Published online Jul 28, 2015. doi: 10.3748/wjg.v21.i28.8569
Peer-review started: December 3, 2014
First decision: April 13, 2015
Revised: April 25, 2015
Accepted: June 15, 2015
Article in press: June 16, 2015
Published online: July 28, 2015
Processing time: 240 Days and 9.1 Hours
AIM: To characterize the regeneration-associated stem cell-related phenotype of hepatocyte-derived growth factor receptor (HGFR)-expressing cells in active ulcerative colitis (UC).
METHODS: On the whole 38 peripheral blood samples and 38 colonic biopsy samples from 18 patients with histologically proven active UC and 20 healthy control subjects were collected. After preparing tissue microarrays and blood smears HGFR, caudal type homeobox 2 (CDX2), prominin-1 (CD133) and Musashi-1 conventional and double fluorescent immunolabelings were performed. Immunostained samples were digitalized using high-resolution Mirax Desk instrument, and analyzed with the Mirax TMA Module software. For semiquantitative counting of immunopositive lamina propria (LP) cells 5 fields of view were counted at magnification × 200 in each sample core, then mean ± SD were determined. In case of peripheral blood smears, 30 fields of view with 100 μm diameter were evaluated in every sample and the number of immunopositive cells (mean ± SD) was determined. Using 337 nm UVA Laser MicroDissection system at least 5000 subepithelial cells from the lamina propria were collected. Gene expression analysis of HGFR, CDX2, CD133, leucine-rich repeat-containing G-protein coupled receptor 5 (Lgr5), Musashi-1 and cytokeratin 20 (CK20) were performed in both laser-microdisscted samples and blood samples by using real time reverse transcription polymerase chain reaction (RT-PCR).
RESULTS: By performing conventional and double fluorescent immunolabelings confirmed by RT-PCR, higher number of HGFR (blood: 6.7 ± 1.22 vs 38.5 ± 3.18; LP: 2.25 ± 0.85 vs 9.22 ± 0.65; P < 0.05), CDX2 (blood: 0 vs 0.94 ± 0.64; LP: 0.75 ± 0.55 vs 2.11 ± 0.75; P < 0.05), CD133 (blood: 1.1 ± 0.72 vs 8.3 ± 1.08; LP: 11.1 ± 0.85 vs 26.28 ± 1.71; P < 0.05) and Musashi-1 (blood and LP: 0 vs scattered) positive cells were detected in blood and lamina propria of UC samples as compared to controls. HGFR/CDX2 (blood: 0 vs 1 ± 0.59; LP: 0.8 ± 0.69 vs 2.06 ± 0.72, P < 0.05) and Musashi-1/CDX2 (blood and LP: 0 vs scattered) co-expressions were found in blood and lamina propria of UC samples. HGFR/CD133 and CD133/CDX2 co-expressions appeared only in UC lamina propria samples. CDX2, Lgr5 and Musashi-1 expressions in UC blood samples were not accompanied by CK20 mRNA expression.
CONCLUSION: In active UC, a portion of circulating HGFR-expressing cells are committed to the epithelial lineage, and may participate in mucosal regeneration by undergoing mesenchymal-to-epithelial transition.
Core tip: HGFR+ cells in the circulation and lamina propria of active ulcerative colitis (UC) could also co-express caudal type homeobox 2 (CDX2), an epithelial stem cell marker, thus suggesting that HGFR+ cells have committed to the epithelial lineage. The presence of CD133/CDX2 and Musashi-1/CDX2 double positive cells in the subepithelial layer supports that mesenchymal-to-epithelial transition might be a crucial event in tissue regeneration of active UC.
- Citation: Sipos F, Constantinovits M, Valcz G, Tulassay Z, Műzes G. Association of hepatocyte-derived growth factor receptor/caudal type homeobox 2 co-expression with mucosal regeneration in active ulcerative colitis. World J Gastroenterol 2015; 21(28): 8569-8579
- URL: https://www.wjgnet.com/1007-9327/full/v21/i28/8569.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i28.8569
The luminal border of the colonic wall is lined by an epithelial monolayer, which has several physiological functions including water- and electrolyte absorption, and barrier defence against luminal pathogens. Due to the high turnover of shedding epithelial cells their continuous replacement is required from a local stem cell pool even in the healthy colon. Stem cells are located at the crypt base, and their progenies migrate towards the luminal surface where they undergo terminal differentiation to secretory (Paneth, enteroendocrine, and goblet cells) or absorptive (epithelial) cells[1-3]. When tissue injury occurs, like in inflammatory bowel disease (IBD) or graft-versus host-disease, capacity of the local intestinal stem cell niche is not sufficient enough for a proper tissue repair[1,4,5]. The classic concept of tissue repair holds that upon entering the damaged tissues inflammatory cells signal resident tissue-specific progenitor cells for mitosis[1,4-6]. However, mesenchymal stem cells can also contribute to tissue repair after their mobilization, migration, and engraftment of the damaged area enhanced definitely by inflammation[6,7]. In addition, circulating immature stem cells seem to participate in regeneration of several different tissues as well[1,4-7].
Recently we have found that during severe inflammatory damage of the colon intraepithelial CD45+ marrow-derived cells within the colonic lymphoid aggregates (LA) contribute to epithelial regeneration[8]. It has also been observed that LAs may determine the migration route of stem cells as well[8]. Furthermore, in ulcerative colitis (UC) we have found hepatocyte-derived growth factor receptor (HGFR) positive subepithelial cells within the LA to be involved in the induction of mucosal repair[9]. The presence of the homeobox gene CDX2 and cytokeratin (CK) positive cells detected in LAs suggested that mesenchymal-to-epithelial (MET) transition is located to LAs[9]. Additionally, an elevated number of HGFR+ peripheral blood cells were observed in severely active UC[9], however their importance and function have not yet been investigated.
In case of HGFR+ cells their possible origin and the route of migration in point of the blood stream and the lamina propria (LP) has not yet been elucidated. It is also unclear, whether these cells are committed or not to the epithelial lineage. For better understanding the role of HGFR+ cells in mucosal regeneration, in the present immunocytochemical study we assayed the injury-associated stem cell-related phenotype of HGFR+ cells in peripheral blood and colonic tissue of patients with active UC and compared to that of controls.
After informed consent, precolonoscopic blood samples and colonic biopsy samples were taken from Caucasian patients complaining on abdominal pain or cramps, frequent stool, bloody diarrhea, and fever. Two times 6 mL of peripheral blood were taken and collected into Vacutainer tubes containing EDTA (BD Bioscience) and 9 mL into Paxgene Blood RNA Tubes (Qiagen). The first 6 mL blood was discarded to avoid skin epithelial cell contamination. The second 6 mL blood was stored at 4 °C, while the Paxgene tubes at -20 °C. Biopsy samples were collected for routine histological evaluations and for RNA isolation. On the whole 38 peripheral blood samples and 38 tissue samples from 18 patients with histologically proven active UC (male: 10; female: 8; average age: 30.5 years) and 20 healthy control subjects (male: 10; female: 10; average age 35.6 years) were investigated. The diagnosis of UC in an active stage was based on clinical symptoms (> 6 bloody stools per day, presence/absence of fever, tachycardia, abdominal pain), conventional laboratory abnormalities (elevated erythrocyte sedimentation rate and CRP, thrombocytosis, mild/moderate anemia, presence/absence of hypokalemia and/or hypomagnesemia, negative stool culture), and the result of colonoscopy. In active UC the average Mayo severity index score of patients was 11.61. None of the patients had been given corticosteroids, antibiotics or immunosuppressive treatment prior to taking samples. Control samples were collected from subjects with negative endoscopy and normal histology who underwent colonoscopy because of a positive fecal occult blood test (FOBT). The FOBT has been performed for screening purposes. In the healthy control group, apart from internal hemorrhoids, there were no pathological colonic alterations. The percentage of left/right-colon-sided biopsies was 90%/10%, respectively in normal, and 100%/0% in UC tissue samples. None of the patients participated in this study suffered from any other forms of inflammatory or tumorous diseases. For real-time RT-PCR validation, the biopsy samples collected were immediately snap frozen in Tissue-Tek OCT compound medium (Ted Pella Inc., CA, United States) at -80 °C.
Cores of 1 mm diameter were collected from selected areas of formalin fixed, paraffin embedded (FFPE) tissue blocks made from 18 UC and 20 normal colon samples of 38 patients by repeating each sample at least once, and were then placed into 80 samples recipient block. Tissue sections of 4 μm thickness were cut from the TMA blocks, mounted on adhesive glass slides and immunostained following endogenous hydrogen peroxidase blocking (0.5% H2O2-methanol) and heat induced epitope retrieval in 150 mL of pH 6.0 TRS buffer (Target Retrieval Solution, S1699; in case of anti-Met, anti-CDX2 clone ZC007, and anti-CD133 antibodies) or pH 8.0 1 mmol/L EDTA buffer (in case of anti-CDX2 clone AMT28 antibody) using a commercial microwave oven at 300 W power for 45 min.
Peripheral blood smears of all UC and control EDTA blood samples were performed. The blood smears were fixed in acetone at -20 °C for 5 min, and stored at -20 °C until immunostainings.
TMA slides and blood smears were incubated with rabbit polyclonal anti-Met culture supernatant antibody (Santa Cruz Biotechnology Inc.; Clone: C-12; 1:100 dilution in PBS), monoclonal mouse anti-human CDX2 antibodies (Clone: AMT28; BioSystems; 1:100 dilution in PBS; Clone: ZC007; Invitrogen; 5 μg/mL working concentration, diluted in PBS) and monoclonal anti-human CD133/1-biotin antibody (Clone: AC133; Miltenyi; 1:100 dilution in PBS) at 37 °C for 60 min. After rinsing 3 times with PBS, samples incubated with anti-Met antibody were finally treated with an antirabbit EnVision polymerHRP conjugate kit (K4003, DAKO) for 40 min. Secondary immunodetection in the cases of samples incubated with anti-CDX2 and anti-CD133 antibodies was performed with EnVision System Labelled Polymer-HRP K4001 (Anti-Mouse 1/1; DAKO), as described in the manual. Signal conversion was carried out with Liquid DAB+Substrate Chromogen System (DAKO). After the final rinsing in PBS, hematoxylin co-staining was performed. Cores from normal human smooth muscle from colonic muscularis propria were used as negative controls.
TMA slides and blood smears were then digitalized using high-resolution MIRAX DESK instrument (Zeiss, Gottingen, Germany), and analyzed with the MIRAX TMA Module software (Zeiss).
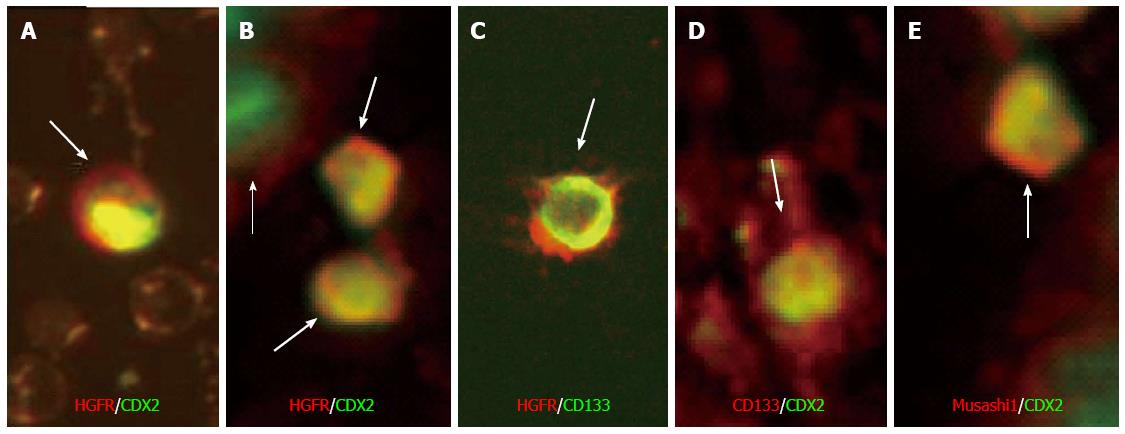
Fot the detection of co-expression of the assayed proteins HGFR/CDX2, HGFR/CD133, CD133/CDX2 and Musashi-1/CDX2 double immunofluorescent labelings were performed.
HGFR/CDX2, HGFR/CD133, Musashi-1/CDX2 labelings: TMA slides and peripheral blood smears were covered with rabbit polyclonal anti-Met culture supernatant antibody (Santa Cruz Biotechnology Inc.; Clone: C-12; 1:100 dilution in PBS) or anti-Musashi-1 monoclonal antibody (EP1302, Abcam, 1:100 dilution in PBS) at 37 °C for 60 min. After rinsing them thrice in PBS, diluted goat polyclonal anti-Rabbit IgG Antibody, biotin-SP conjugate (Millipore Merck; Clone: AP132B; 1:500 dilution in PBS) was added to each sample, and they were incubated at 37 °C for 30 min. After washing them thrice in PBS, samples were covered with diluted Texas Red Streptavidin (1 μL streptavidin in 100 μL of PBS; Jackson Immuno Research Laboratories Inc.) and were also incubated at 37 °C for 30 min. Then samples were incubated with monoclonal mouse anti-human CDX2 antibody (Clone: AMT28; BioSystems; 1:100 dilution in PBS) or monoclonal anti-human CD133/1-biotin antibody (Clone: AC133; Miltenyi; 1:100 dilution in PBS) at 37 °C for 60 min. After rinsing them thrice in PBS, samples were covered with 100x diluted FITC-labeled anti-mouse IgG antibody (Sigma-Aldrich), and were incubated at 37 °C for 30 min. After PBS rinsing, samples were covered with antifading VectaShield (Vector Laboratories Inc.) and coverslips.
CD133/CDX2 labeling: TMA slides and peripheral blood smears were covered with monoclonal anti-human CD133/1-biotin antibody (Clone: AC133; Miltenyi; 1:100 dilution in PBS) at 37 °C for 60 min. After rinsing them thrice in PBS, diluted Biotin-SP-Conjugated Affinipure Goat anti-mouse IgG (1 μL biotin in 100 μL of PBS; Jackson Immuno Research Laboratories Inc.) was added to each sample, and they were incubated at 37 °C for 30 min. After rinsing them thrice in PBS, samples were covered with diluted Texas Red Streptavidin (1 μL streptavidin in 100 μL of PBS; Jackson Immuno Research Laboratories Inc.), and were incubated at 37 °C for 30 min. After washing them thrice in PBS, samples were incubated with monoclonal mouse anti-human CDX2 antibody (Clone: AMT28; BioSystems; 1:100 dilution in PBS) at 37 °C for 60 min. After rinsing them thrice in PBS, samples were covered with 100x diluted FITC-labeled anti-mouse IgG antibody (Sigma-Aldrich) and were incubated at 37 °C for 30 min. After PBS rinsing, samples were covered with antifading VectaShield (Vector Laboratories Inc.) and coverslips.
Finally, immunofluorescently labelled TMA slides and blood smears were digitalized as described previously.
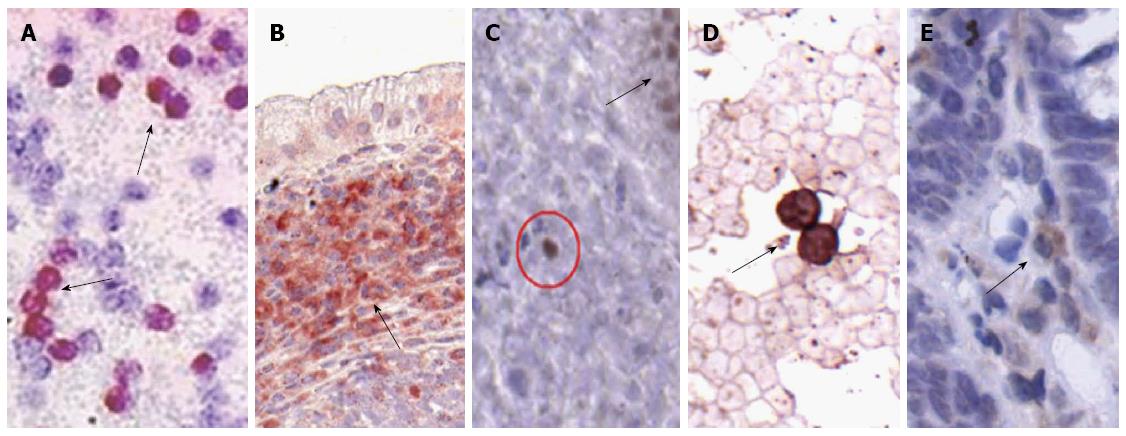
In case of HGFR and CD133 immunolabelings, cells with diffuse, moderate-strong cytoplasmic and/or membrane staining were encountered. In case of CDX2 and Musashi-1, cells showing strong nuclear immunoreactivity were encountered. In normal samples all lymphoid aggregates, in UC samples at least 2 lymphoid aggregates within the lamina propria were examined. The percentage of immunoreactive cells was determined, except in cases where immunopositive cells were found only scattered in the observed area. For semiquantitative counting of immunopositive lamina proprial cells 5 fields of view were counted at magnification × 200 in each sample core, then mean ± SD were determined. In case of peripheral blood smears, 30 fields of view with 100 μm diameter were evaluated in every sample and the number of immunopositive cells (mean ± SD) was determined.
From the biopsy samples stored in Tissue-Tek, 4 μm thick cryostat sections were cut, and placed onto 180 °C heat pre-treated (RNAse free) glass slides. Sections were stained with methylene blue dissolved in diethyl-pyrocarbonate-treated RNAse free water. From one biopsy sample, at least 5000 subepithelial cells (from 2 to 4 cryostat section/sample) from the lymphoid aggregates were then laser capture microdissected by using a 337 nm UVA Laser microdissection system (PALM, Carl Zeiss MicroImaging GmbH, Germany).
Total RNA was extracted using the RNeasy Mini Kit (Qiagen) for biopsy samples and the Paxgene Blood RNA Kit (Qiagen) for peripheral blood samples according to the manufacturer’s instructions. After quantitative (Nanodrop) and qualitative analysis (Bioanalyzer Pico 600 chip kit RNA program; RIN > 8 in all cases), reverse transcription was performed by using 1µg of total RNA (High Capacity cDNA Reverse Transcription Kit, Applied Biosystems, United States).
HGFR (ID: Hs.00179845_m1), CDX2 (Hs.174249) and CD133 (PROM1) (ID: Hs.614737) triplicated Taqman real-time polymerase chain reactions were used to measure mRNA expression of the observed parameters using an Applied Biosystems Micro Fluidic Card System. The measurements were performed using an ABI PRISM 7900HT Sequence Detection System as described in the product’s User Guide (http://www.appliedbiosystems.com, California; United States).
For the examination of leucine-rich repeat-containing G-protein-coupled receptor 5 (Lgr5) (F: TGCTCTTCACCAACTGCATC; R: CTCAGGCTCACCAGATCCTC), Musashi-1 (F: TACGCCAGCCGGAGTTATAC; R: ATTGGTCCGTAGGCAGTGAG) and cytokeratin (CK-)20 (F: CTGATGCAGATTCGGAGTAACA; R: TCTCTCTTCCAGGGTGCTTAAC) gene expression changes triplicated quantitative real-time (qRT) PCR was performed using Probes Master and SYBR green (Roche GmbH, Germany). Gene expression levels for each individual sample were normalized to GAPDH expression. Mean relative gene expression was determined and differences were calculated using the 2-ΔC(t) method. The whole cycle number was 45.
Regarding CK20 RT-PCR, SW480 colon carcinoma cells (90 cells/9 mL blood in Paxgene tube) and crypt epithelial cells (90 cells/5000 laser microdissected subepithelial cells) were used as positive controls.
All routine colonic biopsy specimens and blood samples from the patients were taken after informed consent and ethical permission was obtained for participation in the study.
The data were expressed as the mean ± SD. For the statistics, Student’s t-test was used. P < 0.05 was considered as statistically significant.
The number of HGFR+ and CD133+ cells in the peripheral blood samples and colonic biopsies of patients with active UC was significantly higher comparing to healthy controls. CDX2+ cells were found only in the blood samples of active UC patients, i.e., no CDX2 immunoreactivity was detected in the peripheral blood of healthy controls. In the LP, CDX2 immunoreactivity displayed high specificity to epithelial cell nuclei, however we observed some sporadic CDX2+ cells in the stroma involving infrequently LAs, and without connection to the crypts. In UC tissue samples the number of CDX2+ cells was significantly higher than in controls. In consideration of the unexpected presence of CDX2+ cells in the LP we repeated the anti-CDX2 immunostaining with another antibody (clone ZC007, Invitrogen) to avoid unspecific immunoreactions, and moreover, we performed Musashi-1 immunohistochemistry as well. After analyzing the results of the distinct CDX2 immunoreactions no significant differences were found between the numbers of CDX2-ZC007 and CDX2-AMT28 immunoreactive cells. Some stromal cells showing only Musashi-1 positivity with cytoplasmic and/or nuclear localisation were also detected.
In blood samples of UC patients Musashi-1 positive cells were found only sporadically. Immunopositive cells are presented in Figure 1.
Using double immunofluorescent labeling HGFR/CDX2 double immunopositive cells were found only in blood samples of UC patients, however they were detectable in tissue samples of both controls and UC patients. The number of HGFR/CDX2 double immunopositive cells was significantly higher in LP of active UC patients as compared to healthy controls. In UC samples some stromal CDX2+ cells also showed weak-to-moderate Musashi-1 positivity. We detected no HGFR-/CDX2+ cells in blood or colonic tissue of patients with UC, which indicates that in UC all CDX2+ cells in peripheral blood and lamina propria expressed HGFR simultaneously. Regarding co-expression of Musashi-1/CDX2 in UC blood smears only few numbers of double immunopositive cells were found. HGFR/CD133, and CD133/CDX2 double immunoreactive cells were detected sporadically in the LP of active UC. Double immunoreactive cells are presented in Figure 2. The number of immunopositive cells is indicated in Table 1.
| Average number of cells | Peripheral blood (normal/UC) | Lamina propria (normal/UC) |
| HGFR+ | 6.7 ± 1.22/38.5 ± 3.181 | 2.25 ± 0.85/9.22 ± 0.651 |
| CDX2+(Clone: AMT28)3 | 0/0.94 ± 0.6412 | 0.75 ± 0.55/2.11 ± 0.7512 |
| CDX2+(Clone: ZC007)3 | 0/1.0 ± 0.5912 | 0.8 ± 0.52/2.16 ± 0.7112 |
| CD133+ | 1.1 ± 0.72/8.3 ± 1.081 | 11.1 ± 0.85/26.28 ± 1.711 |
| Musashi-1+ | 0/scattered | 0/scattered |
| HGFR+/CDX2+(Clone: AMT28) | 0/1.0 ± 0.591 | 0.8 ± 0.69/2.06 ± 0.721 |
| HGFR+/CD133+ | 0 | 0/scattered |
| Musashi-1+/CDX2+(Clone: AMT28) | 0/scattered | 0/scattered |
| CD133+/CDX2+(Clone: AMT28) | 0 | 0/scattered |
Real-time RT-PCRs for evaluating HGFR, CD133, CDX2, Musashi-1, Lgr5 and CK20 gene expressions were also performed. The expression values in active UC blood samples and laser-microdissected LP tissues were compared with the values of healthy subjects.
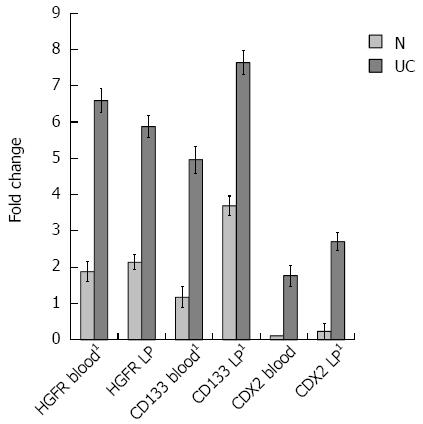
According to the 2-ddCT method, foldchanges of HGFR, CD133 and CDX2 expressions were significantly higher in all UC samples (P < 0.005) than in healthy controls with the exception of CDX2 in peripheral blood, where it displayed an increasing tendency in UC as compared to controls. ddCTs represent the difference between the average threshold cycle differences (dCT) of normal and UC samples.
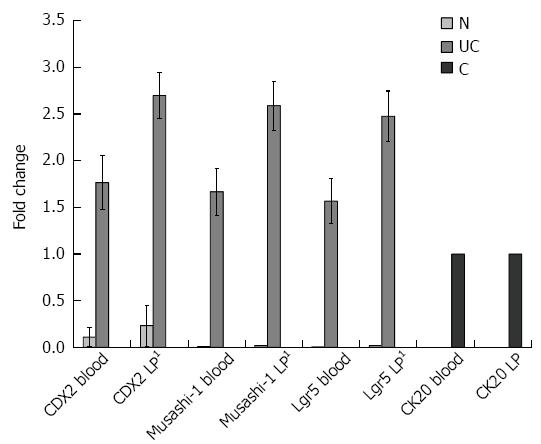
Though CDX2 is a known crypt epithelial stem cell marker, to exclude contamination with epithelial cells Musashi-1, Lgr5, and CK20 mRNA expressions were also analyzed in blood and LP samples. Similarly to CDX2, foldchanges of Musashi-1 and Lgr5 expressions were significantly higher in the LP of UC patients than in controls (P < 0.005 in all cases). Musashi-1 and Lgr5 gene expressions showed an increasing tendency in UC blood samples compared to controls. No detectable CK20 gene expression was present in UC and normal samples. Foldchanges are visualized in Figures 3 and 4.
The concept of mucosal repair means that inflammatory cells enter the damaged area and signal local progenitor cells for mitosis. In addition, upon damage signals multipotent mesenchymal stem cells may also contribute to tissue repair after their mobilization, migration, and engraftment of the inflamed mucosa[6]. Furthermore, circulating immature cells with a potential of stem cell capacity are also likely to participate in colonic mucosal regeneration[10]. In UC, following inflammatory mucosal damage successful epithelial regeneration demands the complex interaction and participation of a local and marrow-derived stem cell pool[11]. The course and regulation of mucosal repair sorely depend on the balance between pro- and anti-inflammatory cytokines influenced mainly by LAs and ILFs[12]. These lymphoid elements are thought to be involved in controling and organizing of homing, development, and differentiation of stem cells, as well[13].
In the present study we assayed the regeneration-associated stem cell-related phenotype of HGFR+ cells both in peripheral blood and colonic tissue samples of patients with clinically active UC.
The proto-oncogene Met is known to encode the high-affinity transmembrane tyrosine kinase receptor for hepatocyte growth factor (HGF). C-Met/HGFR and its family members promote mainly migration and invasion of cancer cells. Signaling within and beyond this pathway seems to be an important factor regarding systemic spread of metastases through induction of epithelial-to-mesenchymal transition (EMT)[14]. HGFR and its ligand, HGF serve as potential mitogenic factors for epithelial cells, enhancing cell motility, with morphogenetic effects, and a crucial role in wound healing within the digestive tract[15-17]. In experimental colitis of rats the HGF/HGFR system was found to be a potential therapeutic target to facilitate intestinal repair[18]. Though HGFR is predominantly expressed on epithelial cells, it is also implicated in hematopoiesis[19]. Expression of HGFR by the hematopoieic compartment was detected in progenitor cells, macrophages, B cells, and dendritic cells. HGFR signaling has been proposed to take part in the development of monocytes-macrophages, and in homing of B cells to lymphoid tissues[20,21]. Basically the HGF/HGFR system is indispensable during embryonic development. HGF is a pleiotropic factor that promotes several cellular functions, including survival, tissue protection, regeneration, and exerts anti-inflammatory activities[16]. Moreover, HGF was found to regulate various immune functions, like cytokine production, cellular migration, and adhesion[22-27]. Nonetheless, under normal conditions the HGF/HGFR signaling seems to be dispensable, since previous studies demonstrated no structural and functional abnormalities in different c-met-knockout cell types[28-31].
HGF is essential in paracrine signaling of mesenchymal and epithelial cells, particularly during embryogenesis, regeneration, and also in carcinogenesis[32]. In adults, HGFR signaling might be involved both in tissue repair and invasive tumor growth, depending mainly on the type of tissues.
Both chronic fibrosis and tumorigenesis can be originated from a sustained healing response due to chronic tissue injury. In fact, chronic fibrosis may directly predispose to cancer[33]. In UC, there is an active and persistent immune-mediated inflammation, therefore the cycle of continuous mucosal injury and repair may lead to an increased risk of colonic wall fibrosis and thus, to the development of cancer. HGF and HGFR expressions are both elevated in the inflamed mucosa of UC patients[34], and additionally, increased plasma HGF levels were found in human UC, and in colitic mice[34,35]. After inducing tissue injury and/or inflammatory cytokines stromal fibroblasts, vascular endothelial and smooth muscle cells, neutrophils, and mast cells represent the major sources of HGF[36]. Fibroblast-like stromal cells from human lymphoid tissues constitutively produce HGF, which is affected by activated T cells[37]. Emerging evidences indicate that HGF possesses potent anti-apoptotic capacity, as well[38-40]. Within the mucosa the HGF-HGFR signaling facilitates colonic mucosal remodeling and significantly lessens inflammation[9,18], breaking the cycle of injury and repair. Conversely, HGFR is overexpressed in UC-associated colorectal cancer[34], suggesting that the HGF/HGFR system is unequivocally involved in carcinogenesis. According to these data, it is likely that the pro- or anti-carcinogenic effects of the HGF/HGFR system is context-dependent influenced mainly by the duration of inflammation, i.e., acute or chronic. During active inflammation of the colonic mucosa the HGF/HGFR system usually favors mucosal healing. The high number of HGFR+ subepithelial and circulating cells found in our study may be the consequence of an increased demand of cells with extensive mucosal reparative capacity.
Although in DSS-colitic rats both an increase in serum HGF level and expression of colonic HGF mRNA were observed, the HGF protein level in colitic mucosa was still reduced[41]. HGF also interacts with heparin-like low-affinity receptors, like the heparin sulfate proteoglycans, which are expressed on the cell surface and within extracellular matrix that retain HGF in tissues[42-44]. In case of severe mucosal damage, when loss of the epithelial layer and the surrounding extracellular matrix can be detected, the local HGF concentration in the injured colonic tissue might be reduced.
Considering our results, the elevated local expression of both mRNA and protein of the high-affinity HGFR may function to retain and bind all the available local HGF for reducing the inflammation, and to promote mucosal regeneration. The mechanism underlying the protective mucosal actions of HGFR signaling in UC is likely attributable to its ability to inhibit apoptosis and inflammation.
In the intestine, CDX2, an intestine-specific transcription factor essential for the regulation of genes related to epithelial functions, is involved in maintaining epithelial homeostasis[45,46], and controling the expression of a number of downstream genes, some of which fundamentally contribute to inflammation[47]. Further, CDX2 was shown to inhibit cell growth and migration in vitro, as well as dissemination of colon tumor cells in vivo[48]. Until now only limited attentions have been paid for the investigation of the relation of CDX2 to intestinal inflammation. In a recent study diminished epithelial CDX2 expression was described in UC[49]. Moreover, TNF-α was suggested to down-regulate the expression of CDX2 in the inflamed mucosa[50]. On the contrary, however, we found that the number of CDX2+ submucosal cells in active UC was slightly elevated. Although CDX2 expression may be influenced by the hypoxia inducible factor 1 (HIF1) expressed during inflammation[51,52], it remains unclear how the expression of CDX2 is regulated exactly within the affected tissues.
Mesenchymal myofibroblasts are involved in mucosal healing process, and epithelial regeneration (re-epitheliazation). Upon expression of Wnt proteins, and secretion of growth factors, like epidermal growth factor, HGF, and bone morphogenic factor-4 myofibroblasts considerably promote the induction of CDX2[53]. Furthermore, myofibroblasts support the local stem cell niche via multiple mechanisms, including interactions via the Wnt/β-catenin and Notch pathways to regulate stem cell behavior[54,55].
In case of our UC patients the parallel presence of HGFR+/CDX2+ double-positive cells in circulation and colonic subepithelium could be indicative for The involvement of HGFR+ cells in regeneration of the damaged mucosa.
Since detection of CDX2+ cells in the LP and blood samples of UC patients was an unexpected result, we performed Musashi-1 immunolabeling, and Musashi-1 and Lgr5 RT-PCR assays. Both Musashi-1 and Lgr5 are considered as markers of intestinal epithelial stem cells[56-58], and Lgr5 is a target of the Wnt pathway, as well[58]. Musashi-1 is co-localizes with Notch genes and augments Notch signaling, essential for maintaining cellular progenitor state[59]. On the other hand, Musashi-1+ blood cells might even represent circulating smooth muscle cell precursors[60]. Lgr5+ cells are mainly intestinal stem cells or crypt basal cells (CBC) being able to give rise to all intestinal epithelial lineages, indicating a self-renewing population of stem cells[59]. Moreover, Lgr5 might also be implicated in cancer stemness[61].
The parallel presence of CDX2, Musashi-1 and Lgr5 in UC blood and LP samples along with the absence of CK20 expression may indicate that a portion of circulating HGFR+ cells has already been committed to the epithelial lineage. However, further experiments are required to understand the role of CDX2 in the regenerative phase of UC, and the exact origin of CDX2+ (Musashi-1+ and Lgr5+) cells.
CD133 (Prominin-1), a 5-transmembrane domain glycoprotein expressed by hematopoietic and mesenchymal stem cells, other organ-specific stem cells and tumor initiating cells, is currently regarded as one of the most significant markers of colonic cancer stem cells, as well[62-64]. The molecule is designated for its prominent location on protrusions of cell membranes[65,66]. Additionally, CD133 can also be expressed by epithelial stem cells[67], and in this respect represents another target for Wnt signaling pathways. Therefore, CD133 is of importance yet in intestinal regeneration, and, further, in decreasing inflammation[68].
The expression of CD133 within the inflamed colonic epithelium is significantly lower in UC patients with a longer duration of the disease[69]. The risk of developing colorectal cancer is considerably higher in patients suffering from UC, especially in those with long-standing disease, i.e., over 10 years[70]. Thus, the decreased CD133 level in the inflamed mucosa could be associated with the development of UC-related colorectal cancer. In our study the number of CD133+ cells were significantly higher in the blood and subepithelium of patients with newly diagnozed UC in an active phase of the disease. The fact that HGFR+/CD133+ cells were detected only in the lamina propria suggests that a portion of HGFR+ cells in LAs may be originated from a local stem cell pool rather than being immigrating cells. CD133 may also influence cellular polarity, migration, and the interaction of stem cells with their neighboring ones and the extracellular matrix[71], and thus promote mucosal healing.
Some Lgr5+ cells co-express CD133, and these CD133+ cells can generate the entire intestinal epithelium[59]. In addition to Lgr5 and CD133, other potential intestinal stem cell markers have been identified, like Musashi-1, expressed by both quiescent label-retaining cells and actively cycling CBCs[59]. In general, during tissue repair stem cells might display a great transient plasticity, so upon a dynamic interplay they can change their current phenotype to ensure successful tissue regeneration[72].
The co-expression of HGFR and CD133 on the surface of colonic subepithelial cells may further indicate the potential involvement of HGFR+ cells in UC-associated carcinogenesis. In the colonic mucosa LAs and ILFs are supposed to have a special organizer role in epithelial repair[12]. Our finding, that CD133 and CDX2 are co-expressed in cells of LAs also supports that MET is primarily localized to the area of these lymphoid structures. It is still questionable whether the complex mucosal healing is related to the recruitment of a quiescent local stem cell population or requires renewal by bone marrow cells, and hence, remain to be determined. One cannot exclude the possibility that stromal cell-derived factor 1 or vascular endothelial growth factor have a role in the chemoattraction of marrow derived cells to the lamina propria. In our study, we did not study these factors. However, HGF alone may also serve as a chemoattractant of circulating HGFR+ cells since it has been reported that HGF level is elevated in the inflamed colonic mucosa[34]. Although it is apparent that the intestinal epithelium responds to inflammation and mucosal injury by initiating a repair response, the specific effects on the turnover of epithelial stem- or progenitor cells, and the exact mechanism how the inflammatory milieu may perturb epithelial differentiation and/or function, are still obscure. Furthermore, the bidirectional interactions between stem cells and their niche are of special importance to determine stem cell behavior, and thus leading toward self-renewal or differentiation.
In conclusion, based on our present results the elevated number of HGFR+ subepithelial and circulating cells within the inflammatory colon may be an indicator of the increased demand of cells with high mucosal regenerative capacity. The presence of HGFR/CDX2 double immunoreactive cells along with the detectable expression of Musashi-1 and Lgr5 in the peripheral blood and LP of patients with UC highlight on the potential involvement of HGFR+ cells in colonic mucosal healing related to severe inflammatory injury. Moreover, the data indicate that HGFR+ cells in the circulation are already committed to the epithelial lineage. The presence of HGFR/CD133 double immunopositive cells in the LP suggests that a portion of HGFR+ cells may be originated from a local stem cell pool, and besides repair functions, they could potentially be involved in UC-associated carcinogenesis as well. In addition, CD133 could also affect the regulation of cell motility, another crucial element of wound healing. The detection of CD133+/CDX2+ cells along with the presence of Musashi-1 in subepithelial lymphoid aggregates support that mesenchymal-to-epithelial transition, an essential event in epithelial regeneration is primarily located to those lymphoid structures. In conclusion, both HGFR and CDX2 seem to be definitely involved in colonic mucosal regeneration of clinically active UC patients, however further investigations are needed to determine their definite role and function, and relation to local and bone marrow stem cell pools.
The authors would like to thank the members of the Endoscopic Unit, and Cell Analysis Laboratory, 2nd Department of Internal Medicine, and the 1st Department of Pathology and Experimental Oncology, Semmelweis University for their technical support. The authors also thank Ms. Anika Scott for her careful language assistance.
In ulcerative colitis hepatocyte-derived growth factor receptor (HGFR) positive subepithelial cells within lymphoid aggregates are supposed to be involved in the induction of mucosal repair. An elevated number of HGFR+ peripheral blood cells were observed in severely active ulcerative colitis, however their importance and function have not yet been investigated. In case of HGFR+ cells their possible origin, the route of migration in point of the blood stream and the lamina propria, and whether these cells are committed or not to the epithelial lineage have not yet been elucidated.
Previous chromosomal chimerism experiments have already proved that part of epithelial stem cells are originated from a local stem cell pool, while another part of them is of marrow origin.
This is the first study evaluating the injury-associated stem cell-related phenotype of HGFR+ cells in peripheral blood and colonic tissue of patients with active ulcerative colitis and compared to that of controls.
The presence of HGFR/CDX2 double immunoreactive cells in the peripheral blood and lamina propria of ulcerative colitis patients highlight on the potential involvement of HGFR+ cells in colonic mucosal healing related to severe inflammatory injury. The data also indicate that HGFR+ cells in the circulation are committed to the epithelial lineage. The presence of HGFR/CD133 double immunopositive cells in the lamina propria suggests that a portion of HGFR+ cells may be originated from a local stem cell pool.
Mesenchymal-to-epithelial transition (MET), a crucial physiologic event converts motile mesenchymal cells to polarized epithelial cells, thus favors -among others- epithelial regeneration. Within pathological circumstances, increasing evidence indicates that MET may regulate epithelial carcinogenesis, as well.
Authors demonstrated that higher number of HGFR, CDX2, CD133 and Musashi-1 positive cells were detected, and that HGFR/CDX2 and Musashi-1/CDX2 co-expressions were found in blood and lamina propria of UC samples. These results are interesting. Previous studies have established that HGFR+ cells may have a role in mucosal healing in ulcerative colitis. However, as the authors point out, the migration pathway of HGFR+ cells from the blood stream to the lamina propria has not been elucidated.
P- Reviewer: Keyashian K, Kamiya T, Tsujikawa T S- Editor: Yu J L- Editor: A E- Editor: Zhang DN
| 1. | Matsumoto T, Okamoto R, Yajima T, Mori T, Okamoto S, Ikeda Y, Mukai M, Yamazaki M, Oshima S, Tsuchiya K. Increase of bone marrow-derived secretory lineage epithelial cells during regeneration in the human intestine. Gastroenterology. 2005;128:1851-1867. [PubMed] |
| 2. | van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol. 2009;71:241-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1156] [Cited by in RCA: 1327] [Article Influence: 82.9] [Reference Citation Analysis (0)] |
| 3. | Ricci-Vitiani L, Pagliuca A, Palio E, Zeuner A, De Maria R. Colon cancer stem cells. Gut. 2008;57:538-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Barry FP, Murphy JM. Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol. 2004;36:568-584. [PubMed] |
| 5. | Okamoto R, Yajima T, Yamazaki M, Kanai T, Mukai M, Okamoto S, Ikeda Y, Hibi T, Inazawa J, Watanabe M. Damaged epithelia regenerated by bone marrow-derived cells in the human gastrointestinal tract. Nat Med. 2002;8:1011-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 287] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 6. | Springer ML, Brazelton TR, Blau HM. Not the usual suspects: the unexpected sources of tissue regeneration. J Clin Invest. 2001;107:1355-1356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Zipori D. Mesenchymal stem cells: harnessing cell plasticity to tissue and organ repair. Blood Cells Mol Dis. 2004;33:211-215. [PubMed] |
| 8. | Valcz G, Krenács T, Sipos F, Patai AV, Wichmann B, Leiszter K, Tóth K, Balogh Z, Csizmadia A, Hagymási K. Lymphoid aggregates may contribute to the migration and epithelial commitment of bone marrow-derived cells in colonic mucosa. J Clin Pathol. 2011;64:771-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Sipos F, Muzes G, Valcz G, Galamb O, Tóth K, Leiszter K, Krenács T, Tulassay Z, Molnár B. Regeneration associated growth factor receptor and epithelial marker expression in lymphoid aggregates of ulcerative colitis. Scand J Gastroenterol. 2010;45:440-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Yen TH, Wright NA. The gastrointestinal tract stem cell niche. Stem Cell Rev. 2006;2:203-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 198] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 11. | Valcz G, Krenács T, Sipos F, Leiszter K, Tóth K, Balogh Z, Csizmadia A, Muzes G, Molnár B, Tulassay Z. The role of the bone marrow derived mesenchymal stem cells in colonic epithelial regeneration. Pathol Oncol Res. 2011;17:11-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Sipos F, Muzes G, Galamb O, Spisák S, Krenács T, Tóth K, Tulassay Z, Molnár B. The possible role of isolated lymphoid follicles in colonic mucosal repair. Pathol Oncol Res. 2010;16:11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Sipos F, Valcz G, Molnár B. Physiological and pathological role of local and immigrating colonic stem cells. World J Gastroenterol. 2012;18:295-301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Bhardwaj V, Cascone T, Cortez MA, Amini A, Evans J, Komaki RU, Heymach JV, Welsh JW. Modulation of c-Met signaling and cellular sensitivity to radiation: potential implications for therapy. Cancer. 2013;119:1768-1775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 15. | Kanda H, Tajima H, Lee GH, Nomura K, Ohtake K, Matsumoto K, Nakamura T, Kitagawa T. Hepatocyte growth factor transforms immortalized mouse liver epithelial cells. Oncogene. 1993;8:3047-3053. [PubMed] |
| 16. | Nakamura T, Sakai K, Nakamura T, Matsumoto K. Hepatocyte growth factor twenty years on: Much more than a growth factor. J Gastroenterol Hepatol. 2011;26 Suppl 1:188-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 360] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 17. | Sakai K, Aoki S, Matsumoto K. Hepatocyte growth factor and Met in drug discovery. J Biochem. 2015;157:271-284. [PubMed] |
| 18. | Tahara Y, Ido A, Yamamoto S, Miyata Y, Uto H, Hori T, Hayashi K, Tsubouchi H. Hepatocyte growth factor facilitates colonic mucosal repair in experimental ulcerative colitis in rats. J Pharmacol Exp Ther. 2003;307:146-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 78] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Kmiecik TE, Keller JR, Rosen E, Vande Woude GF. Hepatocyte growth factor is a synergistic factor for the growth of hematopoietic progenitor cells. Blood. 1992;80:2454-2457. [PubMed] |
| 20. | Beilmann M, Odenthal M, Jung W, Vande Woude GF, Dienes HP, Schirmacher P. Neoexpression of the c-met/hepatocyte growth factor-scatter factor receptor gene in activated monocytes. Blood. 1997;90:4450-4458. [PubMed] |
| 21. | van der Voort R, Taher TE, Keehnen RM, Smit L, Groenink M, Pals ST. Paracrine regulation of germinal center B cell adhesion through the c-met-hepatocyte growth factor/scatter factor pathway. J Exp Med. 1997;185:2121-2131. [PubMed] |
| 22. | Kurz SM, Diebold SS, Hieronymus T, Gust TC, Bartunek P, Sachs M, Birchmeier W, Zenke M. The impact of c-met/scatter factor receptor on dendritic cell migration. Eur J Immunol. 2002;32:1832-1838. [PubMed] |
| 23. | Okunishi K, Dohi M, Nakagome K, Tanaka R, Mizuno S, Matsumoto K, Miyazaki J, Nakamura T, Yamamoto K. A novel role of hepatocyte growth factor as an immune regulator through suppressing dendritic cell function. J Immunol. 2005;175:4745-4753. [PubMed] |
| 24. | Benkhoucha M, Santiago-Raber ML, Schneiter G, Chofflon M, Funakoshi H, Nakamura T, Lalive PH. Hepatocyte growth factor inhibits CNS autoimmunity by inducing tolerogenic dendritic cells and CD25+Foxp3+ regulatory T cells. Proc Natl Acad Sci USA. 2010;107:6424-6429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 171] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 25. | Beilmann M, Vande Woude GF, Dienes HP, Schirmacher P. Hepatocyte growth factor-stimulated invasiveness of monocytes. Blood. 2000;95:3964-3969. [PubMed] |
| 26. | Galimi F, Cottone E, Vigna E, Arena N, Boccaccio C, Giordano S, Naldini L, Comoglio PM. Hepatocyte growth factor is a regulator of monocyte-macrophage function. J Immunol. 2001;166:1241-1247. [PubMed] |
| 27. | Singhal E, Kumar P, Sen P. A novel role for Bruton’s tyrosine kinase in hepatocyte growth factor-mediated immunoregulation of dendritic cells. J Biol Chem. 2011;286:32054-32063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 28. | Schmidt C, Bladt F, Goedecke S, Brinkmann V, Zschiesche W, Sharpe M, Gherardi E, Birchmeier C. Scatter factor/hepatocyte growth factor is essential for liver development. Nature. 1995;373:699-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1076] [Cited by in RCA: 1034] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 29. | Uehara Y, Minowa O, Mori C, Shiota K, Kuno J, Noda T, Kitamura N. Placental defect and embryonic lethality in mice lacking hepatocyte growth factor/scatter factor. Nature. 1995;373:702-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 811] [Cited by in RCA: 790] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 30. | Ma H, Saenko M, Opuko A, Togawa A, Soda K, Marlier A, Moeckel GW, Cantley LG, Ishibe S. Deletion of the Met receptor in the collecting duct decreases renal repair following ureteral obstruction. Kidney Int. 2009;76:868-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 31. | Huh CG, Factor VM, Sánchez A, Uchida K, Conner EA, Thorgeirsson SS. Hepatocyte growth factor/c-met signaling pathway is required for efficient liver regeneration and repair. Proc Natl Acad Sci USA. 2004;101:4477-4482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 616] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 32. | Stephens P, Hiscox S, Cook H, Jiang WG, Zhiquiang W, Thomas DW. Phenotypic variation in the production of bioactive hepatocyte growth factor/scatter factor by oral mucosal and skin fibroblasts. Wound Repair Regen. 2001;9:34-43. [PubMed] |
| 33. | Schäfer M, Werner S. Cancer as an overhealing wound: an old hypothesis revisited. Nat Rev Mol Cell Biol. 2008;9:628-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 630] [Cited by in RCA: 703] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 34. | Kitamura S, Kondo S, Shinomura Y, Isozaki K, Kanayama S, Higashimoto Y, Minami T, Kiyohara T, Yasunaga Y, Ishikawa H. Expression of hepatocyte growth factor and c-met in ulcerative colitis. Inflamm Res. 2000;49:320-324. [PubMed] |
| 35. | Matsuno M, Shiota G, Umeki K, Kawasaki H, Kojo H, Miura K. Induction of plasma hepatocyte growth factor in acute colitis of mice. Inflamm Res. 1997;46:166-167. [PubMed] |
| 36. | Jiang WG, Martin TA, Parr C, Davies G, Matsumoto K, Nakamura T. Hepatocyte growth factor, its receptor, and their potential value in cancer therapies. Crit Rev Oncol Hematol. 2005;53:35-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 189] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 37. | Skibinski G, Skibinska A, James K. The role of hepatocyte growth factor and its receptor c-met in interactions between lymphocytes and stromal cells in secondary human lymphoid organs. Immunology. 2001;102:506-514. [PubMed] |
| 38. | Zhang J, Yang J, Liu Y. Role of Bcl-xL induction in HGF-mediated renal epithelial cell survival after oxidant stress. Int J Clin Exp Pathol. 2008;1:242-253. [PubMed] |
| 39. | Herrero-Fresneda I, Torras J, Franquesa M, Vidal A, Cruzado JM, Lloberas N, Fillat C, Grinyó JM. HGF gene therapy attenuates renal allograft scarring by preventing the profibrotic inflammatory-induced mechanisms. Kidney Int. 2006;70:265-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 40. | Giannopoulou M, Dai C, Tan X, Wen X, Michalopoulos GK, Liu Y. Hepatocyte growth factor exerts its anti-inflammatory action by disrupting nuclear factor-kappaB signaling. Am J Pathol. 2008;173:30-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 102] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 41. | Ortega-Cava CF, Ishihara S, Kawashima K, Rumi MA, Kazumori H, Adachi K, Kinoshita Y. Hepatocyte growth factor expression in dextran sodium sulfate-induced colitis in rats. Dig Dis Sci. 2002;47:2275-2285. [PubMed] |
| 42. | Hernandez J, Zarnegar R, Michalopoulos GK. Characterization of the effects of human placental HGF on rat hepatocytes. J Cell Physiol. 1992;150:116-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 43. | Naldini L, Weidner KM, Vigna E, Gaudino G, Bardelli A, Ponzetto C, Narsimhan RP, Hartmann G, Zarnegar R, Michalopoulos GK. Scatter factor and hepatocyte growth factor are indistinguishable ligands for the MET receptor. EMBO J. 1991;10:2867-2878. [PubMed] |
| 44. | Komada M, Miyazawa K, Ishii T, Kitamura N. Characterization of hepatocyte-growth-factor receptors on Meth A cells. Eur J Biochem. 1992;204:857-864. [PubMed] |
| 45. | Suh E, Traber PG. An intestine-specific homeobox gene regulates proliferation and differentiation. Mol Cell Biol. 1996;16:619-625. [PubMed] |
| 46. | Suh E, Chen L, Taylor J, Traber PG. A homeodomain protein related to caudal regulates intestine-specific gene transcription. Mol Cell Biol. 1994;14:7340-7351. [PubMed] |
| 47. | Beck F, Chawengsaksophak K, Waring P, Playford RJ, Furness JB. Reprogramming of intestinal differentiation and intercalary regeneration in Cdx2 mutant mice. Proc Natl Acad Sci USA. 1999;96:7318-7323. [PubMed] |
| 48. | Gross I, Duluc I, Benameur T, Calon A, Martin E, Brabletz T, Kedinger M, Domon-Dell C, Freund JN. The intestine-specific homeobox gene Cdx2 decreases mobility and antagonizes dissemination of colon cancer cells. Oncogene. 2008;27:107-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 82] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 49. | Zheng X, Tsuchiya K, Okamoto R, Iwasaki M, Kano Y, Sakamoto N, Nakamura T, Watanabe M. Suppression of hath1 gene expression directly regulated by hes1 via notch signaling is associated with goblet cell depletion in ulcerative colitis. Inflamm Bowel Dis. 2011;17:2251-2260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 115] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 50. | Coskun M, Olsen AK, Holm TL, Kvist PH, Nielsen OH, Riis LB, Olsen J, Troelsen JT. TNF-α-induced down-regulation of CDX2 suppresses MEP1A expression in colitis. Biochim Biophys Acta. 2012;1822:843-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 51. | Zheng J, Sun X, Wang W, Lu S. Hypoxia-inducible factor-1alpha modulates the down-regulation of the homeodomain protein CDX2 in colorectal cancer. Oncol Rep. 2010;24:97-104. [PubMed] |
| 52. | Giatromanolaki A, Sivridis E, Maltezos E, Papazoglou D, Simopoulos C, Gatter KC, Harris AL, Koukourakis MI. Hypoxia inducible factor 1alpha and 2alpha overexpression in inflammatory bowel disease. J Clin Pathol. 2003;56:209-213. [PubMed] |
| 54. | Ochicha O, Pringle JH, Mohammed AZ. Immunohistochemical study of epithelial-myofibroblast interaction in Barrett metaplasia. Indian J Pathol Microbiol. 2010;53:262-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 55. | Kabiri Z, Greicius G, Madan B, Biechele S, Zhong Z, Zaribafzadeh H, Edison J, Wu Y, Bunte R, Williams BO. Stroma provides an intestinal stem cell niche in the absence of epithelial Wnts. Development. 2014;141:2206-2215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 276] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 56. | Potten CS, Booth C, Tudor GL, Booth D, Brady G, Hurley P, Ashton G, Clarke R, Sakakibara S, Okano H. Identification of a putative intestinal stem cell and early lineage marker; musashi-1. Differentiation. 2003;71:28-41. [PubMed] |
| 57. | Montgomery RK, Breault DT. Small intestinal stem cell markers. J Anat. 2008;213:52-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 58. | Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3854] [Cited by in RCA: 4336] [Article Influence: 240.9] [Reference Citation Analysis (0)] |
| 59. | Piscaglia AC. Intestinal stem cells and celiac disease. World J Stem Cells. 2014;6:213-229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 60. | Bobryshev YV, Tran D, Botelho NK, Lord RV, Orekhov AN. Musashi-1 expression in atherosclerotic arteries and its relevance to the origin of arterial smooth muscle cells: histopathological findings and speculations. Atherosclerosis. 2011;215:355-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 61. | Nakata S, Phillips E, Goidts V. Emerging role for leucine-rich repeat-containing G-protein-coupled receptors LGR5 and LGR4 in cancer stem cells. Cancer Manag Res. 2014;6:171-180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 62. | Sun J, Zhang C, Liu G, Liu H, Zhou C, Lu Y, Zhou C, Yuan L, Li X. A novel mouse CD133 binding-peptide screened by phage display inhibits cancer cell motility in vitro. Clin Exp Metastasis. 2012;29:185-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 63. | Horst D, Kriegl L, Engel J, Kirchner T, Jung A. CD133 expression is an independent prognostic marker for low survival in colorectal cancer. Br J Cancer. 2008;99:1285-1289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 222] [Cited by in RCA: 256] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 64. | Tondreau T, Meuleman N, Delforge A, Dejeneffe M, Leroy R, Massy M, Mortier C, Bron D, Lagneaux L. Mesenchymal stem cells derived from CD133-positive cells in mobilized peripheral blood and cord blood: proliferation, Oct4 expression, and plasticity. Stem Cells. 2005;23:1105-1112. [PubMed] |
| 65. | Corbeil D, Röper K, Hellwig A, Tavian M, Miraglia S, Watt SM, Simmons PJ, Peault B, Buck DW, Huttner WB. The human AC133 hematopoietic stem cell antigen is also expressed in epithelial cells and targeted to plasma membrane protrusions. J Biol Chem. 2000;275:5512-5520. [PubMed] |
| 66. | Miraglia S, Godfrey W, Yin AH, Atkins K, Warnke R, Holden JT, Bray RA, Waller EK, Buck DW. A novel five-transmembrane hematopoietic stem cell antigen: isolation, characterization, and molecular cloning. Blood. 1997;90:5013-5021. [PubMed] |
| 67. | Shmelkov SV, Butler JM, Hooper AT, Hormigo A, Kushner J, Milde T, St Clair R, Baljevic M, White I, Jin DK. CD133 expression is not restricted to stem cells, and both CD133+ and CD133- metastatic colon cancer cells initiate tumors. J Clin Invest. 2008;118:2111-2120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 430] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 68. | Karim BO, Rhee KJ, Liu G, Yun K, Brant SR. Prom1 function in development, intestinal inflammation, and intestinal tumorigenesis. Front Oncol. 2014;4:323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 69. | Yasuda H, Tanaka K, Okita Y, Araki T, Saigusa S, Toiyama Y, Yokoe T, Yoshiyama S, Kawamoto A, Inoue Y. CD133, OCT4, and NANOG in ulcerative colitis-associated colorectal cancer. Oncol Lett. 2011;2:1065-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 70. | Lakatos L, Mester G, Erdelyi Z, David G, Pandur T, Balogh M, Fischer S, Vargha P, Lakatos PL. Risk factors for ulcerative colitis-associated colorectal cancer in a Hungarian cohort of patients with ulcerative colitis: results of a population-based study. Inflamm Bowel Dis. 2006;12:205-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 155] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 71. | Shmelkov SV, St Clair R, Lyden D, Rafii S. AC133/CD133/Prominin-1. Int J Biochem Cell Biol. 2005;37:715-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 276] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 72. | Strauss R, Hamerlik P, Lieber A, Bartek J. Regulation of stem cell plasticity: mechanisms and relevance to tissue biology and cancer. Mol Ther. 2012;20:887-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |