Published online Jul 28, 2015. doi: 10.3748/wjg.v21.i28.8541
Peer-review started: January 20, 2015
First decision: March 26, 2015
Revised: April 16, 2015
Accepted: May 20, 2015
Article in press: May 21, 2015
Published online: July 28, 2015
Processing time: 192 Days and 11 Hours
Benign hepatic tumors are commonly observed in adults, but rarely reported in children. The reasons for this remain speculative and the exact data concerning the incidence of these lesions are lacking. Benign hepatic tumors represent a diverse group of epithelial and mesenchymal tumors. In pediatric patients, most benign focal liver lesions are inborn and may grow like the rest of the body. Knowledge of pediatric liver diseases and their imaging appearances is essential in order to make an appropriate differential diagnosis. Selection of the appropriate imaging test is challenging, since it depends on a number of age-related factors. This paper will discuss the most frequently encountered benign liver tumors in children (infantile hepatic hemangioendothelioma, mesenchymal hamartoma, focal nodular hyperplasia, nodular regenerative hyperplasia, and hepatocellular adenoma), as well as a comparison to the current knowledge regarding such tumors in adult patients. The current emphasis is on imaging features, which are helpful not only for the initial diagnosis, but also for pre- and post-treatment evaluation and follow-up. In addition, future perspectives of contrast-enhanced ultrasound (CEUS) in pediatric patients are highlighted, with descriptions of enhancement patterns for each lesion being discussed. The role of advanced imaging tests such as CEUS and magnetic resonance imaging, which allow for non-invasive assessment of liver tumors, is of utmost importance in pediatric patients, especially when repeated imaging tests are needed and radiation exposure should be avoided.
Core tip: Focal liver lesions (FLL) are commonly observed in adults, but rarely reported in children. The reasons for this remain speculative. Most benign focal liver lesions are inborn and may grow like the rest of the body. The current paper deals with FLLs in pediatric patients, as well as a comparison to the current knowledge regarding such tumors in adult patients.
- Citation: Chiorean L, Cui XW, Tannapfel A, Franke D, Stenzel M, Kosiak W, Schreiber-Dietrich D, Jüngert J, Chang JM, Dietrich CF. Benign liver tumors in pediatric patients - Review with emphasis on imaging features. World J Gastroenterol 2015; 21(28): 8541-8561
- URL: https://www.wjgnet.com/1007-9327/full/v21/i28/8541.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i28.8541
Primary hepatic tumors are rare in children, where they account for about 5%-6% of all intra-abdominal masses and represent between 0.5% and 2.0% of all pediatric neoplasms[1]. They are a diverse group of epithelial and mesenchymal tumors, which constitute the third most common group of childhood solid abdominal tumors. The incidence is 0.4 to 1.9 per million children each year and can vary with patient age. Liver masses in children can be benign, malignant, or indeterminate. About one-third of pediatric primary liver masses are benign[2-11].
In the literature, the most frequently described benign liver tumors in the pediatric age group are: infantile hepatic hemangioendothelioma (IHH), mesenchymal hamartoma (MHL), focal nodular hyperplasia (FNH), nodular regenerative hyperplasia (NRH), and hepatic adenoma (HA)[12,13]. Other lesions are: hepatic cysts, hemangioma, benign lipomatous tumors (angiomyolipoma and lipoma), and benign biliary tumors (biliary cystadenoma, bile duct hamartoma or adenoma, and papillary adenoma). However, incidence data are derived from surgical studies, and so data concerning “true incidences” are lacking. So far, no data are available concerning the prevalence of hemangioma or cysts in screened, asymptomatic children.
The diagnosis of pediatric liver tumors is made on the basis of clinical features, serum α-fetoprotein (AFP) level, age of the child, and imaging characteristics. The role of imaging, like in adulthood, is to determine the organ of origin and the character and extent of the lesion[14,15]. Knowledge of pediatric liver diseases and their imaging appearances is essential in order to make an appropriate differential diagnosis[12]. Challenges exist for the non-invasive detection and characterization of focal liver lesions (FLLs) in the pediatric population. The selection of the appropriate imaging test depends on a number of factors, such as: (1) children require imaging strategies with a higher resolution due to smaller anatomic structures; (2) children may be unable to tolerate or hold still for an imaging test, so they may require sedation or anesthesia; and (3) the use of imaging tests with ionizing radiation should be minimized given that children are more sensitive to the long-term effects of radiation exposure than adults[16].
Differentiation of masses is still complex, and biopsy or resection for histological diagnosis sometimes becomes necessary[17-21]. The incidence of complications after percutaneous liver biopsy in pediatric patients was 6.83%, of which 2.4% were major complications, as reported by Scheimann et al[22].
This paper will discuss benign liver tumors in children, with an emphasis on imaging features and future perspectives of contrast-enhanced ultrasound (CEUS) in pediatric patients (Table 1).
| US | Computed tomography | Magnetic resonance imaging |
| Infantile hepatic hemangioendothelioma | ||
| 2D | Pre-contrast examination | T1-weighted images |
| Hypoechoic1 | Hypodense1, well-defined | Hypointense1 |
| Doppler US | Post-contrast examination | ± hyperintense foci2 |
| Enlarged hepatic arteries and veins, shunts | Intense, homogeneous enhancement3/peripheral enhancement4 with progressive centripetal filling-in pattern | T2-weighted images |
| CEUS | Hyperintense | |
| Peripheral enhancement, with centripetal filling-in | Enhancement pattern | |
| Centripetal filling-in | ||
| Mesenchymal hamartoma | ||
| 2D | Cystic components | Cystic components |
| Cystic and solid5 components in various amounts6 | Water attenuation | T1-weighted images |
| Thin/thick and mobile septa | Non-enhancement | Variable signal intensity on (depending on the protein content) |
| Parietal nodules | ||
| Rare features7 | T2-weighted images | |
| High signal intensity | ||
| Doppler US | Solid components | Solid components |
| Linear blood flow within solid portions and septa | Hypodense | Hypointense on both T1- and T2-weighted images (fibrosis) |
| Surrounding portal and hepatic veins may be displaced | Post-contrast enhancement | |
| CEUS | Post-contrast mild enhancement (and within the septa) | |
| Solid component may present arterial and portal venous enhancement | ||
| Focal nodular hyperplasia | ||
| 2D | Pre-contrast examination | T1-weighted images |
| Well-circumscribed | Isodense/slightly hypodense | Isointense/slightly hypointense to the liver |
| Variable echogenicity | Hypoattenuating central scar | Hypointense central scar |
| Stellate hyperechoic central scar | Arterial and early portal phases | T2-weighted images |
| Doppler US | Homogeneous enhancement, earlier and more intense than the surrounding liver parenchyma | Isointense/slightly hyperintense |
| Spoke-wheel pattern | Hypoattenuating central scar | Hyperintense central scar |
| CEUS | Late portal and delayed phases | Arterial phase |
| Arterial and portal venous enhancement | Isoattenuating to the liver | Hyperintense lesion |
| Delayed enhancement of the central scar | Non-enhanced central scar | |
| Portal venous phase | ||
| Slightly hyperintense/isointense | ||
| Delayed phase | ||
| Enhancement of the central scar | ||
| Nodular regenerative hyperplasia | ||
| 2D | Pre-contrast examination | T1-weighted images |
| Multiple, tiny, well-circumscribed, hypo-/isoechoic nodules8 | Hypo-9/isoattenuating to the liver | Slightly hyperintense |
| ± sonolucent rim | Post-contrast images | ± foci of high signal intensity11 |
| CEUS | Nodules do not enhance9 | ± hyper-/hypointense rim |
| Arterial and portal venous enhancement similar to the surrounding liver parenchyma | Diffuse or peripheral rim-like enhancement10 | Fat suppressed images decreased signal intensity12 |
| T2-weighted images | ||
| Iso-/hypointense | ||
| ± hyperintense rim | ||
| Enhancement pattern | ||
| Similar to normal liver parenchyma13 | ||
| Hepatic adenoma | ||
| 2D | Pre-contrast examination | T1- and T2-weighted images |
| Heterogeneous | Hypoattenuating, sharply delineated spherical mass ± pseudocapsule | Heterogeneous, predominantly hyperintense20 |
| Hyperechoic14/hypoechoic15/cyst-like16 | Heterogeneous content17 | In-phase and out-of-phase T1 sequence |
| Doppler US | Arterial phase | Signal loss (fatty components) |
| Central vessels with a triphasic pattern/continuous venous waveform | Homogeneous18/heterogeneous enhancement19 | Enhancement pattern |
| CEUS | Portal venous and delayed phases | Early arterial enhancement |
| Only arterial enhancement as the distinctive feature to FNH | Isoattenuating/rapid “wash-out” | Isointense during portal and venous phases21 |
| Hepatocyte-specific contrast agents | ||
| Hypointense on hepatocyte phase imaging | ||
US is the least costly and most widely used method for evaluating children with liver tumors. It can provide real-time assessment without ionizing radiation, and it is often the first imaging modality of choice because of its wide availability and because it offers quick, non-invasive evaluation of the liver parenchyma. US examination accurately excludes a mass when it is not present or, if the mass is present, it evaluates whether it is cystic or solid, as well as assessing the vascular flow through the use of Doppler technique. The size, number, and appearance of FLLs can be readily determined, narrowing the differential diagnosis. Moreover, it helps to evaluate hepatic and portal venous involvement[16,23-26]. US-guided liver biopsy in children is a procedure with a low rate of major complications and a high rate of minor bleeding that does not require intervention[27].
Although CEUS has higher diagnostic efficacy in FLLs than baseline US, its use is still off-label in children. Given that CEUS is a candidate for consideration as the primary imaging tool for FLLs assessment in adults (with documented safety issues)[28], it should therefore be considered as a primary modality of choice in children as well[28,29]. However, to date there is little data available concerning the use of CEUS in the pediatric age group. Although some magnetic resonance imaging (MRI) and computed-tomography (CT) contrast medias are available for children, the remainder are also off-label (e.g., liver specific MR contrast media)[24].
CT depicts liver lesions and their involvement with adjacent structures with excellent spatial resolution in adults and older children, giving improved anatomic location and FLL characterization[30]. Multiphase contrast-enhanced CT improves diagnostic specificity by further characterizing liver lesions, albeit at the expense of increased radiation exposure[16]. The potential risks of radiation exposure have to be especially considered in children[31-34]. The need for sedation is decreased due to shorter imaging times[35,36].
MRI contrast agents that preferentially target the liver may be helpful in characterizing liver masses in select children. The imaging approach is non-invasive, radiation free, relatively rapid to perform, and provides anatomical and functional information[16].
Off-label use raises controversies about access to innovations, as well as pharmacovigilance and liability. The SHI Modernization Act internationally took a pioneering role by introducing an expert committee to clarify rules for off-label use in Germany. Two new instruments were introduced in 2006: Annex 9 defines drugs eligible for off-label-use and Annex 10 lists drugs with a prescription ability following the assessment of the IQWiG (Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen; Institute for Quality and Efficiency in Health Care). The judging committee is affiliated to the Federal Institute of Pharmaceuticals and Medical Devices and consists of nominated representatives from the Institute, from scientific medical societies, physicians’ associations, manufacturers, sickness funds, SHI medical review boards, representatives of pharmacists, and patient interest groups. Based on a jurisdiction from the Federal Social Court on the criteria for access to off-label use drugs, the committee started with defining rules and conditions for the prescription and SHI-financing of oncological medications that are not yet licensed for the required indication. Despite these efforts, there are still many problems not yet solved, such as CEUS. CEUS has a number of distinct advantages over CT and MRI. It can be performed immediately, without any preliminary laboratory testing, and can be carried out in a variety of scenarios (e.g., bedside, operatory room, and CT suite). It can be applied independently of renal function and operates in real time, so that rapid changes can be captured without radiation exposure[37].
US Contrast Agents registered in Europe are licensed only for cardiac or, in the case of SonoVue®, liver and vascular applications. SonoVue® is safe and effective for the examination of almost all organs, and has published European guidelines[38-41]. Published clinical recommendations on the use of CEUS are based on comprehensive literature surveys, including results from prospective clinical trials. Despite this, many indications are still off-label[37-41].
The current legal requirements for registration of pharmaceutical products in Germany and the rest of Europe are strict. In order for a new indication to be registered, the manufacturer must provide data on its safety and efficacy, with a dedicated phase III trial specifically designed to achieve registration approval. Diagnostic agents, including contrast microbubbles, are not exempt to this rule, which is designed to protect patients from the misuse of drugs or diagnostic agents, but, on some occasions, may limit the potential benefits to patients. In fact, applications for indications do not only follow clinical or scientific needs, but also the financial expectations of the producer (and health care funders)[37,42].
Off-label use is of utmost importance in pediatrics because many drugs are not tested by randomized trials in children, which also means that they are not specifically licensed for use in children. Licensed drugs are often prescribed outside the terms of the product license (off-label) in relation to age, indication, dose frequency, route of administration, or formulation. Over two thirds (67%) of 624 children admitted to wards in five European hospitals received drugs prescribed in an unlicensed or off-label manner and 39% of the 2262 drug prescriptions given to children were off-label. Thus, licensed drugs for adults may only be used in children after the parents (or legal representatives) have been adequately informed and specific consent has been obtained (except in cases of emergency). To be able to use any medication or agent routinely in children, a dedicated trial demonstrating its efficacy and safety has to be performed. Until this happens the agent will remain for off-label use only, with all the procedures this involves. Unfortunately, these rules, which are designed to protect patients, may on some occasions limit the potential benefit to patients if, for instance, an operator refuses to perform off-label indications. This may clash with clinical need[37,42].
Generally, drugs not licensed at all for the German pharmaceutical market or not licensed for the respective indication may not be prescribed by any physician except under clinical trial conditions or individual clinical advice. Sickness funds may not fund clinical research and basically may not cover prescriptions of unlicensed drugs or unlicensed indications. Only a time-consuming and individual clarification process with the sickness fund may offer a solution[37,42].
Infantile hepatic hemangioendothelioma (IHH) represents a relatively common liver tumor in children, accounting for 12% of all pediatric liver tumors in surgical studies. About half of cases occur as solitary masses, with the other half being multifocal[7,12,43,44]. All races are affected, with an increased risk for fair-skinned individuals. Female infants are three times more likely to develop IHH than male infants[45]. There is an increased prevalence in patients with hemi-hypertrophy and Beckwith-Wiedemann syndrome[46].
Approximately one third of cases are detected in the first month of life, while less than 5% of cases are diagnosed beyond 1 year of age. They have a peak presentation at 6 months of age, with hepatomegaly or an abdominal mass[7,18,47-52]. The natural course is typical, with presentation shortly after birth, rapid proliferation in the first year of life, and spontaneous involution over a period of years[53].
AFP levels are usually within normal reference ranges for age. Some cases with increased serum levels have been reported, possibly due to the entrapping of hepatocytes within the IHH tumor[54-56].
IHH were subdivided into well-demarked and infiltrative growth patterns. Histologically, two types of IHH exist. Type I IHH is composed of proliferating small capillary-like vessels (bloodless or dilated) lined by bland or plump endothelial cells[57]. Vascular channels are separated by variable connective tissue. In some cases, extramedullary hematopoiesis is present. Involution and regression with thrombosis and fibrosis with calcification are common features. The histopathological characteristics of type II IHH are equivalent to those found in angiosarcomas with aggressive behavior.
Previously grouped together as infantile hepatic hemangioendothelioma, pediatric benign hepatic vascular tumors have recently been shown to be at least two different lesions: hepatic infantile hemangioma and congenital hepatic vascular malformation with associated capillary proliferation (HVMCP). The first type (infantile or juvenile hemangioma) often presents as multiple masses (then referred to as hemangiomatosis), and usually involutes and regresses. If the neonatal hemangiomatosis is diffuse, including skin and liver manifestations, the prognosis is seriously worse due to an increased risk of hemorrhage[58]. The other type has been called a vascular malformation. GLUT1 endothelial reactivity distinguishes the two entities histologically: hepatic infantile hemangiomas stain positively for GLUT1, whereas the hepatic vascular malformations do not exhibit GLUT1 immunoreactivity[59].
US: US examination has an important role in the detection and localization of liver masses, as well as in follow-up[57]. IHH, presenting as a hypoechoic liver mass, may be detected at prenatal US, along with polyhydramnios. Postnatal US appearance of solitary IHH is that of a predominantly round and hypoechoic mass, sometimes with an inhomogeneous structure due to tiny echogenic foci having posterior acoustic shadowing and representing calcifications (seen in up to 36% of cases). Inhomogeneity may also be due to hemorrhage, necrosis, or fibrosis, in cases of larger lesions. In cases of diffuse disease, an enlarged liver may be the only sonographic finding[12,51,60].
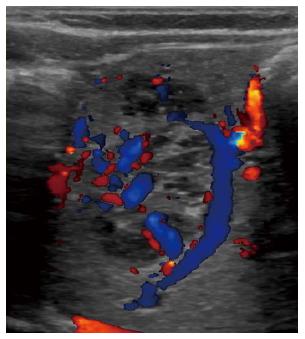
The color and spectral Doppler analysis of IHH demonstrates a variety of flow patterns, with enlarged intralesional arteries and veins, direct arteriovenous or portovenous shunts, and large feeding and draining vessels[57,60]. Kassarjian et al[60] showed abnormal color flow patterns of IHH in 60% of patients and the presence of shunting was confirmed in 44%. Paltiel et al[57] studied 13 children with IHH and revealed that the range of the peak Doppler shift overlapped with those previously reported in the literature for malignant liver tumors. Follow-up of the treated lesions demonstrated a decrease of flow velocity in the feeding arteries and resolution of arteriovenous shunts, which was also associated with a decrease in the size of the masses[57,61].
Apart from IHH, hypoechoic infantile hemangiomas are most commonly detected incidentally. They present as multiple masses throughout the liver (hemangiomatosis) and are often associated with multiple cutaneous hemangiomas (> 5). The hyperechoic appearance of adult hemangioma is uncommon in young children[12] (Figure 1).
CEUS: Tumor-specific vascularization patterns such as a nodular peripheral enhancement and partial or complete centripetal fill-in pattern in hemangiomas could be assessed in the majority of lesions, but not all[62]. It is vital for diagnosis that the nodules are hyperenhanced during all phases and that bubble destruction is avoided[63].
CT: CT has been the main diagnostic tool in the past, but this is no longer true due partially to its diminished resolution in infants and small children, but mainly due to significant radiation exposure[31]. In patients with IHH, CT shows a well-defined hypodense liver mass on non-enhanced examination, sometimes with speckled calcifications (in up to 50% of cases). After contrast agent administration, IHH enhances in a similar way with adult hemangiomas: peripheral enhancement during arterial time, with progressive centripetal fill-in demonstrated during portal and venous phases. On delayed enhanced images, IHH shows a characteristic persistent enhancement, which is a distinct feature compared with other liver tumors. If the mass is large with central hemorrhage, necrosis, or fibrosis, enhancement will be inhomogeneous. Small multifocal lesions enhance intensely and homogeneously. The appearance may be mistaken for metastases. Diffuse involvement presents with innumerable lesions with centripetal enhancement[12,23,51,60,61,64-66].
MRI: IHH are generally hypointense on pre-contrast T1-weighted images, sometimes with hyperintense foci reflecting hemorrhage[51]. T2-weighted and gradient-echo sequences, as well as dynamic gadolinium-enhanced imaging, allow confident diagnosis[60,67,68]. On T2-weighted sequence, IHH has a similar appearance to that of adult hemangiomas, being markedly hyperintense due to its vascular nature. Larger lesions may be inhomogeneous due to hemorrhage, necrosis, central thrombosis, or fibrosis, while smaller lesions usually have homogeneous signal intensity. The enhancement pattern is similar to that seen on contrast-enhanced CT, with a centripetal fill-in pattern[51,60,61].
Arteriography is currently reserved for patients with complications from arteriovenous shunts in whom the use of embolization is considered[12,51,67].
Scintigraphy, both with technetium 99m (99mTc)-labeled sulfur colloid and 99mTc-tagged red blood cells, was previously considered but that is no longer the case, showing the lesion as a cold spot because of a lack of Kupffer cells within the tumor[12,51,69,70].
US, associated with share wave elastography, is an additive tool to characterize hemangiomas and differentiate liver hemangiomas from malignant liver tumors in the pediatric population[71].
The treatment algorithm is based on imaging features and on the presence or absence of complications[72]. The prognosis of lesions is dependent on size and the effect on heart function, and is usually good if heart failure is managed successfully in cases of lesions with a high shunt volume. Spontaneous regression is frequent, but death may still occur within the first 6 mo of life because of cardiac failure or replacement of normal hepatic parenchyma[70,73,74].
Although IHH is usually a benign lesion, it may show malignant potential, with cases of malignant sarcomatous transformation being reported[6,64,68,75-78]. Histology was not proved to be predictive of malignant potential, although older children are usually considered to be at higher risk. This points to the need for regular evaluation of this tumor until complete regression[79].
Mesenchymal hamartoma of the liver (MHL) is the second most common benign hepatic tumor in children according to surgical studies, accounting for 8% of all pediatric hepatic tumors[13,80,81]. The rate in imaging experience is much lower. The term ‘mesenchymal hamartoma’ was introduced by Edmondson in 1956, describing a cystic mesenchymal lesion, chiefly composed of connective tissue and containing much serous fluid[82]. Before, it had been described in the literature under various names, including pseudocystic mesenchymal tumor, giant cell lymphangioma, cystic hamartoma, bile cell fibroadenoma, hamartoma, and cavernous lymphangiomatoid tumor[19]. It is a rare benign tumor, usually presenting within the first 2 years of life (80% of cases), with a median age at presentation of 10 mo. Nearly all lesions (95% of cases) are diagnosed by the age of 5 years[80,81]. Cases of MHL in fetus have been reported[83], with rare cases in adults also being reported[84,85]. The male to female ratio is 3:2[64,80,81]. The right lobe is affected more frequently than the left one (6:1). Sometimes it may be pedunculated, with a thin or thick pedicle[13,80,81]. The tumor is often large, with Kim et al finding tumors to be larger than 10 cm in 85% of patients[86].
The etiology is uncertain. Typically, MHL are not associated with other anomalies, but associations with congenital heart disease, gut malrotation, omphalocele, myelomeningocele, Beckwith-Wiedemann syndrome, biliary atresia, and abnormalities of chromosome 19 have been reported[21,87,88]. A rare case of a giant MHL in a neonate, associated with malrotation of bowel, has recently been presented in the literature[89].
Abdominal swelling with or without a large, firm, and smooth abdominal mass is usually the predominant clinical feature. Abdominal pain, anorexia, vomiting, fever, constipation, diarrhea, and poor weight gain or weight loss have also been reported. Being a space occupying lesion, if the mass is very large it may compress adjacent organs, resulting in various complications, such as ascites, jaundice, or even congestive heart failure[10,21,44,81,82,90-101]. Visible engorged veins over the anterior abdominal wall and lower limb edema due to inferior vena cava compression may sometimes be noticed[19,21]. Respiratory distress may be caused by upward shift on the diaphragm[10,19].
No specific panel of laboratory tests is characteristic of MHL[102]. Although liver function tests usually remain within normal limits, liver enzymes may be moderately elevated[21,103]. AFP is usually within normal ranges[85,95,104,105], but may be also mildly elevated, presumably originating from the peripheral hepatocyte component of the lesion[56,95,105-109]. If serum AFP level is elevated at diagnosis, its level will decrease to normal after complete resection of the tumor[21,95,101].
Microscopically, MHL consists of spindle cells in a myxoid background, with occasional areas of extramedullary hematopoiesis, all in a disordered arrangement of mesenchyme, malformed bile ducts, and cords of normal-appearing hepatocytes. It has a porous nature which permits accumulation of fluid within cystic spaces, leading to tumor enlargement of up to 30 cm[21,81,84,99,110,111]. If this happens rapidly, it may cause respiratory distress[112-114]. Cytogenetically, these tumors are characterized by translocations involving 19q13.4.
Depending upon the amount and nature of the myxoid stroma, it may present as a cystic, mixed, or solid mass.
The imaging appearance of MHL depends on its pathologic appearance and constitutes a wide spectrum, from a predominantly cystic, avascular mass with thin or thick septa, to a predominantly solid (stromal or mesenchymal), hypovascular mass containing only few small cysts[110].
US: Prenatal US usually detects MHL during the last trimester of pregnancy (mean gestational age: 35 wk)[50]. It is associated with rising AFP or human chorionic gonadotropin levels in the maternal serum and polyhydramnios[21,50,83,115]. The most commonly described presentation was that of a fetal abdominal cystic mass, of which the organ of origin can be hard to determine[50,116].
On post-natal US, MHL has a wide spectrum of sonographic features depending on whether it is in its cystic, mixed, or solid form. The cystic form presents as a well-defined multicystic mass. The cysts are anechoic or nearly anechoic, and are variable in size, separated by thin or thick echogenic and mobile septa. Sometimes, internal debris with fluid-fluid level inside the cysts may be seen, as well as low-level echoes within the fluid, presumably reflecting gelatinous contents. US findings of round hyperechoic parietal nodules within the cystic spaces are specific for MHL. In the mixed form, variable amounts of solid components are also noted, being usually isoechoic to the liver and minimally vascularized. The mixed tumors are formed by multiple tiny anechoic areas with posterior enhancement and intervening isoechoic to hyperechoic solid tissue, leading to a sieve-like appearance at high-resolution US. Portions with very small cysts may appear completely solid at US. When the solid component is much better represented, the septa between the cysts may be irregularly thickened, and the solid portion may present with heterogeneous hyperechogenicity. Few cases of MHL appearing as a well-defined homogeneous echogenic mass have been described. The surrounding liver parenchyma is normal, but the surrounding portal and hepatic veins may be displaced. Color and power Doppler may detect relatively little, with linear blood flow limited to the solid portions and septa. A true capsule is generally not present, and the tumor can grow to a large size[12,21,107,117-121]. Calcification, significant necrosis, and bleeding into the lesion are rare[21,64,86].
CEUS: CEUS shows isoenhancement in the non-cystic parts of the lesion in all phases[122]. The cystic parts are non-enhancing.
CT: The CT appearance of MHL is that of a complex cystic mass, with cystic components of water attenuation being unenhanced after contrast agent administration. Stromal components hypoattenuate to the surrounding liver parenchyma, and, after administration of iodinated intravenous contrast material, the solid components and the septa present enhancement[12,64,110,123].
MRI: The amount of cystic versus stromal components and the protein content of the fluid cysts are factors that influence the MRI features of MHL. The cystic portions present high signal intensity on T2-weighted images, and variable signal intensity on T1-weighted images, depending on the protein content. Owing to fibrosis, solid portions may appear hypointense to the adjacent liver parenchyma on both T1- and T2-weighted images. After gadolinium injection, solid parts may show mild enhancement, as well as enhancement within the septa[15,44,64,110,123].
Differential diagnosis enrolls entities with varying amounts of solid and cystic components[124]. The cystic form of MHL must be distinguished from hydatid cyst, IHH, hepatoblastoma, and biliary rhabdomyosarcoma, which can all overlap considerably in their appearance[124,125]. Cases of cystic MHL mimicking a hydatid cyst have been described in the literature[103], with some children being inappropriately treated for presumed hydatid disease[94,124]. The differential diagnosis of the solid form of MHL includes various hepatic tumors, such as hepatoblastoma, hepatocellular carcinoma (HCC), IHH, HA, and FNH[86].
Some imaging features of MHL are useful elements for differential diagnosis: the absence of a capsule, a multi-septated cystic appearance (rarely seen in other pediatric hepatic tumors, and the absence of intratumoral calcifications, which were very rarely reported in MHL, but can be frequently detected in hepatoblastomas (over 50%) or IHHs (up to 40%)[44].
Diagnosis is typically made during infancy, sometimes incidentally, and the prognosis is excellent. Complete resection, as an anatomic hepatic lobectomy or non-anatomically with a rim of normal tissue, is curative in most cases, and long-term follow-up is satisfactory[10]. In cases considered unresectable, surgical options include enucleation and marsupialization[8,19,102,126]. Very rarely, liver transplantation may be needed[21]. Recently, laparoscopic liver resection for MHL has been reported with successful results[127]. Since recurrence and malignant transformation have been rarely observed, careful follow-up is warranted[128].
Focal nodular hyperplasia (FNH) is defined as a nodule composed of benign-appearing hepatocytes (“focal liver cirrhosis”) with bile duct proliferations and vascular anomalies occurring in healthy liver parenchyma[129,130]. The term FNH was introduced by Edmondson in 1958[131]. In the general population, these types of ductal plate malformations are commonly observed and diagnosis is feasible using CEUS. However, surgery for FNH is rarely indicated.
Pediatric cases of FNH are unusual, accounting for only 15% of all reported cases of FNH and representing 2% to 7% of all pediatric hepatic tumors and 0.02% of all pediatric tumors[11,13,132-134]. FNH has been reported in all pediatric age groups, including early childhood, prenatal, and neonatal periods, but there is an age prevalence in 7-8 year-old children[135-139]. There is a female predominance in the pediatric age group, as is the cases in adults as well[11,12,133,140,141]. In a large series of 172 cases of pediatric FNH, Lautz et al[142] found that 66% (113/172) of cases occurred in females. Multiple FNH lesions are found in about one third of cases[132].
The pathogenesis of FNH is largely unknown. Evidence of polyclonality in different DNA studies excludes a neoplastic lesion nature and further supports the hypothesis of a possible reactive hyperplastic response of liver cells to local vessel abnormalities[143]. Vascular malformations, such as hypoplasia or agenesis of the portal vein, vascular dysplasia, Budd-Chiari syndrome, hereditary hemorrhagic telangiectasia, and vascular injuries, such as thrombosis, vasculitis, intimal hyperplasia, high sinusoidal pressure, or increased flow after pediatric oncological therapy, have all been suggested as the underlying mechanism[144-150].
The majority of FNHs are asymptomatic[132,133], with only 20%-36% of cases showing symptoms, as reported by different authors[140,142]. When present, the clinical manifestations of FNH in children are non-specific, with the commonest being that of a palpable abdominal mass associated or unassociated with abdominal pain[11,12]. Lautz et al[142] found that pediatric FNH patients with a history of malignancy were significantly less likely to be symptomatic, had lesions much smaller in size, and fewer patients required resection of the lesion as compared with patients without a malignancy history. It also seems that pediatric patients with a history of malignancy are more likely to have multiple FNH nodules and less likely to have central scars, while pediatric patients without a history of malignant lesions are more likely to have FNH lesions with central scars[151-153].
Laboratory testing results often do not show clinical significance, and tumor markers, such as AFP, are usually within normal ranges for age[12,140,154,155].
FNH of the liver is a non-neoplastic lesion characterized by three classical histological findings: abnormal architecture (“focal cirrhosis”), bile duct proliferation, and malformed vessels[129]. Portal triads are lacking. The diameter of the lesion is extremely variable, from less than 1 cm to more than 15 cm, but is usually less than 5 cm and located near the liver surface[64,135]. The central scar contains thick fibrous areas with large vessels showing dysplastic features (e.g., fibromuscular hyperplasia). Atypical hepatocytes and atypical mitotic figures are lacking. In the literature, some subtypes of FNH are described (e.g., the telangiectatic form with multiple dilated vascular channels in the center of the mass that is currently handled as hepatocellular adenoma). However, these different types may not have any clinical consequences.
US: The classical appearance of FNH on US is that of a homogeneous, well-circumscribed mass, with variable echogenicity, central or eccentric vascular supply, and a typical stellate hyperechoic central scar. Calcifications are rare[12,19,132,135,140,156]. On color and power Doppler sonography, increased blood flow in the central scar extending to the periphery in a spoke-wheel pattern may be seen in 50% of cases, with the flow being predominantly arterial, with high speed and low resistance.
CEUS: A predominant arterial and portal venous enhancement pattern is typical in 96% of cases[63,122,123,135,157-162].
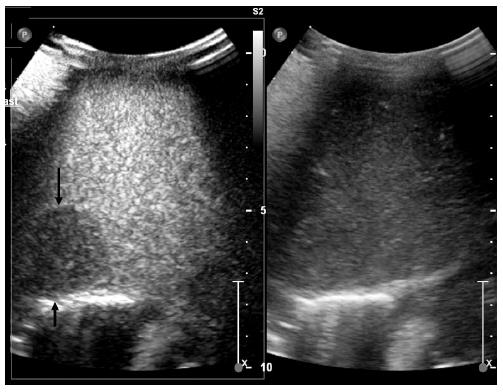
Tumor-specific vascularization patterns, such as a wheel-spoke pattern and homogeneous arterial hyperenhancement followed by isoenhancement in the portal and late venous phases in FNH, or a nodular peripheral enhancement and partial or complete fill-in pattern in hemangiomas, could be assessed in the majority of lesions, but not all[62]. Differentiation from a malignant lesion can be easily made by the use of CEUS, since malignant tumors present with hypoenhancement during the portal and/or late venous phases (Figures 2 and 3).
CT: On an unenhanced CT scan, FNH has a typical appearance of a well-circumscribed mass with a lobular outline, is isodense/slightly hypodense to the surrounding liver parenchyma, and has a hypoattenuating central scar. It has a typical homogeneous appearance, which is a very helpful feature for distinguishing FNH from malignant tumors, which are more likely to appear heterogeneous due to hemorrhage, necrosis, and calcifications. After contrast agent injection, the lesion exhibits a characteristic pattern of enhancement. It typically enhances homogeneously in the arterial and early portal venous phases, earlier and more intense than the adjacent parenchyma due to its arterial supply, and becomes isoattenuating to the liver in the late portal venous and delayed phases. The stellate scar is typically hypoattenuating on early contrast-enhanced images and demonstrates enhancement on delayed images. An enhancing artery may be seen within the hypoattenuating scar on arterial phase images. In cases of atypical appearances, such as the absence of a central scar, rapid washout of contrast material in the portal venous phase, lack of enhancement of the central scar on delayed images, early draining veins, and partial peripheral rim-like enhancement on delayed images, a biopsy is mandatory in order to establish the correct diagnosis[12,123,156,161,163-166]. The need for acquisitions in several phases, with a consequently high dose of radiation, raises questions as to whether this is an appropriate diagnostic method in children[44].
Due to its pathological features, mostly being composed of hepatocytes, FNH may be unapparent on conventional US or unenhanced CT, except for a potential mass effect on adjacent structures. In these cases, the presence of the central scar is a very useful diagnostic tool. The presence of the central scar is also a useful diagnostic sign in patients with a solidary hypervascular lesion and a history of malignancy. In these cases, liver metastasis must be ruled out, but since most metastases do not exhibit a central scar, identifying this feature may be of great diagnostic help[163,167].
MRI: On MRI images, FNH typically appears homogeneous and isointense/slightly hypointense to the liver on T1-weighted images and isointense/slightly hyperintense on T2-weighted images. The central scar is hypointense on T1-weighted images and hyperintense on T2-weighted images (in 75% of cases) owing to edema within the myxomatous tissue of the scar. After gadolinium administration, the mass enhances homogeneously, being hyperintense to the liver in the arterial phase. During the portal venous phase, the mass may rest slightly hyperintense to the liver, or it may become isointense. The central scar, in general, demonstrates enhancement during the delayed phase. The presence of a hyperintense T2-weighted central scar with delayed enhancement is particularly helpful in distinguishing FNH from fibrolamellar carcinoma, which also demonstrates a central scar but with different features (hypointense on T2-weighted images due to its collagenous content and does not enhance during delayed images)[12,64,123,161,168-171]. MRI with hepatobiliary-specific contrast agents is an excellent diagnostic choice for characterizing FNH, as it usually allows the separation of FNH from other liver lesions on MRI. FNH contains normal hepatocytes, which readily take up hepatobiliary-specific contrast agents, but because of the malformed bile ducts present in FNH the hepatocytes fail to excrete it. Thus, FNH lesions readily enhance on the arterial phase of imaging and continue to enhance for an extended period, remaining high in signal intensity long after other liver lesions have washed-out (compared with the normal surrounding liver parenchyma). This allows a confident differential diagnosis between FNH and metastases, or other malignant tumors[16,172-174].
CEUS and MRI are extremely reliable for the differentiation of benign and malignant lesions and for the diagnosis of liver hemangiomas and FNHs[175].
The scintigraphic appearance of FNH is characteristic, with 99mTc sulfur colloid imaging presenting normal uptake in 60%-75% of lesions, owing to the presence of Kupffer cells. This feature is very helpful in distinguishing FNH from HA and malignant tumors[19,161,176].
The natural history of FNH is characterized by the absence of malignant transformation. Complications such as tumor rupture, necrosis, and hemorrhage are rare[11,12,64,140,177-182]. The mass remains stable in about 2/3 of cases, while in about 1/3-1/4 of cases it may show gradual spontaneous improvement that can even go as far as complete remission. An increase in number or size is rare[183,184]. Di Stasi et al[185] detected a reduction in size or complete resolution of FNH in 50% of patients that were followed-up by US for a mean period of 33 mo.
If a confident diagnosis of FNH can be made using imaging methods, no treatment is needed in asymptomatic patients. Cases will be followed-up with serial US every 6-12 mo. In patients with clinical, biochemical, or imaging features that are not typical of FNH, a histologic diagnosis is necessary. Only cases of voluminous masses with tumor enlargement or which will become symptomatic will be considered for resection[11,132,147,154,178,181,186-188]. Recurrences are very rare after surgical removal[135]. Surgical treatment will also be considered in pediatric patients in whom malignancy cannot be ruled out confidently. In order to avoid unnecessary surgical resection, it is important to differentiate by imaging FNH from other FLLs[12,142,189,190].
Nodular regenerative hyperplasia (NRH) is a rare benign process of the liver, first defined by Steiner in 1959. The normal hepatic architecture is entirely replaced by small diffuse regenerative nodules of hepatocytes surrounded by atrophic liver, in the absence of fibrosis. The nodules vary in size, from a few millimeters to several centimeters. NRH is considered a major cause of portal hypertension in young, non-cirrhotic patients[12,191-196].
It occurs predominantly in adult patients between 25 and 60 years of age[191,197], with rare cases in children and even fetuses[130,140,197-207]. Stocker demonstrated in his study that, of a large series of 716 pediatric tumors, NRH cases represented 4.5% of cases and 2.1% of liver tumors from birth to two years of age[13]. From the age of 5 to 20 years, it is the 4th most common liver tumor in children after HCC, FNH, and undifferentiated embryonal sarcoma[202].
The vascular hypothesis considers microcirculatory disturbances, related either to lesions of small intrahepatic venous or arterial vessels, or to primary alterations of the sinusoidal wall, with consecutive obliteration or thrombus, as being the basic pathologic lesion. Vascular disorders lead to successive episodes of atrophy, followed by compensatory regeneration. This theory has been also considered as the underlying cause of other benign nodular lesions, such as FNH. However, the precise mechanisms by which circulatory disturbances could cause these lesions have not yet been elucidated.
The disease may be idiopathic, but associations with various systemic diseases or cytotoxic and immunosuppressive drug intake, which can induce thrombotic venopathy, have been reported[145,192,195,200,208]. These consist of: myeloproliferative syndromes, lymphoproliferative syndromes, pancytopenia, thrombocytopenia, idiopathic thrombocytopenic purpura, chronic vascular disorders, congenital heart disease, multiple organ malformations, chronic Budd-Chiari syndrome, Felty syndrome, Vater syndrome, Donohue syndrome, Krabbe disease, Still’s disease, polyarteritis nodosa, scleroderma, calcinosis cutis, Raynaud’s phenomenon, sclerodactyly, telangiectasia, lupus erythematosus, antiphospholipid syndrome, hypersplenism, hepatic/abdominal/retroperitoneal tumor, sacrococcygeal teratoma, AIDS, and use of steroids or antineoplastic medication[123,140,193,195,198,201-207,209-213]. It is often associated with patients suffering from inflammatory bowel disease treated with thioguanine or azathioprine.
Rare pediatric cases are mostly in association with the congenital absence of the portal vein[214]. Devarbhavi et al[215] reported 14 cases of patients who developed NRH occurring de novo following liver transplantation, from which two pediatric patients had symptomatic NRH: one 4.8-year-old male child who underwent liver transplantation for biliary atresia and an 18 year-old male patient who underwent liver transplantation for primary sclerosing cholangitis. The results of this study suggest that development of NRH post liver transplantation occurs in approximately 1% of transplanted patients. Familial cases have also been described[123], with Dumortier et al[216] reporting familial occurrence of NRH in three families.
During the initial stages, most patients have no symptoms attributable to NRH, leading to a challenging diagnosis. The disease is discovered incidentally during abdominal imaging performed for various reasons. In these cases, no further interventions are required since the progression of the disease is slow or absent.
In advanced stages, portal hypertension is the most often associated finding (in up to 50% of cases), with NRH being one of the major causes of non-cirrhotic portal hypertension in the Western world, apart from portal vein thrombosis due to umbilical catheterization. In all cases of abnormal liver enzymes, both in pediatric and adult patients, NRH should be considered in the differential diagnosis. NRH should also be considered in young patients with portal hypertension and no evidence of portal vein thrombosis[12,191,196,202].
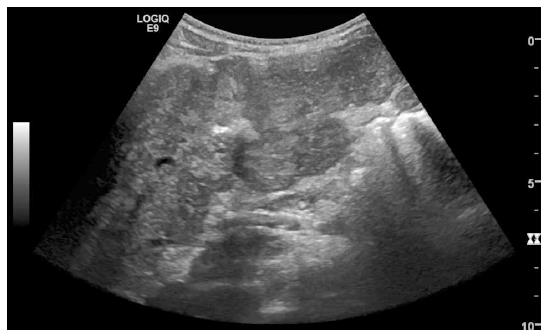
Histopathology is the diagnostic standard, demonstrating diffuse regenerative micronodular transformation of the liver (multiple monoacinar regenerative nodules that involve most areas of the liver) without parietal thickening of portal venules or a central scar. Fibrosis is absent or minimal in the perisinusoidal or periportal areas on reticulin staining. Nodules are between 1-3 mm in diameter, but can rarely be larger, and may even coalesce into a large tumor. Granularity of the hepatic surface may resemble micronodular cirrhosis, but the absence of fibrous septa distinguishes NRH from the regenerative nodules encountered in the cirrhotic liver[129,193,196,200,214,217-219] (Figure 4).
Particular attention should be given to the size of the biopsy sample, since histologic features of NRH may be lacking or incomplete if the sample is too small[140,220].
Evidence of central hemorrhage or infarction may be noted in larger lesions[140].
The radiological features of NRH are relatively non-specific, with imaging methods for it have poor sensitivity and specificity[64,221]. The appearance of NRH at imaging is variable and depends in part on the size of the nodules. Tiny nodules diffusely distributed within the liver may not be detected by imaging. If the nodules coalesce, they may become evident by imaging. Findings related to portal hypertension, including esophagogastric varicose veins, ascites, and splenomegaly, may be observed[200].
US: NRH lesions are multiple, tiny, well-circumscribed, homogeneous, and hypo-/isoechoic on US, but due to their small size or isoechogenicity, the nodules are often not visible. A diffusely heterogeneous echotexture of the hepatic parenchyma or distortion of normal hepatic architecture may be the only sonographic finding. When lesions present a sonolucent rim, they may be hard to distinguish from metastases. Hyperechoic nodules have been reported in very rare cases of NRH, sometimes with hypoechoic centers. This finding is possibly related to prior hemorrhage[199,200,222]. No specific enhancement pattern has been described by CEUS so far[39,40,162].
CT: On CT scan, regenerative nodules are usually hypoattenuating to the normal liver on pre-contrast images, although they may be also isoattenuating. They usually do not enhance after intravenous administration of iodinated contrast material, remaining isodense or hypodense in both arterial and portal venous phases. This distinguishes NRH from FNH and HAs. Occasionally, they may enhance diffusely or demonstrate peripheral rim-like enhancement.
Differential diagnosis with regenerative nodules of cirrhosis may be challenging on both US and CT imaging[64,195,199,200,202,206,213,214,221,222].
MRI: On MRI, regenerative nodules are commonly homogeneous and slightly hyperintense on T1- weighted images, and may contain foci of high signal intensity compatible with hemorrhage. On T2-weighted images, the lesions are iso-/hypointense to normal liver. Sometimes, a T1 hyper- or hypointense or T2 hyperintense rim may be noted. On fat-suppressed T1-weighted images, decreased signal intensity may be observed due to intra-cellular fat, similar to findings in HA. The nodules may present gadolinium enhancement preferentially in the portal venous phase, similar to normal liver parenchyma. This is an important feature for distinguishing NRH nodules from other FLLs which may present similar imaging aspects (like FNH, HA, or metastatic disease) and demonstrate arterial phase enhancement, while NRH does not. However, reports are few and the utility of MRI in the diagnosis of NRH is still controversial[12,199,213,222-224]. The sensitivity and specificity of MRI for the diagnosis of NRH are 70%-80% when using gadolinium contrast, as reported by Zech et al[225], with more disappointing results reported by Laharie et al[226].
Once established, treatment is directed toward the causative factor. When portal hypertension develops, often associated with esophageal varicose veins (with/without bleeding) and ascites, therapy is directed to its management[216]. In most cases, the disease is slowly progressive and the prognosis is better than that of other chronic liver diseases. Sometimes, the rate of nodular growth may be accelerated for unknown reasons[227]. Once diagnosed, follow-up of these children is mandatory due to possible future complications. Of these, portal hypertension is mostly encountered, but cases of malignant transformation of a long standing disease have been reported[202].
Hepatic adenoma (HA) is a spherical or ovoid, well-circumscribed, benign tumor of the liver[140]. It constitutes 2% to 4% of all liver tumors in children[228,229], and up to 21% of all pediatric benign liver tumors[8,230-232]. HAs are solitary masses in approximately 70%-80% of cases. Multiple HAs are more frequently observed in predisposed children[140,233]. Liver adenomatosis has been defined as a separate entity, consisting of over 10 adenomas per patient without underlying glycogen storage disease or steroid use[12,234,235]. Sex ratio is variable in different studies, the female predominance observed in adults not being a rule in children[230]. Still, some reports suggest that pediatric patients mainly consist of girls over 10 years old, most of whom have a history of oral contraceptive use[81,236]. The mean age at diagnosis is around 14 years. Rare cases have also been described in younger children[232] and even in utero[229]. HA before the age of one year is exceptional, with the youngest reported case being of a three-week-old patient with multiple congenital anomalies[237].
During childhood, HAs are more frequently associated with predisposing factors such as glycogen storage diseases type I and III, anabolic androgenic steroid treatments with or without Fanconi anemia, seizure disorder patients who are on carbamazepine therapy, congenital or surgical portosystemic shunts, germline mutation of the HNF-1α gene, familial adenomatosis polyposis, Hurler syndrome, Turcot syndrome, Lynch syndrome, immunodeficiency syndrome, tyrosinemia, galactosemia, and diabetes mellitus[147,229,232,234,238-243]. An exceptional case of a patient with polycystic ovarian syndrome and HA has been reported[244], and a previously unreported co-occurrence of ovarian Sertoli-Leydig cell tumor with HA in a 14 year old girl has been published[245].
In individuals with glycogen storage disease I, HA tends to occur at a relatively younger age, and patients are prone to having multiple lesions and hemorrhages[152,228]. A study of the genotype-phenotype profile of HA in children concerning glycogen storage disease type I showed a high frequency of β-catenin mutations and a lack of HNF-1α inactivation[246]. In children with Fanconi anemia and in those under androgen therapy with or without Fanconi anemia, liver tumors can occur in about 3% of patients[247], with most being HAs[248,249].
Although rare, a few cases of spontaneous HAs not associated with any hormonal or metabolic abnormalities have been reported in children. In these cases, HAs can be found incidentally during imaging for unrelated pathology. A biopsy of the non-tumoral liver may be necessary in order to depict underlying liver disease[1,64,229,250,251].
Patients are usually asymptomatic. They may present with an abdominal mass, with or without acute or chronic abdominal pain[12].
The results of liver function tests are usually normal, with no elevation of AFP level[12].
Histologically, HA is a well-circumscribed lesion of 1-15 cm in diameter, frequently presenting areas of necrosis, hemorrhage, myxoid stroma, or calcifications. They are usually unencapsulated, although a fibrous pseudocapsule of compressed adjacent hepatic parenchyma may be present. HA is composed of benign-appearing hepatocytes, which may contain increased amounts of fat and glycogen, and are organized in sheets or cords. It is characterized by scattered thin-walled vascular channels within the mass and the absence of portal and central veins and bile ducts or connective tissue. A predisposition to hemorrhage may be due to the peritumoral arteries, as well as poor connective tissue support. In most cases, histologic assessment of the tumor is necessary in order to adapt its management[1,12,140,240,252]. The classification into genetic subcategories of HAs is so far limited to adult cases. Data concerning genetic abnormalities in HAs of infants are so far lacking.
Very little data on the imaging features of HA are available for children, making diagnosis based on imaging appearance challenging. The appearance of HA on imaging varies depending on its pathologic composition. Those without hemorrhage and necrosis are homogeneous and similar in appearance to the adjacent normal liver parenchyma, while the presence of intratumoral hemorrhage or intracellular fat produces distinguishing imaging features[12].
US: The sonographic appearance of HA is that of a well-delineated and heterogeneous solid mass. Lesions with high lipid content or recent hemorrhage may be hyperechoic to the normal liver parenchyma. After time, old blood will become hypoechoic, mimicking a cyst. In predisposed children with diffuse fatty infiltration of the liver in glycogen storage diseases, HAs may be hypoechoic to the surrounding liver parenchyma. US is the screening tool for predisposed children with glycogen storage diseases and should be performed at least annually. The main concern is the detection of malignant transformation, and can be suspected when an increase in size or a change in tumor aspect are detected[1,228,253]. Doppler might show variable peritumoral arterial and venous waveforms[12,254].
CEUS: In contrast to FNH, which has a predominantly central arterial flow, HA has homogenous enhancing vessels in the arterial time and non-enhancing in the portal venous phase due to a lack of portal veins.
HNF-1α-inactivated HAs and inflammatory HAs have characteristic CEUS patterns. Delayed washout, which is otherwise an unusual finding in benign hepatic lesions, is of particular interest, and is characteristic for the inflammatory HA subtype[255].
CT: At CT, HAs are typically spherical, sharply delineated masses, with a heterogeneous structure due to hemorrhage, fat, or necrosis. A pseudocapsule may be seen in up to 25%-30% of cases[240,252]. Most lesions are hypoattenuating on unenhanced images compared to normal liver, with heterogeneous content due to areas of fat (seen at CT in 7%-10% of cases), and calcifications (seen at CT in 5%-15% of cases)[240,252,253]. Areas of recent hemorrhage with a hyperattenuating appearance are seen in 15%-43% of cases[165,240,252,256]. The presence of hemorrhage and heterogeneous attenuation is often the key to a correct diagnosis[165].
After intravenous iodinate contrast agent administration, HA enhances heterogeneously (due to necrosis, fat, hemorrhage, and calcification) during the arterial phase, being hyperattenuating compared to the normal liver. It then becomes isoattenuating in the portal venous and delayed phases or present with rapid wash-out. Smaller lesions usually present homogeneous enhancement[12,23].
HA does not present a central scar. This aspect is helpful for differential diagnosis with FNH, which also enhances during the arterial phase and presents a central scar. The pattern of enhancement is also different between the two lesions: HA presents with heterogeneous enhancement, while FNH typically enhances homogeneously[257].
MRI: Besides CEUS, MRI is the best technique to diagnose HA. On MRI sequences, HA patterns will depend on the amount of fat, hemorrhage, and necrosis within the mass. Generally, HAs are heterogeneous, predominantly hyperintense on T1- and T2-weighted images, and with signal loss on “in-phase” and “out-of-phase” T1-sequence due to fatty components. Sequences with fat saturation may also be useful, but less sensitive. However, this finding is not specific for HA, as 40% of HCCs also histologically contain fat. The hyperintensity on T1- and T2-weighted images may also be due to hemorrhage. The enhancement pattern after intravenous administration of gadolinium contrast material is similar to that seen on a CT scan. HA present early arterial enhancement in most cases and then become isointense to the liver on portal venous and delayed phase images. It may also present late wash-out, but not as important as that seen in cases of HCCs. HCCs also present with increased enhancement during the arterial phase as compared to HAs. An enhanced pseudocapsule can be visible on delayed acquisition. The kinetics of enhancement is also important in the follow-up of HAs, since a change in the enhancement pattern may raise suspicions of malignant transformation. Diffusion sequences may be helpful for the detection of small lesions[1,12,152,228,238,240,251,258-260].
Experience with hepatocyte-specific agents, such as gadobenate dimeglumine, suggests that the lack of bile ducts can be used to distinguish between HAs and FNHs. Because of their lack of biliary ducts, HAs do not enhance and are typically hypointense to liver parenchyma on hepatocyte phase imaging, while FNH often demonstrates hyperenhancement during that phase[16,261].
Nuclear medicine studies show non-specific findings. At hepatobiliary scintigraphy, because of the lack of bile ducts, HAs demonstrate increased uptake or retention of the radiotracer[262].
With the discontinuation of oral contraceptives or the institution of dietary therapy for glycogen storage disease, some HAs spontaneously regress, while others remain stable or enlarge. Complete surgical resection of HAs is recommended whenever technically feasible, especially if the tumor is larger than 5 cm because of the risk of rupture and hemorrhage. In cases of hemorrhage, embolization can be performed. In cases of multiple HAs, resection of the largest tumor and close follow-up of the remaining lesions is the management of choice. Surgical treatment is also indicated because of some reported cases of HCC arising in both solitary and multiple HA cases, particularly in those greater than 4 cm in size. Percutaneous radiofrequency ablation is an alternative to surgical resection[1,230,232,251,262,263]. Potential complications of HA include hemorrhage and malignant transformation[244]. Hemorrhage is one of the most important complication of HA, occurring in approximately 10% of patients[238]. The risk of bleeding increases with increasing tumor size and with the duration of contraceptive use. It may occur inside the lesion, usually in HAs larger than 4 cm and mixed with necrotic changes, or it can occur outside the lesion, with consecutive subcapsular hematoma and/or hemoperitoneum. Clinically, it will present with severe abdominal pain and possible hemodynamic disorders or even hypovolemic shock. Fatal cases have been reported in young patients with familial adenomatosis related to HNF-1α mutation and Fanconi anemia, with some occurring even after androgen therapy discontinuation[238,264-266].
The malignant transformation of HAs is extremely rare in children, with reported cases being mainly associated with glycogen storage disease, Fanconi anemia with steroid therapy, and congenital portosystemic shunts. Still, in these cases of associated pathologies, potential regression of HA has been also reported, especially under metabolic control, androgen withdraw, or closure of the shunt, respectively[1,239,267-271].
Focal liver lesions are commonly observed in adults, but should also be recognized in children. Exact data concerning the incidence of these common lesions (hemangioma, FNH, or cysts) are lacking in children. Most benign liver tumors are inborn and may grow like the rest of the body. Exact descriptions of radiological and histopathological features may help to differentiate these lesions more clearly.
The use of CEUS in pediatric patients for characterizing liver lesions which remain indeterminate on grey-scale US is a possible option to be adopted in the future, and has the potential to reduce exposure to ionizing radiation[272].
P- Reviewer: Chen Z, Floria M, Fusai GK, Kambadakone A S- Editor: Ma YJ L- Editor: Rutherford A E- Editor: Zhang DN
| 1. | Franchi-Abella S, Branchereau S. Benign hepatocellular tumors in children: focal nodular hyperplasia and hepatocellular adenoma. Int J Hepatol. 2013;2013:215064. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 2. | Bakshi P, Srinivasan R, Rao KL, Marwaha RK, Gupta N, Das A, Nijhawan R, Rajwanshi A. Fine needle aspiration biopsy in pediatric space-occupying lesions of liver: a retrospective study evaluating its role and diagnostic efficacy. J Pediatr Surg. 2006;41:1903-1908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Davey MS, Cohen MD. Imaging of gastrointestinal malignancy in childhood. Radiol Clin North Am. 1996;34:717-742. [PubMed] |
| 4. | Donnelly LF, Bisset GS. Pediatric hepatic imaging. Radiol Clin North Am. 1998;36:413-427. [PubMed] |
| 5. | Ehren H, Mahour GH, Isaacs H. Benign liver tumors in infancy and childhood. Report of 48 cases. Am J Surg. 1983;145:325-329. [PubMed] |
| 6. | Emre S, McKenna GJ. Liver tumors in children. Pediatr Transplant. 2004;8:632-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 89] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 7. | Jha P, Chawla SC, Tavri S, Patel C, Gooding C, Daldrup-Link H. Pediatric liver tumors--a pictorial review. Eur Radiol. 2009;19:209-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Luks FI, Yazbeck S, Brandt ML, Bensoussan AL, Brochu P, Blanchard H. Benign liver tumors in children: a 25-year experience. J Pediatr Surg. 1991;26:1326-1330. [PubMed] |
| 9. | Mowat AP. Liver disorders in children: the indications for liver replacement in parenchymal and metabolic diseases. Transplant Proc. 1987;19:3236-3241. [PubMed] |
| 10. | Pandey A, Gangopadhyay AN, Sharma SP, Kumar V, Gupta DK, Gopal SC, Patne SC. Long-term follow up of mesenchymal hamartoma of liver--single center study. Saudi J Gastroenterol. 2011;17:20-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Reymond D, Plaschkes J, Lüthy AR, Leibundgut K, Hirt A, Wagner HP. Focal nodular hyperplasia of the liver in children: review of follow-up and outcome. J Pediatr Surg. 1995;30:1590-1593. [PubMed] |
| 12. | Chung EM, Cube R, Lewis RB, Conran RM. From the archives of the AFIP: Pediatric liver masses: radiologic-pathologic correlation part 1. Benign tumors. Radiographics. 2010;30:801-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 115] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 13. | Stocker JT. Hepatic tumors in children. Clin Liver Dis. 2001;5:259-81, viii-ix. [PubMed] |
| 14. | Boechat MI, Kangarloo H, Gilsanz V. Hepatic masses in children. Semin Roentgenol. 1988;23:185-193. [PubMed] |
| 15. | Powers C, Ros PR, Stoupis C, Johnson WK, Segel KH. Primary liver neoplasms: MR imaging with pathologic correlation. Radiographics. 1994;14:459-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 106] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Courtier JL, Perito ER, Rhee S, Tsai P, Heyman MB, MacKenzie JD. Targeted MRI contrast agents for pediatric hepatobiliary disease. J Pediatr Gastroenterol Nutr. 2012;54:454-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Charny CK, Jarnagin WR, Schwartz LH, Frommeyer HS, DeMatteo RP, Fong Y, Blumgart LH. Management of 155 patients with benign liver tumours. Br J Surg. 2001;88:808-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 95] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Kochin IN, Miloh TA, Arnon R, Iyer KR, Suchy FJ, Kerkar N. Benign liver masses and lesions in children: 53 cases over 12 years. Isr Med Assoc J. 2011;13:542-547. [PubMed] |
| 19. | Meyers RL. Tumors of the liver in children. Surg Oncol. 2007;16:195-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 161] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 20. | Shamaly H, Abu-Nassar Z, Groisman GM, Shamir R. Hepatic hemangloendothelioma: the need for early diagnosis and resection. Isr Med Assoc J. 2006;8:585-586. [PubMed] |
| 21. | Stringer MD, Alizai NK. Mesenchymal hamartoma of the liver: a systematic review. J Pediatr Surg. 2005;40:1681-1690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 113] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 22. | Scheimann AO, Barrios JM, Al-Tawil YS, Gray KM, Gilger MA. Percutaneous liver biopsy in children: impact of ultrasonography and spring-loaded biopsy needles. J Pediatr Gastroenterol Nutr. 2000;31:536-539. [PubMed] |
| 23. | Dong Q, Chen JJ. CT Scan of Pediatric Liver Tumors. CT Scanning-Techniques and Applications. Croatia: InTech 2011; . |
| 24. | Pan FS, Xu M, Wang W, Zhou LY, Xie XY. Infantile hepatic hemangioendothelioma in comparison with hepatoblastoma in children: clinical and ultrasound features. Hepat Mon. 2013;13:e11103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Roebuck D. Focal liver lesion in children. Pediatr Radiol. 2008;38 Suppl 3:S518-S522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Varich L. Ultrasound of Pediatric Liver Masses. Ultrasound Clin. 2010;5:137. |
| 27. | Westheim BH, Østensen AB, Aagenæs I, Sanengen T, Almaas R. Evaluation of risk factors for bleeding after liver biopsy in children. J Pediatr Gastroenterol Nutr. 2012;55:82-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 28. | Piscaglia F, Bolondi L. The safety of Sonovue in abdominal applications: retrospective analysis of 23188 investigations. Ultrasound Med Biol. 2006;32:1369-1375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 596] [Cited by in RCA: 532] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 29. | Grothues D, Knoppke B, Pfister ED, Vermehren J, Rauschenfels S, Gebel M, Melter M. Contrast enhanced ultrasound (CEUS) has a high diagnostic value in children with focal liver lesions (FLL). J Pediatr Gastroenterol Nutr. 2006;42:E77. |
| 30. | Yekeler E. Pediatric abdominal applications of multidetector-row CT. Eur J Radiol. 2004;52:31-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | Brenner DJ, Hall EJ. Computed tomography--an increasing source of radiation exposure. N Engl J Med. 2007;357:2277-2284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6212] [Cited by in RCA: 5783] [Article Influence: 321.3] [Reference Citation Analysis (3)] |
| 32. | Hricak H, Brenner DJ, Adelstein SJ, Frush DP, Hall EJ, Howell RW, McCollough CH, Mettler FA, Pearce MS, Suleiman OH. Managing radiation use in medical imaging: a multifaceted challenge. Radiology. 2011;258:889-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 212] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 33. | Miglioretti DL, Johnson E, Williams A, Greenlee RT, Weinmann S, Solberg LI, Feigelson HS, Roblin D, Flynn MJ, Vanneman N. The use of computed tomography in pediatrics and the associated radiation exposure and estimated cancer risk. JAMA Pediatr. 2013;167:700-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1033] [Cited by in RCA: 1020] [Article Influence: 85.0] [Reference Citation Analysis (0)] |
| 34. | Smith-Bindman R, Miglioretti DL, Johnson E, Lee C, Feigelson HS, Flynn M, Greenlee RT, Kruger RL, Hornbrook MC, Roblin D. Use of diagnostic imaging studies and associated radiation exposure for patients enrolled in large integrated health care systems, 1996-2010. JAMA. 2012;307:2400-2409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 568] [Cited by in RCA: 596] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 35. | Pappas JN, Donnelly LF, Frush DP. Reduced frequency of sedation of young children with multisection helical CT. Radiology. 2000;215:897-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 61] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 36. | White KS. Reduced need for sedation in patients undergoing helical CT of the chest and abdomen. Pediatr Radiol. 1995;25:344-346. [PubMed] |
| 37. | Dietrich CF, Mäurer M, Riemer-Hommel P. Challenges for the German Health Care System – Pharmaceuticals. Endo heute. 2014;27:45-53. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 38. | Claudon M, Cosgrove D, Albrecht T, Bolondi L, Bosio M, Calliada F, Correas JM, Darge K, Dietrich C, D’Onofrio M. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) - update 2008. Ultraschall Med. 2008;29:28-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 556] [Cited by in RCA: 498] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 39. | Claudon M, Dietrich CF, Choi BI, Cosgrove DO, Kudo M, Nolsøe CP, Piscaglia F, Wilson SR, Barr RG, Chammas MC. Guidelines and good clinical practice recommendations for Contrast Enhanced Ultrasound (CEUS) in the liver - update 2012: A WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultrasound Med Biol. 2013;39:187-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 501] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 40. | Claudon M, Dietrich CF, Choi BI, Cosgrove DO, Kudo M, Nolsøe CP, Piscaglia F, Wilson SR, Barr RG, Chammas MC. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) in the liver--update 2012: a WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultraschall Med. 2013;34:11-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 222] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 41. | Piscaglia F, Nolsøe C, Dietrich CF, Cosgrove DO, Gilja OH, Bachmann Nielsen M, Albrecht T, Barozzi L, Bertolotto M, Catalano O. The EFSUMB Guidelines and Recommendations on the Clinical Practice of Contrast Enhanced Ultrasound (CEUS): update 2011 on non-hepatic applications. Ultraschall Med. 2012;33:33-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 721] [Cited by in RCA: 678] [Article Influence: 52.2] [Reference Citation Analysis (0)] |
| 42. | Schreiber-Dietrich D, Dietrich CF. Contrast enhanced ultrasound (CEUS) and off-label use (in children). Ultraschall Med. 2012;33:295-296. [PubMed] |
| 43. | Glass RBJ, Kuhn JP, Slovis TL, Haller JO. Caffey’s pediatric diagnostic imaging. Pediatr Radiol. 2004;34:518-518. [DOI] [Full Text] |
| 44. | Helmberger TK, Ros PR, Mergo PJ, Tomczak R, Reiser MF. Pediatric liver neoplasms: a radiologic-pathologic correlation. Eur Radiol. 1999;9:1339-1347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 84] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 45. | Powell TG, West CR, Pharoah PO, Cooke RW. Epidemiology of strawberry haemangioma in low birthweight infants. Br J Dermatol. 1987;116:635-641. [PubMed] |
| 46. | Ishak KGA PP, Niederau C, Nakanuma Y. Mesenchymal tumours of the liver. World Health Organization classification of tumours of the digestive system. Lyon: IARC Press 2000; 191-198. |
| 47. | Chandra RS, Stoeker JT. The liver, gallbladder, and biliary tree. Pediatric pathology. Philadelphia: Lippincott 1992; 703-789. |
| 48. | Dehner LP, Ishak KG. Vascular tumors of the liver in infants and children. A study of 30 cases and review of the literature. Arch Pathol. 1971;92:101-111. [PubMed] |
| 49. | Feng ST, Chan T, Ching AS, Sun CH, Guo HY, Fan M, Meng QF, Li ZP. CT and MR imaging characteristics of infantile hepatic hemangioendothelioma. Eur J Radiol. 2010;76:e24-e29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 50. | Isaacs H. Fetal and neonatal hepatic tumors. J Pediatr Surg. 2007;42:1797-1803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 127] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 51. | Keslar PJ, Buck JL, Selby DM. From the archives of the AFIP. Infantile hemangioendothelioma of the liver revisited. Radiographics. 1993;13:657-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 52. | Moon SB, Kwon HJ, Park KW, Yun WJ, Jung SE. Clinical experience with infantile hepatic hemangioendothelioma. World J Surg. 2009;33:597-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 53. | Bowers RE, Graham EA, Thominson KM. The natural history of strawberry nevus. ArchDermatol. 1960;82:667-670. |
| 54. | Kapoor G, Kurkure PA, Chinoy R, Borwankar S, Advani S. Interpretation of serum alpha-feto protein in an infant with hepatomegaly. Indian Pediatr. 1996;33:65-69. [PubMed] |
| 55. | Kim TJ, Lee YS, Song YS, Park CK, Shim SI, Kang CS, Lee KY. Infantile hemangioendothelioma with elevated serum alpha fetoprotein: report of 2 cases with immunohistochemical analysis. Hum Pathol. 2010;41:763-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 56. | Singal AK, Agarwala S. Tumour markers in pediatric solid tumors. J Indian Assoc Pediatr Surg. 2005;10:183-190. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 57. | Paltiel HJ, Patriquin HB, Keller MS, Babcock DS, Leithiser RE. Infantile hepatic hemangioma: Doppler US. Radiology. 1992;182:735-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 58. | Gottschling S, Schneider G, Meyer S, Reinhard H, Dill-Mueller D, Graf N. Two infants with life-threatening diffuse neonatal hemangiomatosis treated with cyclophosphamide. Pediatr Blood Cancer. 2006;46:239-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 59. | Hegde SV, Dillman JR, Lopez MJ, Strouse PJ. Imaging of multifocal liver lesions in children and adolescents. Cancer Imaging. 2013;12:516-529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 60. | Kassarjian A, Zurakowski D, Dubois J, Paltiel HJ, Fishman SJ, Burrows PE. Infantile hepatic hemangiomas: clinical and imaging findings and their correlation with therapy. AJR Am J Roentgenol. 2004;182:785-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 112] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 61. | Christison-Lagay ER, Burrows PE, Alomari A, Dubois J, Kozakewich HP, Lane TS, Paltiel HJ, Klement G, Mulliken JB, Fishman SJ. Hepatic hemangiomas: subtype classification and development of a clinical practice algorithm and registry. J Pediatr Surg. 2007;42:62-7; discussion 67-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 216] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 62. | Strobel D, Seitz K, Blank W, Schuler A, Dietrich CF, von Herbay A, Friedrich-Rust M, Bernatik T. Tumor-specific vascularization pattern of liver metastasis, hepatocellular carcinoma, hemangioma and focal nodular hyperplasia in the differential diagnosis of 1,349 liver lesions in contrast-enhanced ultrasound (CEUS). Ultraschall Med. 2009;30:376-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 118] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 63. | Dietrich CF, Maddalena ME, Cui XW, Schreiber-Dietrich D, Ignee A. Liver tumor characterization--review of the literature. Ultraschall Med. 2012;33 Suppl 1:S3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 64. | Horton KM, Bluemke DA, Hruban RH, Soyer P, Fishman EK. CT and MR imaging of benign hepatic and biliary tumors. Radiographics. 1999;19:431-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 162] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 65. | Lucaya J, Enriquez G, Amat L, Gonzalez-Rivero MA. Computed tomography of infantile hepatic hemangioendothelioma. AJR Am J Roentgenol. 1985;144:821-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 66. | Siegel MJ. Pediatric liver imaging. Semin Liver Dis. 2001;21:251-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 67. | Burrows PE, Dubois J, Kassarjian A. Pediatric hepatic vascular anomalies. Pediatr Radiol. 2001;31:533-545. [PubMed] |
| 68. | Mortelé KJ, Vanzieleghem B, Mortelé B, Benoit Y, Ros PR. Solitary hepatic infantile hemangioendothelioma: dynamic gadolinium-enhanced MR imaging findings. Eur Radiol. 2002;12:862-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 69. | Park CH, Hwang HS, Hong J, Pak MS. Giant infantile hemangioendothelioma of the liver. Scintigraphic diagnosis. Clin Nucl Med. 1996;21:293-295. [PubMed] |
| 70. | Schiff ER, Maddrey WC, Sorrell MF. Schiff’s Diseases of the Liver. 11th ed. Publisher: Wiley-Blackwell 2011; 1264. |
| 71. | Özmen E, Adaletli I, Kayadibi Y, Emre Ş, Kiliç F, Dervişoğlu S, Kuruğoğlu S, Şenyüz OF. The impact of share wave elastography in differentiation of hepatic hemangioma from malignant liver tumors in pediatric population. Eur J Radiol. 2014;83:1691-1697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 72. | Daller JA, Bueno J, Gutierrez J, Dvorchik I, Towbin RB, Dickman PS, Mazariegos G, Reyes J. Hepatic hemangioendothelioma: clinical experience and management strategy. J Pediatr Surg. 1999;34:98-105; discussion 105-6. [PubMed] |
| 73. | Hobbs KE. Hepatic hemangiomas. World J Surg. 1990;14:468-471. [PubMed] |
| 74. | Wiederkehr JC, Coelho IM, Avilla SG, Wiederkehr BA, Wiederkehr HA. Liver Tumors in Infancy. Hepatic Surgery. Croatia: Intech 2013; . |
| 75. | Zenge JP, Fenton L, Lovell MA, Grover TR. Case report: infantile hemangioendothelioma. Curr Opin Pediatr. 2002;14:99-102. [PubMed] |
| 76. | Weinberg AG, Finegold MJ. Primary hepatic tumors of childhood. Hum Pathol. 1983;14:512-537. [PubMed] |
| 77. | Selby DM, Stocker JT, Ishak KG. Angiosarcoma of the liver in childhood: a clinicopathologic and follow-up study of 10 cases. Pediatr Pathol. 1992;12:485-498. [PubMed] |
| 78. | Achilleos OA, Buist LJ, Kelly DA, Raafat F, McMaster P, Mayer AD, Buckels JA. Unresectable hepatic tumors in childhood and the role of liver transplantation. J Pediatr Surg. 1996;31:1563-1567. [PubMed] |
| 79. | Riley MR, Garcia MG, Cox KL, Berquist WE, Kerner JA. Hepatic infantile hemangioendothelioma with unusual manifestations. J Pediatr Gastroenterol Nutr. 2006;42:109-113. [PubMed] |
| 80. | Ishak KGG, Stocker JT. Benign mesenchymal tumors and pseudotumors. Atlas of tumor pathology. Washington DC: Armed Forces Institute of Pathology 2001; 71-157. |
| 81. | Stocker JT, Ishak KG. Mesenchymal hamartoma of the liver: report of 30 cases and review of the literature. Pediatr Pathol. 1983;1:245-267. [PubMed] |
| 82. | Edmondson HA. Differential diagnosis of tumors and tumor-like lesions of liver in infancy and childhood. AMA J Dis Child. 1956;91:168-186. [PubMed] |
| 83. | Kamata S, Nose K, Sawai T, Hasegawa T, Kuroda S, Sasaki T, Okada A, Tawara M. Fetal mesenchymal hamartoma of the liver: report of a case. J Pediatr Surg. 2003;38:639-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 84. | Cook JR, Pfeifer JD, Dehner LP. Mesenchymal hamartoma of the liver in the adult: association with distinct clinical features and histological changes. Hum Pathol. 2002;33:893-898. [PubMed] |
| 85. | Wada M, Ohashi E, Jin H, Nishikawa M, Shintani S, Yamashita M, Kano M, Yamanaka N, Nishigami T, Shimoyama T. Mesenchymal hamartoma of the liver: report of an adult case and review of the literature. Intern Med. 1992;31:1370-1375. [PubMed] |
| 86. | Kim SH, Kim WS, Cheon JE, Yoon HK, Kang GH, Kim IO, Yeon KM. Radiological spectrum of hepatic mesenchymal hamartoma in children. Korean J Radiol. 2007;8:498-505. [PubMed] |
| 87. | Cajaiba MM, Sarita-Reyes C, Zambrano E, Reyes-Múgica M. Mesenchymal hamartoma of the liver associated with features of Beckwith-Wiedemann syndrome and high serum alpha-fetoprotein levels. Pediatr Dev Pathol. 2007;10:233-238. [PubMed] |
| 88. | Sharif K, Ramani P, Lochbühler H, Grundy R, de Ville de Goyet J. Recurrent mesenchymal hamartoma associated with 19q translocation. A call for more radical surgical resection. Eur J Pediatr Surg. 2006;16:64-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 89. | Ramareddy RS, Alladi A. Neonatal mesenchymal hamartoma of liver: an unusual presentation. J Clin Neonatol. 2012;1:211-213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 90. | Yen JB, Kong MS, Lin JN. Hepatic mesenchymal hamartoma. J Paediatr Child Health. 2003;39:632-634. [PubMed] |
| 92. | Raffensperger JG, Gonzalez-Crussi F, Skeehan T. Mesenchymal hamartoma of the liver. J Pediatr Surg. 1983;18:585-587. [PubMed] |
| 93. | Narasimhan KL, Radotra BD, Harish J, Rao KL. Conservative management of giant hepatic mesenchymal hamartoma. Indian J Gastroenterol. 2004;23:26. [PubMed] |
| 94. | Karpelowsky JS, Pansini A, Lazarus C, Rode H, Millar AJ. Difficulties in the management of mesenchymal hamartomas. Pediatr Surg Int. 2008;24:1171-1175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 95. | Ito H, Kishikawa T, Toda T, Arai M, Muro H. Hepatic mensenchymal hamartoma of an infant. J Pediatr Surg. 1984;19:315-317. [PubMed] |
| 96. | Ishak KGG, Stocker JT. Tumors of the Liver and Intrahepatic Bile Ducts. Atlas of Tumor Pathology (AFIP) 3rd Series. 2nd ed. Washington DC: American Registry of Pathology 2001; 356. |
| 97. | Guzzetta PC. Non malignant tumors of the liver. Pediatric Surgery. Philadelphia PA: Mosby Elsevier 2006; 495-501. |
| 98. | George JC, Cohen MD, Tarver RD, Rosales RN. Ruptured cystic mesenchymal hamartoma: an unusual cause of neonatal ascites. Pediatr Radiol. 1994;24:304-305. [PubMed] |
| 99. | Dehner LP, Ewing SL, Sumner HW. Infantile mesenchymal hamartoma of the liver. Histologic and ultrastructural observations. Arch Pathol. 1975;99:379-382. [PubMed] |
| 100. | Dehner LP. Hepatic tumors in the pediatric age group: a distinctive clinicopathologic spectrum. Perspect Pediatr Pathol. 1978;4:217-268. [PubMed] |
| 101. | Alwaidh MH, Woodhall CR, Carty HT. Mesenchymal hamartoma of the liver: a case report. Pediatr Radiol. 1997;27:247-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 102. | Siddiqui MA, McKenna BJ. Hepatic mesenchymal hamartoma: a short review. Arch Pathol Lab Med. 2006;130:1567-1569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 103. | Atas E, Demirkaya M, Kesik V, Balamtekin N, Kocaoglu M. Mesenchymal Hamartoma of the Liver Mimicking Hydatid Cyst. Pediatr Therapeut. 2012;2. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 104. | Sonobe H, Ohtsuki Y, Enzan H, Kurashige T, Matsuura K, Kaneko A, Ogata T. An unusual case of solid hamartoma in the liver. Acta Pathol Jpn. 1988;38:75-82. [PubMed] |
| 105. | Boman F, Bossard C, Fabre M, Diab N, Bonnevalle M, Boccon-Gibod L. Mesenchymal hamartomas of the liver may be associated with increased serum alpha foetoprotein concentrations and mimic hepatoblastomas. Eur J Pediatr Surg. 2004;14:63-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 106. | Unal E, Koksal Y, Akcoren Z, Tavl L, Gunel E, Kerimoglu U. Mesenchymal hamartoma of the liver mimicking hepatoblastoma. J Pediatr Hematol Oncol. 2008;30:458-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 107. | Gow KW, Lee L, Pruthi S, Patterson K, Healey PJ. Mesenchymal hamartoma of the liver. J Pediatr Surg. 2009;44:468-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 108. | Chang HJ, Jin SY, Park C, Park YN, Jang JJ, Park CK, Suh YL, Yu E, Kang DY, Bae HI. Mesenchymal hamartomas of the liver: comparison of clinicopathologic features between cystic and solid forms. J Korean Med Sci. 2006;21:63-68. [PubMed] |
| 109. | Andronikou S, Soin S, Nafoos O, Platt K, Lakhoo K. Hepatic mesenchymal hamartoma mimicking hemangioma on multiple-phase gadolinium-enhanced MRI. J Pediatr Hematol Oncol. 2006;28:322-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 110. | Ros PR, Goodman ZD, Ishak KG, Dachman AH, Olmsted WW, Hartman DS, Lichtenstein JE. Mesenchymal hamartoma of the liver: radiologic-pathologic correlation. Radiology. 1986;158:619-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 77] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 111. | Konez O, Goyal M, Vyas PK, Boinapally SB. Mesenchymal hamartoma of the liver. Comput Med Imaging Graph. 2001;25:61-65. [PubMed] |
| 112. | Srouji MN, Chatten J, Schulman WM, Ziegler MM, Koop CE. Mesenchymal hamartoma of the liver in infants. Cancer. 1978;42:2483-2489. [PubMed] |
| 113. | Jaswal TS, Singh S, Purwar P, Sen R, Marwah N, Sharma LK. Mesenchymal hamartoma of the liver--a case report. Indian J Pathol Microbiol. 2003;46:226-228. [PubMed] |
| 114. | Gilbert RF, Waugh DE, Clarke JS. Mesenchymal hamartoma of the liver. South Med J. 1978;71:530-532. [PubMed] |
| 115. | Ramírez-Garrido F, LópezGonzález-Garrido Jde D, Ruíz-López MJ, Sabatell-López RM, Mirás-Baldó MJ, Rodróguez-Fernández A. Prenatal and post-natal imaging of an hepatic mesenchymal hamartoma. Eur J Pediatr. 2003;162:57-58. [PubMed] |
| 116. | Kodandapani S, Pai MV, Kumar V, Pai KV. Prenatal diagnosis of congenital mesenchymal hamartoma of liver: a case report. Case Rep Obstet Gynecol. 2011;2011:932583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 117. | Anil G, Fortier M, Low Y. Cystic hepatic mesenchymal hamartoma: the role of radiology in diagnosis and perioperative management. Br J Radiol. 2011;84:e91-e94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 118. | Gosset N, Christophe C, Damry N, Delaet MH. Predominantly cystic hepatic mesenchymal hamartoma. JBR-BTR. 2008;91:63. [PubMed] |
| 119. | Kirks DR, Griscom NT. Practical Pediatric Imaging: Diagnostic Radiology of Infants and Children. 3rd ed. Boston: Lippincott Williams & Wilkins 1998; 1248. |
| 120. | Koumanidou C, Vakaki M, Papadaki M, Pitsoulakis G, Savvidou D, Kakavakis K. New sonographic appearance of hepatic mesenchymal hamartoma in childhood. J Clin Ultrasound. 1999;27:164-167. [PubMed] [DOI] [Full Text] |
| 121. | Stanley P, Hall TR, Woolley MM, Diament MJ, Gilsanz V, Miller JH. Mesenchymal hamartomas of the liver in childhood: sonographic and CT findings. AJR Am J Roentgenol. 1986;147:1035-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 122. | Dietrich CF, Ignee A, Trojan J, Fellbaum C, Schuessler G. Improved characterisation of histologically proven liver tumours by contrast enhanced ultrasonography during the portal venous and specific late phase of SHU 508A. Gut. 2004;53:401-405. [PubMed] |
| 123. | Mortele KJ, Ros PR. Benign liver neoplasms. Clin Liver Dis. 2002;6:119-145. [PubMed] |
| 124. | Smith SL, Ramli NM, Somers JM. Cystic mesenchymal hamartoma mimicking hepatic hydatid disease. Clin Radiol. 2001;56:599-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 125. | Kaufman RA. Is cystic mesenchymal hamartoma of the liver similar to infantile hemangioendothelioma and cavernous hemangioma on dynamic computed tomography? Pediatr Radiol. 1992;22:582-583. [PubMed] |
| 126. | Narasimharao KL, Narasimhan KL, Katariya S, Suri S, Kaushik S, Mitra SK. Giant hamartoma of liver mimicking malignancy. Postgrad Med J. 1988;64:398-400. [PubMed] |
| 127. | Yoon YS, Han HS, Choi YS, Lee SI, Jang JY, Suh KS, Kim SW, Lee KU, Park YH. Total laparoscopic left lateral sectionectomy performed in a child with benign liver mass. J Pediatr Surg. 2006;41:e25-e28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 128. | Ramanujam TM, Ramesh JC, Goh DW, Wong KT, Ariffin WA, Kumar G, Taib NA. Malignant transformation of mesenchymal hamartoma of the liver: case report and review of the literature. J Pediatr Surg. 1999;34:1684-1686. [PubMed] |
| 129. | International Working Party. Terminology of nodular hepatocellular lesions. Hepatology. 1995;22:983-993. [PubMed] |
| 130. | Herman P, Pugliese V, Machado MA, Montagnini AL, Salem MZ, Bacchella T, D’Albuquerque LA, Saad WA, Machado MC, Pinotti HW. Hepatic adenoma and focal nodular hyperplasia: differential diagnosis and treatment. World J Surg. 2000;24:372-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 100] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 131. | Edmondson HA. Tumors of the liver and intrahepatic bile ducts. Atlas of tumor pathology. Washington DC: Armed Forces Institute of Pathology 1958; . |
| 132. | Bouyn CI, Leclere J, Raimondo G, Le Pointe HD, Couanet D, Valteau-Couanet D, Hartmann O. Hepatic focal nodular hyperplasia in children previously treated for a solid tumor. Incidence, risk factors, and outcome. Cancer. 2003;97:3107-3113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 79] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 133. | Stocker JT, Ishak KG. Focal nodular hyperplasia of the liver: a study of 21 pediatric cases. Cancer. 1981;48:336-345. [PubMed] |
| 134. | Tomlinson GE, Finegold MJ. Tumors of the liver. Principles and practice of pediatric oncology. Philadelphia: Lippincott 2002; 847-864. |
| 135. | Farruggia P, Alaggio R, Cardella F, Tropia S, Trizzino A, Ferrara F, D’Angelo P. Focal nodular hyperplasia of the liver: an unusual association with diabetes mellitus in a child and review of literature. Ital J Pediatr. 2010;36:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 136. | Kang J, Choi HJ, Yu E, Hwang I, Kim YM, Cha HJ. A case report of fetal telangiectatic focal nodular hyperplasia. Pediatr Dev Pathol. 2007;10:416-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 137. | Lack EE, Ornvold K. Focal nodular hyperplasia and hepatic adenoma: a review of eight cases in the pediatric age group. J Surg Oncol. 1986;33:129-135. [PubMed] |
| 138. | Okamura N, Nakadate H, Ishida K, Nakahara S, Isobe Y, Ohbu M, Okayasu I. Telangiectatic focal nodular hyperplasia of the liver in the perinatal period: case report. Pediatr Dev Pathol. 2005;8:581-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 139. | Petrikovsky BM, Cohen HL, Scimeca P, Bellucci E. Prenatal diagnosis of focal nodular hyperplasia of the liver. Prenat Diagn. 1994;14:406-409. [PubMed] |
| 140. | Ishak KGG, Stocker JT. Benign hepatocellular tumors. Atlas of tumor pathology: tumors of the liver and intrahepatic bile ducts. Washington, DC: Armed Forces Institute of Pathology 2001; 9-48. |
| 141. | Luciani A, Kobeiter H, Maison P, Cherqui D, Zafrani ES, Dhumeaux D, Mathieu D. Focal nodular hyperplasia of the liver in men: is presentation the same in men and women? Gut. 2002;50:877-880. [PubMed] |
| 142. | Lautz T, Tantemsapya N, Dzakovic A, Superina R. Focal nodular hyperplasia in children: clinical features and current management practice. J Pediatr Surg. 2010;45:1797-1803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 143. | Scalori A, Tavani A, Gallus S, La Vecchia C, Colombo M. Risk factors for focal nodular hyperplasia of the liver: an Italian case-control study. Am J Gastroenterol. 2002;97:2371-2373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 144. | Wanless IR, Mawdsley C, Adams R. On the pathogenesis of focal nodular hyperplasia of the liver. Hepatology. 1985;5:1194-1200. [PubMed] |
| 145. | Wanless IR. Micronodular transformation (nodular regenerative hyperplasia) of the liver: a report of 64 cases among 2,500 autopsies and a new classification of benign hepatocellular nodules. Hepatology. 1990;11:787-797. [PubMed] |
| 146. | Vilgrain V. Focal nodular hyperplasia. Eur J Radiol. 2006;58:236-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 95] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 147. | Rebouissou S, Bioulac-Sage P, Zucman-Rossi J. Molecular pathogenesis of focal nodular hyperplasia and hepatocellular adenoma. J Hepatol. 2008;48:163-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 146] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 148. | Kumagai H, Masuda T, Oikawa H, Endo K, Endo M, Takano T. Focal nodular hyperplasia of the liver: direct evidence of circulatory disturbances. J Gastroenterol Hepatol. 2000;15:1344-1347. [PubMed] |
| 149. | De Pasquale MD, Monti L, D’Andrea ML, De Ioris MA, Castellano A. Focal nodular hyperplasia and hepatic regenerating nodules in pediatric oncology patients: how much invasive approach is necessary? Ann Hepatol. 2013;12:308-314. [PubMed] |
| 150. | Bralet MP, Terris B, Vilgrain V, Brégeaud L, Molas G, Corbic M, Belghiti J, Fléjou JF, Degott C. Epithelioid hemangioendothelioma, multiple focal nodular hyperplasias, and cavernous hemangiomas of the liver. Arch Pathol Lab Med. 1999;123:846-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 151. | Do RK, Shaylor SD, Shia J, Wang A, Kramer K, Abramson SJ, Price AP, Schwartz LH. Variable MR imaging appearances of focal nodular hyperplasia in pediatric cancer patients. Pediatr Radiol. 2011;41:335-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 152. | Liu QY, Zhang WD, Lai DM, Ou-Yang Y, Gao M, Lin XF. Hepatic focal nodular hyperplasia in children: imaging features on multi-slice computed tomography. World J Gastroenterol. 2012;18:7048-7055. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 153. | Towbin AJ, Luo GG, Yin H, Mo JQ. Focal nodular hyperplasia in children, adolescents, and young adults. Pediatr Radiol. 2011;41:341-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 154. | Jankowska I, Pawłowska J, Nachulewicz P, Teisseyre M, Liberek A, Dzik E, Kmiotek J, Kościesza A, Kalicinski P, Cielecka-Kuszyk J. [Focal nodular hyperplasia - own experiences]. Med Wieku Rozwoj. 2006;10:417-427. [PubMed] |
| 155. | Miller JH, Greenspan BS. Integrated imaging of hepatic tumors in childhood. Part II: Benign lesions (congenital, reparative, and inflammatory). Radiology. 1985;154:91-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 156. | Shamsi K, De Schepper A, Degryse H, Deckers F. Focal nodular hyperplasia of the liver: radiologic findings. Abdom Imaging. 1993;18:32-38. [PubMed] |
| 157. | Bartolozzi C, Lencioni R, Paolicchi A, Moretti M, Armillotta N, Pinto F. Differentiation of hepatocellular adenoma and focal nodular hyperplasia of the liver: comparison of power Doppler imaging and conventional color Doppler sonography. Eur Radiol. 1997;7:1410-1415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 44] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 158. | Dietrich CF. Liver tumor characterization--comments and illustrations regarding guidelines. Ultraschall Med. 2012;33 Suppl 1:S22-S30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 159. | Dietrich CF, Cui XW, Schreiber-Dietrich DG, Ignee A. EFSUMB guidelines 2011: comments and illustrations. Ultraschall Med. 2012;33 Suppl 1:S11-S21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 160. | Dietrich CF, Schuessler G, Trojan J, Fellbaum C, Ignee A. Differentiation of focal nodular hyperplasia and hepatocellular adenoma by contrast-enhanced ultrasound. Br J Radiol. 2005;78:704-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 119] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 161. | Kehagias D, Moulopoulos L, Antoniou A, Hatziioannou A, Smyrniotis V, Trakadas S, Lahanis S, Vlahos L. Focal nodular hyperplasia: imaging findings. Eur Radiol. 2001;11:202-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 162. | Strobel D, Seitz K, Blank W, Schuler A, Dietrich C, von Herbay A, Friedrich-Rust M, Kunze G, Becker D, Will U. Contrast-enhanced ultrasound for the characterization of focal liver lesions--diagnostic accuracy in clinical practice (DEGUM multicenter trial). Ultraschall Med. 2008;29:499-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 175] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 163. | Carlson SK, Johnson CD, Bender CE, Welch TJ. CT of focal nodular hyperplasia of the liver. AJR Am J Roentgenol. 2000;174:705-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 74] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 164. | Choi CS, Freeny PC. Triphasic helical CT of hepatic focal nodular hyperplasia: incidence of atypical findings. AJR Am J Roentgenol. 1998;170:391-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 79] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 165. | Mathieu D, Bruneton JN, Drouillard J, Pointreau CC, Vasile N. Hepatic adenomas and focal nodular hyperplasia: dynamic CT study. Radiology. 1986;160:53-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 112] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 166. | Shirkhoda A, Farah MC, Bernacki E, Madrazo B, Roberts J. Hepatic focal nodular hyperplasia: CT and sonographic spectrum. Abdom Imaging. 1994;19:34-38. [PubMed] |
| 167. | Mahfouz AE, Hamm B, Taupitz M, Wolf KJ. Hypervascular liver lesions: differentiation of focal nodular hyperplasia from malignant tumors with dynamic gadolinium-enhanced MR imaging. Radiology. 1993;186:133-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 101] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 168. | Hussain SM, Terkivatan T, Zondervan PE, Lanjouw E, de Rave S, Ijzermans JN, de Man RA. Focal nodular hyperplasia: findings at state-of-the-art MR imaging, US, CT, and pathologic analysis. Radiographics. 2004;24:3-17; discussion 18-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 200] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 169. | Martí-Bonmatí L, Casillas C, Dosdá R. Enhancement characteristics of hepatic focal nodular hyperplasia and its scar by dynamic magnetic resonance imaging. MAGMA. 2000;10:200-204. [PubMed] |
| 170. | Nagorney DM. Benign hepatic tumors: focal nodular hyperplasia and hepatocellular adenoma. World J Surg. 1995;19:13-18. [PubMed] |
| 171. | Vilgrain V, Fléjou JF, Arrivé L, Belghiti J, Najmark D, Menu Y, Zins M, Vullierme MP, Nahum H. Focal nodular hyperplasia of the liver: MR imaging and pathologic correlation in 37 patients. Radiology. 1992;184:699-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 103] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 172. | Zech CJ, Grazioli L, Breuer J, Reiser MF, Schoenberg SO. Diagnostic performance and description of morphological features of focal nodular hyperplasia in Gd-EOB-DTPA-enhanced liver magnetic resonance imaging: results of a multicenter trial. Invest Radiol. 2008;43:504-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 189] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 173. | Raman SS, Leary C, Bluemke DA, Amendola M, Sahani D, McTavish JD, Brody J, Outwater E, Mitchell D, Sheafor DH. Improved characterization of focal liver lesions with liver-specific gadoxetic acid disodium-enhanced magnetic resonance imaging: a multicenter phase 3 clinical trial. J Comput Assist Tomogr. 2010;34:163-172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 174. | Hammerstingl R, Huppertz A, Breuer J, Balzer T, Blakeborough A, Carter R, Fusté LC, Heinz-Peer G, Judmaier W, Laniado M. Diagnostic efficacy of gadoxetic acid (Primovist)-enhanced MRI and spiral CT for a therapeutic strategy: comparison with intraoperative and histopathologic findings in focal liver lesions. Eur Radiol. 2008;18:457-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 311] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 175. | Seitz K, Bernatik T, Strobel D, Blank W, Friedrich-Rust M, Strunk H, Greis C, Kratzer W, Schuler A. Contrast-enhanced ultrasound (CEUS) for the characterization of focal liver lesions in clinical practice (DEGUM Multicenter Trial): CEUS vs. MRI--a prospective comparison in 269 patients. Ultraschall Med. 2010;31:492-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 103] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 176. | Schmidt E, Udvaros E, Szabó Z, Zámbó K. Varying appearance of focal nodular hyperplasia in nuclear medicine imaging. Clin Nucl Med. 2008;33:71-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 177. | Becker YT, Raiford DS, Webb L, Wright JK, Chapman WC, Pinson CW. Rupture and hemorrhage of hepatic focal nodular hyperplasia. Am Surg. 1995;61:210-214. [PubMed] |
| 178. | Cherqui D, Rahmouni A, Charlotte F, Boulahdour H, Métreau JM, Meignan M, Fagniez PL, Zafrani ES, Mathieu D, Dhumeaux D. Management of focal nodular hyperplasia and hepatocellular adenoma in young women: a series of 41 patients with clinical, radiological, and pathological correlations. Hepatology. 1995;22:1674-1681. [PubMed] |
| 179. | Evans JE, Dick R, Sherlock S. Focal nodular hyperplasia of the liver. Br J Surg. 1980;67:175-177. [PubMed] |
| 180. | Kerlin P, Davis GL, McGill DB, Weiland LH, Adson MA, Sheedy PF. Hepatic adenoma and focal nodular hyperplasia: clinical, pathologic, and radiologic features. Gastroenterology. 1983;84:994-1002. [PubMed] |
| 181. | Pain JA, Gimson AE, Williams R, Howard ER. Focal nodular hyperplasia of the liver: results of treatment and options in management. Gut. 1991;32:524-527. [PubMed] |
| 182. | Sadowski DC, Lee SS, Wanless IR, Kelly JK, Heathcote EJ. Progressive type of focal nodular hyperplasia characterized by multiple tumors and recurrence. Hepatology. 1995;21:970-975. [PubMed] |
| 183. | Bioulac-Sage P, Laumonier H, Laurent C, Zucman-Rossi J, Balabaud C. Hepatocellular adenoma: what is new in 2008. Hepatol Int. 2008;2:316-321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 184. | Dimmick JE, Rogers PCJ, Blair G. Hepatic Tumors. Neoplastic Diseases of Childhood Chur. Switzerland: Harwood Academic 1994; 973-1010. |
| 185. | Di Stasi M, Caturelli E, De Sio I, Salmi A, Buscarini E, Buscarini L. Natural history of focal nodular hyperplasia of the liver: an ultrasound study. J Clin Ultrasound. 1996;24:345-350. [PubMed] [DOI] [Full Text] |
| 186. | Buetow PC, Pantongrag-Brown L, Buck JL, Ros PR, Goodman ZD. Focal nodular hyperplasia of the liver: radiologic-pathologic correlation. Radiographics. 1996;16:369-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 133] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 187. | De Carlis L, Pirotta V, Rondinara GF, Sansalone CV, Colella G, Maione G, Slim AO, Rampoldi A, Cazzulani A, Belli L. Hepatic adenoma and focal nodular hyperplasia: diagnosis and criteria for treatment. Liver Transpl Surg. 1997;3:160-165. [PubMed] |
| 188. | Weimann A, Ringe B, Klempnauer J, Lamesch P, Gratz KF, Prokop M, Maschek H, Tusch G, Pichlmayr R. Benign liver tumors: differential diagnosis and indications for surgery. World J Surg. 1997;21:983-90; discussion 990-1. [PubMed] |
| 189. | Okada T, Sasaki F, Kamiyama T, Nakagawa T, Nakanishi K, Onodera Y, Itoh T, Todo S. Management and algorithm for focal nodular hyperplasia of the liver in children. Eur J Pediatr Surg. 2006;16:235-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 190. | Yang Y, Fu S, Li A, Zhou W, Pan Z, Cui L, Huang G, Wu B, Wu M. Management and surgical treatment for focal nodular hyperplasia in children. Pediatr Surg Int. 2008;24:699-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 191. | Trotter JF, Everson GT. Benign focal lesions of the liver. Clin Liver Dis. 2001;5:17-42, v. [PubMed] |
| 192. | Stromeyer FW, Ishak KG. Nodular transformation (nodular “regenerative” hyperplasia) of the liver. A clinicopathologic study of 30 cases. Hum Pathol. 1981;12:60-71. [PubMed] |
| 193. | Steiner PE. Nodular regenerative hyperplasia of the liver. Am J Pathol. 1959;35:943-953. [PubMed] |
| 194. | Ranstrom S. Miliary hepatocellular adenomatosis. Acta Pathol Microbiol Scand. 1953;33:225-229. [PubMed] |
| 195. | Naber AH, Van Haelst U, Yap SH. Nodular regenerative hyperplasia of the liver: an important cause of portal hypertension in non-cirrhotic patients. J Hepatol. 1991;12:94-99. [PubMed] |
| 196. | Hartleb M, Gutkowski K, Milkiewicz P. Nodular regenerative hyperplasia: evolving concepts on underdiagnosed cause of portal hypertension. World J Gastroenterol. 2011;17:1400-1409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 128] [Cited by in RCA: 122] [Article Influence: 8.7] [Reference Citation Analysis (1)] |
| 197. | Hillaire S, Bonte E, Denninger MH, Casadevall N, Cadranel JF, Lebrec D, Valla D, Degott C. Idiopathic non-cirrhotic intrahepatic portal hypertension in the West: a re-evaluation in 28 patients. Gut. 2002;51:275-280. [PubMed] |
| 198. | Alperstein G, Kahn E, Aiges H, Daum F. Nodular regenerative hyperplasia of the liver: an unusual cause of portal hypertension in childhood. Am J Dis Child. 1981;135:572-574. [PubMed] |
| 199. | Casillas C, Martí-Bonmatí L, Galant J. Pseudotumoral presentation of nodular regenerative hyperplasia of the liver: imaging in five patients including MR imaging. Eur Radiol. 1997;7:654-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 200. | Dachman AH, Ros PR, Goodman ZD, Olmsted WW, Ishak KG. Nodular regenerative hyperplasia of the liver: clinical and radiologic observations. AJR Am J Roentgenol. 1987;148:717-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 62] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 201. | Galdeano S, Drut R. Nodular regenerative hyperplasia of fetal liver: a report of two cases. Pediatr Pathol. 1991;11:479-485. [PubMed] |
| 202. | Mahamid J, Miselevich I, Attias D, Laor R, Zuckerman E, Shaoul R. Nodular regenerative hyperplasia associated with idiopathic thrombocytopenic purpura in a young girl: a case report and review of the literature. J Pediatr Gastroenterol Nutr. 2005;41:251-255. [PubMed] |
| 203. | Mones JM, Saldana MJ. Nodular regenerative hyperplasia of the liver in a 4-month-old infant. Am J Dis Child. 1984;138:79-81. [PubMed] |
| 204. | Moran CA, Mullick FG, Ishak KG. Nodular regenerative hyperplasia of the liver in children. Am J Surg Pathol. 1991;15:449-454. [PubMed] |
| 205. | Pettei MJ, Valderamma E, Levine JJ, Ilowite NT. Childhood nodular regenerative hyperplasia of the liver complicated by severe hypoxemia. J Pediatr Gastroenterol Nutr. 1995;20:343-346. [PubMed] |
| 206. | Trenschel GM, Schubert A, Dries V, Benz-Bohm G. Nodular regenerative hyperplasia of the liver: case report of a 13-year-old girl and review of the literature. Pediatr Radiol. 2000;30:64-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 207. | Tsui WM, So KT. Partial nodular transformation of liver in a child. Histopathology. 1993;22:594-596. [PubMed] |
| 208. | Buffet C, Altman C. [Nodular regenerative hyperplasia]. Gastroenterol Clin Biol. 1994;18:123-132. [PubMed] |
| 209. | Asherson RA, Cervera R. Unusual manifestations of the antiphospholipid syndrome. Clin Rev Allergy Immunol. 2003;25:61-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 210. | Dall’Igna P, Cecchetto G, Perilongo G, Scapinello A, Talenti E, Gamba PG, Zancan L, Guglielmi M. Nodular regenerative hyperplasia of the liver: description of two cases. Med Pediatr Oncol. 1996;26:190-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 211. | de Sousa JM, Portmann B, Williams R. Nodular regenerative hyperplasia of the liver and the Budd-Chiari syndrome. Case report, review of the literature and reappraisal of pathogenesis. J Hepatol. 1991;12:28-35. [PubMed] |
| 212. | Morris JM, Oien KA, McMahon M, Forrest EH, Morris J, Stanley AJ, Campbell S. Nodular regenerative hyperplasia of the liver: survival and associated features in a UK case series. Eur J Gastroenterol Hepatol. 2010;22:1001-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 213. | Rha SE, Lee MG, Lee YS, Kang GH, Ha HK, Kim PN, Auh YH. Nodular regenerative hyperplasia of the liver in Budd-Chiari syndrome: CT and MR features. Abdom Imaging. 2000;25:255-258. [PubMed] |
| 214. | Reshamwala PA, Kleiner DE, Heller T. Nodular regenerative hyperplasia: not all nodules are created equal. Hepatology. 2006;44:7-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 168] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 215. | Devarbhavi H, Abraham S, Kamath PS. Significance of nodular regenerative hyperplasia occurring de novo following liver transplantation. Liver Transpl. 2007;13:1552-1556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 216. | Dumortier J, Boillot O, Chevallier M, Berger F, Potier P, Valette PJ, Paliard P, Scoazec JY. Familial occurrence of nodular regenerative hyperplasia of the liver: a report on three families. Gut. 1999;45:289-294. [PubMed] |
| 217. | Ames JT, Federle MP, Chopra K. Distinguishing clinical and imaging features of nodular regenerative hyperplasia and large regenerative nodules of the liver. Clin Radiol. 2009;64:1190-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 218. | Biecker E, Trebicka J, Fischer HP, Sauerbruch T, Lammert F. Portal hypertension and nodular regenerative hyperplasia in a patient with celiac disease. Z Gastroenterol. 2006;44:395-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 219. | Wang HM, Lo GH, Hsu PI, Lin CK, Chan HH, Chen WC, Lai KH, Wang BW, Lin SL. Nodular regenerative hyperplasia of the liver. J Chin Med Assoc. 2008;71:523-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 220. | Seiderer J, Zech CJ, Diebold J, Schoenberg SO, Brand S, Tillack C, Göke B, Ochsenkühn T. Nodular regenerative hyperplasia: a reversible entity associated with azathioprine therapy. Eur J Gastroenterol Hepatol. 2006;18:553-555. [PubMed] |
| 221. | Mergo PJ, Ros PR. Imaging of diffuse liver disease. Radiol Clin North Am. 1998;36:365-375. [PubMed] |
| 222. | Clouet M, Boulay I, Boudiaf M, Soyer P, Nemeth J, Kiselman R, Rymer R. Imaging features of nodular regenerative hyperplasia of the liver mimicking hepatic metastases. Abdom Imaging. 1999;24:258-261. [PubMed] |
| 223. | Horita T, Tsutsumi A, Takeda T, Yasuda S, Takeuchi R, Amasaki Y, Ichikawa K, Atsumi T, Koike T. Significance of magnetic resonance imaging in the diagnosis of nodular regenerative hyperplasia of the liver complicated with systemic lupus erythematosus: a case report and review of the literature. Lupus. 2002;11:193-196. [PubMed] |
| 224. | Siegelman ES, Outwater EK, Furth EE, Rubin R. MR imaging of hepatic nodular regenerative hyperplasia. J Magn Reson Imaging. 1995;5:730-732. [PubMed] |
| 225. | Zech CJ, Seiderer J, Reinisch W, Ochsenkuhn T, Schima W, Diebold J, Wrba F, Reiser MF, Schoenberg SO. Thioguanin-induced nodular regenerative hyperplasia of the liver-ROC analysis of different MR techniques. Eur Radiol. 2007;17:1898-1905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 226. | Laharie D, Vergniol J, Bioulac-Sage P, Diris B, Poli J, Foucher J, Couzigou P, Drouillard J, de Lédinghen V. Usefulness of noninvasive tests in nodular regenerative hyperplasia of the liver. Eur J Gastroenterol Hepatol. 2010;22:487-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 227. | Nzeako UC, Goodman ZD, Ishak KG. Hepatocellular carcinoma and nodular regenerative hyperplasia: possible pathogenetic relationship. Am J Gastroenterol. 1996;91:879-884. [PubMed] |
| 228. | Daga BV, Shah VR, More RB. CT scan diagnosis of hepatic adenoma in a case of von Gierke disease. Indian J Radiol Imaging. 2012;22:54-57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 229. | Resnick MB, Kozakewich HP, Perez-Atayde AR. Hepatic adenoma in the pediatric age group. Clinicopathological observations and assessment of cell proliferative activity. Am J Surg Pathol. 1995;19:1181-1190. [PubMed] |
| 230. | Fabre M, Yilmaz F, Buendia MA. [Hepatic tumors in childhood: experience on 245 tumors and review of literature]. Ann Pathol. 2004;24:536-555. [PubMed] |
| 231. | Geramizadeh B, Bahador A, Foroutan HR, Banani A, Nikeghbalian S, Malek-Hosseini SA. Pathology of pediatric liver tumors, a single center experience from south of Iran. Indian J Pathol Microbiol. 2010;53:422-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 232. | Wheeler DA, Edmondson HA, Reynolds TB. Spontaneous liver cell adenoma in children. Am J Clin Pathol. 1986;85:6-12. [PubMed] |
| 233. | Flejou JF, Barge J, Menu Y, Degott C, Bismuth H, Potet F, Benhamou JP. Liver adenomatosis. An entity distinct from liver adenoma? Gastroenterology. 1985;89:1132-1138. [PubMed] |
| 234. | Grazioli L, Federle MP, Ichikawa T, Balzano E, Nalesnik M, Madariaga J. Liver adenomatosis: clinical, histopathologic, and imaging findings in 15 patients. Radiology. 2000;216:395-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 110] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 235. | Lewin M, Handra-Luca A, Arrivé L, Wendum D, Paradis V, Bridel E, Fléjou JF, Belghiti J, Tubiana JM, Vilgrain V. Liver adenomatosis: classification of MR imaging features and comparison with pathologic findings. Radiology. 2006;241:433-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 69] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 236. | Siegel MJ, Chung EM, Conran RM. Pediatric liver: focal masses. Magn Reson Imaging Clin N Am. 2008;16:437-52, v. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 237. | Gold JH, Guzman IJ, Rosai J. Benign tumors of the liver. Pathologic examination of 45 cases. Am J Clin Pathol. 1978;70:6-17. [PubMed] |
| 238. | Brancatelli G, Federle MP, Vullierme MP, Lagalla R, Midiri M, Vilgrain V. CT and MR imaging evaluation of hepatic adenoma. J Comput Assist Tomogr. 2006;30:745-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 239. | Franchi-Abella S, Branchereau S, Lambert V, Fabre M, Steimberg C, Losay J, Riou JY, Pariente D, Gauthier F, Jacquemin E. Complications of congenital portosystemic shunts in children: therapeutic options and outcomes. J Pediatr Gastroenterol Nutr. 2010;51:322-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 159] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 240. | Grazioli L, Federle MP, Brancatelli G, Ichikawa T, Olivetti L, Blachar A. Hepatic adenomas: imaging and pathologic findings. Radiographics. 2001;21:877-92; discussion 892-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 193] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 241. | Guérin F, Blanc T, Gauthier F, Abella SF, Branchereau S. Congenital portosystemic vascular malformations. Semin Pediatr Surg. 2012;21:233-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 242. | Kim T, Murakami T, Sugihara E, Hori M, Wakasa K, Nakamura H. Hepatic nodular lesions associated with abnormal development of the portal vein. AJR Am J Roentgenol. 2004;183:1333-1338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 40] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 243. | Lautz TB, Finegold MJ, Chin AC, Superina RA. Giant hepatic adenoma with atypical features in a patient on oxcarbazepine therapy. J Pediatr Surg. 2008;43:751-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 244. | Triantafyllopoulou M, Whitington PF, Melin-Aldana H, Benya EC, Brickman W. Hepatic adenoma in an adolescent with elevated androgen levels. J Pediatr Gastroenterol Nutr. 2007;44:640-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 245. | Rossor T, Quaglia A, Zacharoulis S, Strautnieks S, Davebport M, Hadzic N. Hepatic adenoma mimicking a Leydig-Sertoli cell tumor metastasis. Paediatrics Today. 2013;9:201-204. |
| 246. | Calderaro J, Labrune P, Morcrette G, Rebouissou S, Franco D, Prévot S, Quaglia A, Bedossa P, Libbrecht L, Terracciano L. Molecular characterization of hepatocellular adenomas developed in patients with glycogen storage disease type I. J Hepatol. 2013;58:350-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 120] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 247. | Alter BP. Cancer in Fanconi anemia, 1927-2001. Cancer. 2003;97:425-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 286] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 248. | Ozenne V, Paradis V, Vullierme MP, Vilgrain V, Leblanc T, Belghiti J, Imbert A, Valla DC, Degos F. Liver tumours in patients with Fanconi anaemia: a report of three cases. Eur J Gastroenterol Hepatol. 2008;20:1036-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 249. | Velazquez I, Alter BP. Androgens and liver tumors: Fanconi’s anemia and non-Fanconi’s conditions. Am J Hematol. 2004;77:257-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 143] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 250. | Leese T, Farges O, Bismuth H. Liver cell adenomas. A 12-year surgical experience from a specialist hepato-biliary unit. Ann Surg. 1988;208:558-564. [PubMed] |
| 251. | Vaithianathan R, Philipchandran G, Jayaganesh P. Spontaneous hepatocellular adenoma in paediatric age group - case report. J Clin Diagn Res. 2013;7:2962-2963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 252. | Ichikawa T, Federle MP, Grazioli L, Nalesnik M. Hepatocellular adenoma: multiphasic CT and histopathologic findings in 25 patients. Radiology. 2000;214:861-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 99] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 253. | Hussain SM, van den Bos IC, Dwarkasing RS, Kuiper JW, den Hollander J. Hepatocellular adenoma: findings at state-of-the-art magnetic resonance imaging, ultrasound, computed tomography and pathologic analysis. Eur Radiol. 2006;16:1873-1886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 57] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 254. | Golli M, Van Nhieu JT, Mathieu D, Zafrani ES, Cherqui D, Dhumeaux D, Vasile N, Rahmouni A. Hepatocellular adenoma: color Doppler US and pathologic correlations. Radiology. 1994;190:741-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 255. | Laumonier H, Cailliez H, Balabaud C, Possenti L, Zucman-Rossi J, Bioulac-Sage P, Trillaud H. Role of contrast-enhanced sonography in differentiation of subtypes of hepatocellular adenoma: correlation with MRI findings. AJR Am J Roentgenol. 2012;199:341-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 256. | Welch TJ, Sheedy PF, Johnson CM, Stephens DH, Charboneau JW, Brown ML, May GR, Adson MA, McGill DB. Focal nodular hyperplasia and hepatic adenoma: comparison of angiography, CT, US, and scintigraphy. Radiology. 1985;156:593-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 151] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 257. | Ruppert-Kohlmayr AJ, Uggowitzer MM, Kugler C, Zebedin D, Schaffler G, Ruppert GS. Focal nodular hyperplasia and hepatocellular adenoma of the liver: differentiation with multiphasic helical CT. AJR Am J Roentgenol. 2001;176:1493-1498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 258. | Arrivé L, Fléjou JF, Vilgrain V, Belghiti J, Najmark D, Zins M, Menu Y, Tubiana JM, Nahum H. Hepatic adenoma: MR findings in 51 pathologically proved lesions. Radiology. 1994;193:507-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 115] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 259. | Chung KY, Mayo-Smith WW, Saini S, Rahmouni A, Golli M, Mathieu D. Hepatocellular adenoma: MR imaging features with pathologic correlation. AJR Am J Roentgenol. 1995;165:303-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 81] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 260. | Paulson EK, McClellan JS, Washington K, Spritzer CE, Meyers WC, Baker ME. Hepatic adenoma: MR characteristics and correlation with pathologic findings. AJR Am J Roentgenol. 1994;163:113-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 125] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 261. | Grazioli L, Morana G, Kirchin MA, Schneider G. Accurate differentiation of focal nodular hyperplasia from hepatic adenoma at gadobenate dimeglumine-enhanced MR imaging: prospective study. Radiology. 2005;236:166-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 243] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 262. | Chaib E, Gama-Rodrigues J, Ribeiro MA, Herman P, Saad WA. Hepatic adenoma. Timing for surgery. Hepatogastroenterology. 2007;54:1382-1387. [PubMed] |
| 263. | Rocourt DV, Shiels WE, Hammond S, Besner GE. Contemporary management of benign hepatic adenoma using percutaneous radiofrequency ablation. J Pediatr Surg. 2006;41:1149-1152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 264. | Chiche L, Dao T, Salamé E, Galais MP, Bouvard N, Schmutz G, Rousselot P, Bioulac-Sage P, Ségol P, Gignoux M. Liver adenomatosis: reappraisal, diagnosis, and surgical management: eight new cases and review of the literature. Ann Surg. 2000;231:74-81. [PubMed] |
| 265. | Kumar AR, Wagner JE, Auerbach AD, Coad JE, Dietz CA, Schwarzenberg SJ, MacMillan ML. Fatal hemorrhage from androgen-related hepatic adenoma after hematopoietic cell transplantation. J Pediatr Hematol Oncol. 2004;26:16-18. [PubMed] |
| 266. | van Aalten SM, de Man RA, IJzermans JN, Terkivatan T. Systematic review of haemorrhage and rupture of hepatocellular adenomas. Br J Surg. 2012;99:911-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 121] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 267. | Bioulac-Sage P, Balabaud C, Bedossa P, Scoazec JY, Chiche L, Dhillon AP, Ferrell L, Paradis V, Roskams T, Vilgrain V. Pathological diagnosis of liver cell adenoma and focal nodular hyperplasia: Bordeaux update. J Hepatol. 2007;46:521-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 112] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 268. | Bioulac-Sage P, Laumonier H, Couchy G, Le Bail B, Sa Cunha A, Rullier A, Laurent C, Blanc JF, Cubel G, Trillaud H. Hepatocellular adenoma management and phenotypic classification: the Bordeaux experience. Hepatology. 2009;50:481-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 296] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 269. | Foster JH, Berman MM. The malignant transformation of liver cell adenomas. Arch Surg. 1994;129:712-717. [PubMed] |
| 270. | Parker P, Burr I, Slonim A, Ghishan FK, Greene H. Regression of hepatic adenomas in type Ia glycogen storage disease with dietary therapy. Gastroenterology. 1981;81:534-536. [PubMed] |
| 271. | Stoot JH, van der Linden E, Terpstra OT, Schaapherder AF. Life-saving therapy for haemorrhaging liver adenomas using selective arterial embolization. Br J Surg. 2007;94:1249-1253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 272. | Jacob J, Deganello A, Sellars ME, Hadzic N, Sidhu PS. Contrast enhanced ultrasound (CEUS) characterization of grey-scale sonographic indeterminate focal liver lesions in pediatric practice. Ultraschall Med. 2013;34:529-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |