number | |
| ≤ 14 | 764 (51.3) |
| ≥ 15 | 726 (48.7) |
| Received treatment | |
| Surgery alone | 1171 (78.6) |
| Combined treatment | 319 (21.4) |
A total of 631 patients were still alive at the last follow-up, and the median survival time was 35.5 mo. To investigate the influence of nodal status on therapeutic response, we divided the entire cohort based on their nodal status (N0 vs N1 vs N2-3).
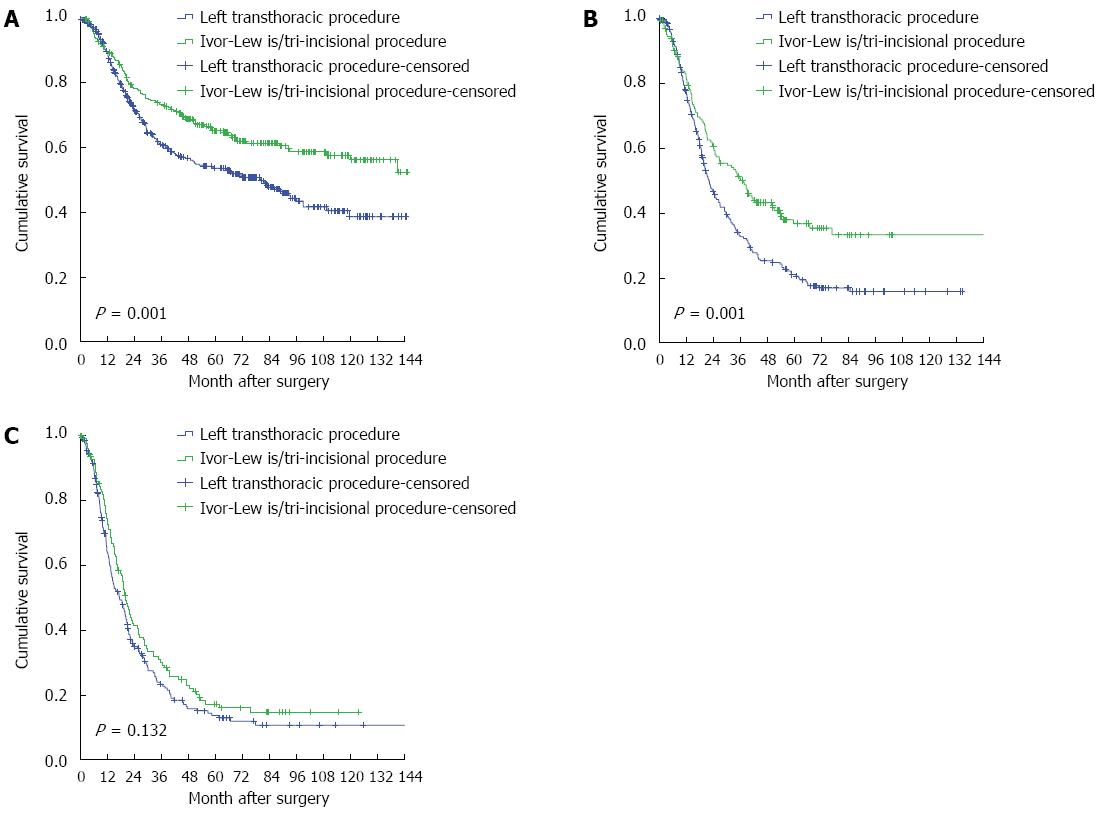
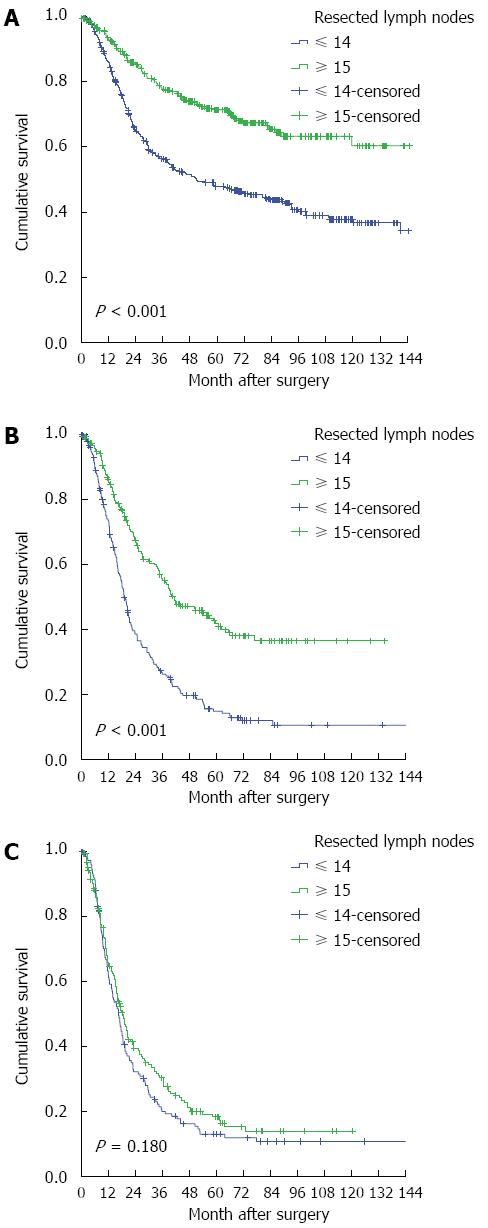
The influence of nodal status on surgery is shown in Table 2. The patients with a status of N0 or N1 who underwent the Ivor-Lewis/tri-incisional procedure presented with significantly better survival compared with those who underwent left transthoracic esophagectomy, with adjusted hazard ratios (HRs) of 0.632 (95%CI: 0.499-0.801) and 0.661 (95%CI: 0.516-0.847), respectively (P < 0.05) (Figure 1). Similar results were also observed for the number of resected LNs (Figure 2). For the patients with an N2-3 status, neither the surgical approach nor the number of resected LNs reached significance by univariate analysis, with unadjusted HRs of 0.826 (95%CI: 0.644-1.058) and 0.849 (95%CI: 0.668-1.078), respectively. The results indicate the poor curative effect of surgery in the patients with an N2-3 status.
| Factors | Surgical approach | Resected lymph nodes (n) | ||
| Left transthoracic | Ivor-Lewis/tri-incisional | ≤14 | ≥15 | |
| In patients with N0 status (n = 727) | ||||
| No. at risk | 445 | 282 | 391 | 336 |
| No. of events | 204 | 106 | 215 | 95 |
| aHR (95%CI) | 1 | 0.632 (0.499-0.801) | 1 | 0.478 (0.375-0.609) |
| P value1 | < 0.001 | < 0.001 | ||
| In patients with N1 status (n = 419) | ||||
| No. at risk | 254 | 165 | 219 | 200 |
| No. of events | 183 | 97 | 174 | 106 |
| aHR (95%CI) | 1 | 0.661 (0.516-0.847) | 1 | 0.464 (0.364-0.592) |
| P value1 | 0.001 | < 0.001 | ||
| In patients with N2-3 status (n = 344) | ||||
| No. at risk | 218 | 126 | 154 | 190 |
| No. of events | 170 | 99 | 127 | 142 |
| uHR (95%CI) | 1 | 0.826 (0.644-1.058) | 1 | 0.849 (0.668-1.078) |
| P value2 | 0.130 | 0.179 | ||
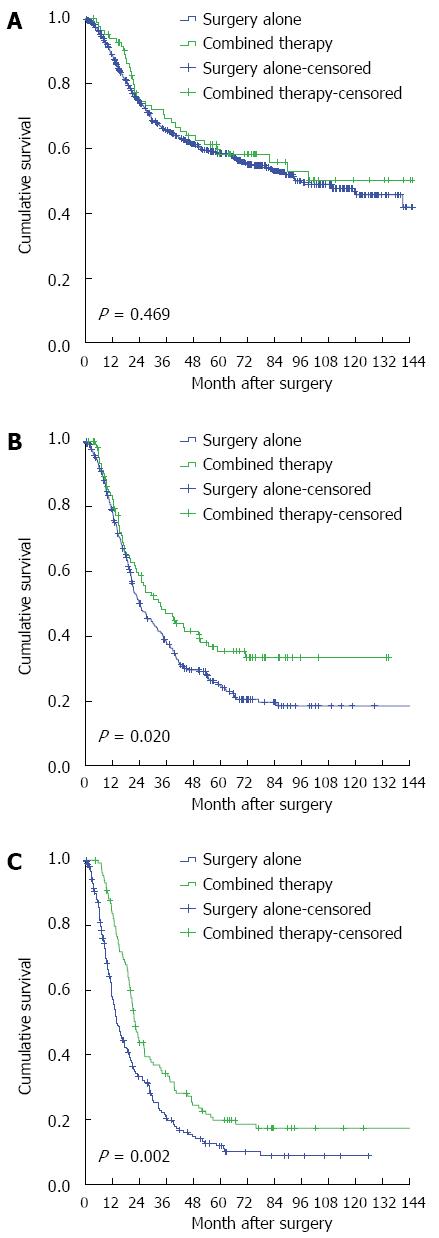
The influence of nodal status on combined therapy is shown in Table 3. Compared with surgery alone, the combined therapy did not generate better outcomes in the patients with N0 status (unadjusted HR = 0.878, 95%CI: 0.617-1.249). For those with an N1 or N2-3 status, survival benefits were observed in those who received the combined therapy, with adjusted HRs of 0.712 (95%CI: 0.537-0.944) and 0.672 (95%CI: 0.521-0.867), respectively (Figure 3). These data supported the use of combined therapy to treat OSCC with LNM.
| Factors | Surgery alone | Combined therapy |
| In patients with N0 status (n = 727) | ||
| No. at risk | 644 | 83 |
| No. of events | 275 | 35 |
| uHR (95%CI) | 1 | 0.878 (0.617-1.249) |
| P value1 | 0.469 | |
| In patients with N1 status (n = 419) | ||
| No. at risk | 313 | 106 |
| No. of events | 217 | 63 |
| aHR (95%CI) | 1 | 0.712 (0.537-0.944) |
| P value2 | 0.018 | |
| In patients with N2-3 status (n = 344) | ||
| No. at risk | 214 | 130 |
| No. of events | 169 | 100 |
| aHR (95%CI) | 1 | 0.672 (0.521-0.867) |
| P value3 | 0.002 | |
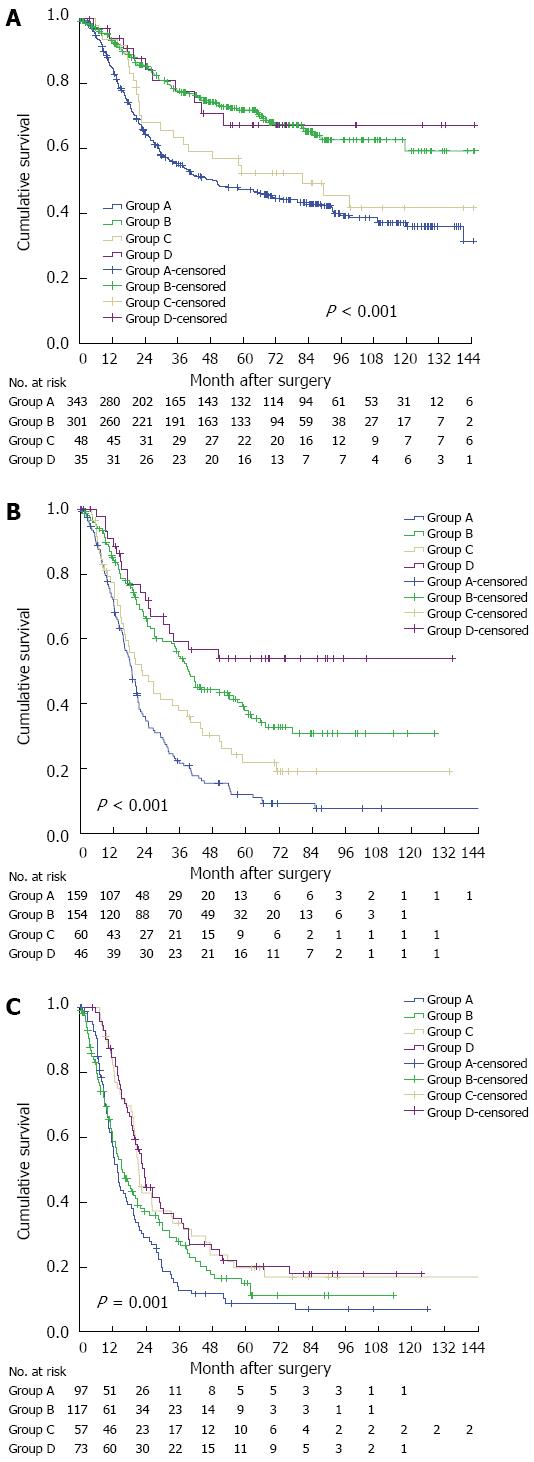
To search for an optimal treatment, extensive surgery was defined as Ivor-Lewis/tri-incisional procedure with resection of ≥ 15 LNs, and limited surgery as the other procedures assessed. Therefore, patients were classified into four groups based on their therapeutic regimens, including Group A (limited surgery alone), Group B (extensive surgery alone), Group C (limited surgery followed by adjuvant treatment), and Group D (extensive surgery followed by adjuvant treatment). The prognosis of patients with N0 status who underwent surgery alone was comparable to that of the combined therapy, and the longest survival times were observed in Group B and Group D (Figure 4A). The optimal treatment for the patients with N1 status was extensive surgery followed by adjuvant treatment (Group D) (Figure 4B). The outcomes of limited surgery for the patients with an N2-3 status were similar to those of extensive surgery, and the best curative effects were observed in Group C and Group D (Figure 4C).
In this study, we investigated the influence of nodal status on therapeutic responses to different treatments for operable OSCC. Based on the results, adopting combined therapy improved the outcomes of the nodal-positive patients, except for those with N0 status. Furthermore, aggressive surgical resection did not improve survival in patients with an N2-3 status.
Surgical resection has long been applied as a mainstay treatment for esophageal cancer[2,6,27]. Previous studies have shown that performing aggressive surgical resection is very important to achieving long-term survival[1,4,5,10,11]. However, recent studies have indicated that the curative effects of surgical resection might be invalid for patients with multiple LNMs. For instance, Tabire et al[6] concluded that the five-year survival rate for patients with ≥ 5 LNMs is merely 9.1% even after McKeown esophagectomy and three-field lymphadenectomy, which is in accordance with Mariette et al[13]. Likewise, Nishimaki et al[9] investigated the outcomes of extended radical esophagectomy in 190 patients and observed that no patients with ≥ 5 LNMs survive beyond five years. Thus, in this study, we first grouped the nodal status according to LNM number (0 vs 1-2 vs 3-4 vs 5-6 vs≥ 7). We found that the positive effect of aggressive surgery on survival is insignificant in patients with 3-4, 5-6, and ≥ 7 LNMs (data not shown), which led us to adopt our current grouping program (N0 vs N1 vs N2-3). Our finding indicated that prognosis could not be improved in patients with an N2-3 status solely by performing more aggressive surgery.
In recent years, there have been many research studies performed in search of an effective multidisciplinary treatment for esophageal cancer[14,15,17-22,24,25,28-30]. In this study, we observed that combined therapy generated better outcomes only in nodal-positive patients compared with surgery alone, which is quite similar with previous studies[14-17]. For example, Ando et al[14] enrolled 242 patients with localized OSCC and found that adjuvant chemotherapy prevents recurrence in patients with LNM, which was further supported by additional studies[15,17]. Further, Lyu et al[16] supported the positive role of adjuvant chemotherapy in enhancing long-term survival in OSCC with LNM.
Recent studies have also reported that neoadjuvant chemoradiotherapy is associated with better outcomes than surgery alone for esophageal cancer, especially in patients with LNM[18-20,22,25,28,30]. Additionally, in the final analysis of the randomized controlled phase III trial FFCD 9901, Mariette et al[21] negated the value of neoadjuvant chemoradiotherapy for stage I or II esophageal cancer. Notably, most of these patients were clinically staged with an N0 status (74.2%). Recently, Okumura et al[23] found that neoadjuvant chemoradiotherapy improves the outcomes of patients with ≥ 4 LNMs, similar to those with 1-3 LNMs treated with surgery alone. Thus, we also support the positive role of neoadjuvant treatment for esophageal cancer with LNM. However, because we did not include patients who underwent neoadjuvant treatment, our results are inconclusive in this regard.
Based on our results, we recommend intraoperative frozen sectioning of suspicious LNs and oppose excessively aggressive surgery for patients with ≥ 3 LNM (at least N2 status), because conservative surgical procedure would decrease perioperative morbidity[12]. In addition, the pathologic stage of OSCC with ≥ 3 LNM is at least IIIA according to the 7th American Joint Committee on Cancer staging system[26], supporting the use of multimodality treatment suggested by our study and previous investigations[14-17]. Although a more aggressive lymphadenectomy might facilitate the acquisition of more accurate staging information in theory[31-34], it would not alter the treatment choice. Furthermore, long-term survival did not differ between the limited dissection and aggressive lymphadenectomy groups in this study.
These finding should be considered in the context of certain weaknesses in our study design. First, the study is retrospective in nature. Second, heterogeneities, including those associated with the basic characteristics of patients or the determination of treatment, were unavoidable due to the long study period, though we conducted subgroup and multivariate analyses to minimize these confounders. However, we believe it is this nonselective and nonmatching population that results in a more generalized significance of this study. Third, because patients who received neoadjuvant treatment were not investigated, further studies are critical to validate our results.
The results presented here indicate the positive role of combined therapy in OSCC with LNM and further suggest that aggressive surgical resection does not improve survival in patients with an N2-3 status.
Esophageal cancer is a frequent cause of death worldwide, and the traditional management has always been surgical resection. In previous decades, great improvements in preoperative examination and surgical technology have led to significantly enhanced long-term survival. However, survival is still unsatisfactory for locally advanced esophageal cancer, which has prompted an evaluation of neoadjuvant (preoperative) and adjuvant (postoperative) combined-modality therapy. Until now, the optimal multimodality therapy has not yet been established.
Lymph node metastasis (LNM) is one of the worst prognostic factors for localized esophageal cancer. Furthermore, LNM decreases the curative effects of surgery and enhances the necessity of multimodality treatment. Therefore, we speculated that the assessment of nodal status might facilitate the selection of the most effective treatment.
With a median follow-up time of 72.2 mo, a total of 1490 patients were analyzed for survival and the median survival time was 35.5 mo. The surgical approach (left transthoracic vs Ivor-Lewis/tri-incisional) was verified as independent prognostic significance in patients with an N0 or N1 status, but not in those with an N2-3 status. Similar results were also observed with the number of resected LNs (≤ 14 vs≥ 15). Compared with surgery alone, combined therapy achieved better outcomes in patients with N1 or N2-3 status, but not in those with an N0 status. For those with N2-3 status, neither the surgical approaches nor the number of resected LNs was significant, and aggressiveness of surgery did not influence the outcome; the longest survival was observed in those patients who received the combined therapy.
Our results demonstrate the positive role of combined therapy in esophageal squamous cell carcinoma with LNM, and that aggressive surgical resection does not improve survival in patients with an N2-3 status.
This retrospective study with a large cohort of patients with esophageal squamous cell carcinoma (n = 1490) contributes to the existing literature regarding surgical therapy of esophageal cancer. It adds relevant data and concludes that aggressive surgery is not justified at an advanced tumor stage. The limitations of this study are clear and declared by the authors. The aspect of a neoadjuvant therapy is not included but discussed and answered in other studies.
P- Reviewer: Kuehn F S- Editor: Qi Y L- Editor: AmEditor E- Editor: Zhang DN
| 1. | Altorki N, Kent M, Ferrara C, Port J. Three-field lymph node dissection for squamous cell and adenocarcinoma of the esophagus. Ann Surg. 2002;236:177-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 2. | Natsugoe S, Yoshinaka H, Shimada M, Sakamoto F, Morinaga T, Nakano S, Kusano C, Baba M, Takao S, Aikou T. Number of lymph node metastases determined by presurgical ultrasound and endoscopic ultrasound is related to prognosis in patients with esophageal carcinoma. Ann Surg. 2001;234:613-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 62] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Ma GW, Situ DR, Ma QL, Long H, Zhang LJ, Lin P, Rong TH. Three-field vs two-field lymph node dissection for esophageal cancer: a meta-analysis. World J Gastroenterol. 2014;20:18022-18030. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 66] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (1)] |
| 4. | Altorki NK, Zhou XK, Stiles B, Port JL, Paul S, Lee PC, Mazumdar M. Total number of resected lymph nodes predicts survival in esophageal cancer. Ann Surg. 2008;248:221-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 226] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 5. | Greenstein AJ, Litle VR, Swanson SJ, Divino CM, Packer S, Wisnivesky JP. Effect of the number of lymph nodes sampled on postoperative survival of lymph node-negative esophageal cancer. Cancer. 2008;112:1239-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 165] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 6. | Tabira Y, Yasunaga M, Tanaka M, Nakano K, Sakaguchi T, Nagamoto N, Ogi S, Kitamura N. Recurrent nerve nodal involvement is associated with cervical nodal metastasis in thoracic esophageal carcinoma. J Am Coll Surg. 2000;191:232-237. [PubMed] |
| 7. | Ando N, Ozawa S, Kitagawa Y, Shinozawa Y, Kitajima M. Improvement in the results of surgical treatment of advanced squamous esophageal carcinoma during 15 consecutive years. Ann Surg. 2000;232:225-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 408] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 8. | Müller JM, Erasmi H, Stelzner M, Zieren U, Pichlmaier H. Surgical therapy of oesophageal carcinoma. Br J Surg. 1990;77:845-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 662] [Cited by in RCA: 624] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 9. | Nishimaki T, Suzuki T, Suzuki S, Kuwabara S, Hatakeyama K. Outcomes of extended radical esophagectomy for thoracic esophageal cancer. J Am Coll Surg. 1998;186:306-312. [PubMed] |
| 10. | Hulscher JB, van Sandick JW, de Boer AG, Wijnhoven BP, Tijssen JG, Fockens P, Stalmeier PF, ten Kate FJ, van Dekken H, Obertop H. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med. 2002;347:1662-1669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1232] [Cited by in RCA: 1144] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 11. | Lerut T, Nafteux P, Moons J, Coosemans W, Decker G, De Leyn P, Van Raemdonck D, Ectors N. Three-field lymphadenectomy for carcinoma of the esophagus and gastroesophageal junction in 174 R0 resections: impact on staging, disease-free survival, and outcome: a plea for adaptation of TNM classification in upper-half esophageal carcinoma. Ann Surg. 2004;240:962-72; discussion 972-4. [PubMed] |
| 12. | D’Journo XB, Doddoli C, Michelet P, Loundou A, Trousse D, Giudicelli R, Fuentes PA, Thomas PA. Transthoracic esophagectomy for adenocarcinoma of the oesophagus: standard versus extended two-field mediastinal lymphadenectomy? Eur J Cardiothorac Surg. 2005;27:697-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Mariette C, Piessen G, Briez N, Triboulet JP. The number of metastatic lymph nodes and the ratio between metastatic and examined lymph nodes are independent prognostic factors in esophageal cancer regardless of neoadjuvant chemoradiation or lymphadenectomy extent. Ann Surg. 2008;247:365-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Ando N, Iizuka T, Ide H, Ishida K, Shinoda M, Nishimaki T, Takiyama W, Watanabe H, Isono K, Aoyama N. Surgery plus chemotherapy compared with surgery alone for localized squamous cell carcinoma of the thoracic esophagus: a Japan Clinical Oncology Group Study--JCOG9204. J Clin Oncol. 2003;21:4592-4596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 518] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 15. | Zhang SS, Yang H, Xie X, Luo KJ, Wen J, Bella AE, Hu Y, Yang F, Fu JH. Adjuvant chemotherapy versus surgery alone for esophageal squamous cell carcinoma: a meta-analysis of randomized controlled trials and nonrandomized studies. Dis Esophagus. 2014;27:574-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 16. | Lyu X, Huang J, Mao Y, Liu Y, Feng Q, Shao K, Gao S, Jiang Y, Wang J, He J. Adjuvant chemotherapy after esophagectomy: is there a role in the treatment of the lymph node positive thoracic esophageal squamous cell carcinoma? J Surg Oncol. 2014;110:864-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 17. | Lee J, Lee KE, Im YH, Kang WK, Park K, Kim K, Shim YM. Adjuvant chemotherapy with 5-fluorouracil and cisplatin in lymph node-positive thoracic esophageal squamous cell carcinoma. Ann Thorac Surg. 2005;80:1170-1175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, Richel DJ, Nieuwenhuijzen GA, Hospers GA, Bonenkamp JJ. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074-2084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3288] [Cited by in RCA: 4077] [Article Influence: 313.6] [Reference Citation Analysis (0)] |
| 19. | Allum WH, Stenning SP, Bancewicz J, Clark PI, Langley RE. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol. 2009;27:5062-5067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 662] [Cited by in RCA: 754] [Article Influence: 47.1] [Reference Citation Analysis (0)] |
| 20. | Berger AC, Farma J, Scott WJ, Freedman G, Weiner L, Cheng JD, Wang H, Goldberg M. Complete response to neoadjuvant chemoradiotherapy in esophageal carcinoma is associated with significantly improved survival. J Clin Oncol. 2005;23:4330-4337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 401] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 21. | Mariette C, Dahan L, Mornex F, Maillard E, Thomas PA, Meunier B, Boige V, Pezet D, Robb WB, Le Brun-Ly V. Surgery alone versus chemoradiotherapy followed by surgery for stage I and II esophageal cancer: final analysis of randomized controlled phase III trial FFCD 9901. J Clin Oncol. 2014;32:2416-2422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 459] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 22. | Urba SG, Orringer MB, Turrisi A, Iannettoni M, Forastiere A, Strawderman M. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol. 2001;19:305-313. [PubMed] |
| 23. | Okumura H, Uchikado Y, Omoto I, Kita Y, Sasaki K, Arigami T, Uenosono Y, Matsushita D, Hiraki Y, Owaki T. The usefulness of neoadjuvant chemoradiation therapy for locally advanced esophageal cancer with multiple lymph-node metastases. Ann Surg Oncol. 2014;21:2845-2849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Liu HC, Hung SK, Huang CJ, Chen CC, Chen MJ, Chang CC, Tai CJ, Tzen CY, Lu LH, Chen YJ. Esophagectomy for locally advanced esophageal cancer, followed by chemoradiotherapy and adjuvant chemotherapy. World J Gastroenterol. 2005;11:5367-5372. [PubMed] |
| 25. | Jin HL, Zhu H, Ling TS, Zhang HJ, Shi RH. Neoadjuvant chemoradiotherapy for resectable esophageal carcinoma: a meta-analysis. World J Gastroenterol. 2009;15:5983-5991. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 78] [Cited by in RCA: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 26. | Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. American joint committee on cacer (AJCC) cancer staging manual.7th ed. Chicago: Springer 2010; 67-72. |
| 27. | Hofstetter W, Swisher SG, Correa AM, Hess K, Putnam JB, Ajani JA, Dolormente M, Francisco R, Komaki RR, Lara A. Treatment outcomes of resected esophageal cancer. Ann Surg. 2002;236:376-84; discussion 384-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 28. | Schena M, La Rovere E, Solerio D, Bustreo S, Barone C, Daniele L, Buffoni L, Bironzo P, Sapino A, Gasparri G. Neoadjuvant chemo-radiotherapy for locally advanced esophageal cancer: a monocentric study. Tumori. 2012;98:451-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 29. | Makino T, Miyata H, Yamasaki M, Fujiwara Y, Takiguchi S, Nakajima K, Higuchi I, Hatazawa J, Mori M, Doki Y. Utility of response evaluation to neo-adjuvant chemotherapy by (18)F-fluorodeoxyglucose-positron emission tomography in locally advanced esophageal squamous cell carcinoma. Surgery. 2010;148:908-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 30. | Greer SE, Goodney PP, Sutton JE, Birkmeyer JD. Neoadjuvant chemoradiotherapy for esophageal carcinoma: a meta-analysis. Surgery. 2005;137:172-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 117] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 31. | Groth SS, Virnig BA, Whitson BA, DeFor TE, Li ZZ, Tuttle TM, Maddaus MA. Determination of the minimum number of lymph nodes to examine to maximize survival in patients with esophageal carcinoma: data from the Surveillance Epidemiology and End Results database. J Thorac Cardiovasc Surg. 2010;139:612-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 107] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 32. | Kawahara K, Maekawa T, Okabayashi K, Shiraishi T, Yoshinaga Y, Yoneda S, Hideshima T, Shirakusa T. The number of lymph node metastases influences survival in esophageal cancer. J Surg Oncol. 1998;67:160-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 33. | Chao YK, Liu HP, Hsieh MJ, Wu YC, Liu YH, Yeh CH, Chang HK, Tseng CK. Impact of the number of lymph nodes sampled on outcome in ypT0N0 esophageal squamous cell carcinoma patients. J Surg Oncol. 2012;106:436-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 34. | Zhang HL, Chen LQ, Liu RL, Shi YT, He M, Meng XL, Bai SX, Ping YM. The number of lymph node metastases influences survival and International Union Against Cancer tumor-node-metastasis classification for esophageal squamous cell carcinoma. Dis Esophagus. 2010;23:53-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |