Published online Jul 21, 2015. doi: 10.3748/wjg.v21.i27.8458
Peer-review started: December 22, 2014
First decision: January 8, 2015
Revised: February 10, 2015
Accepted: March 27, 2015
Published online: July 21, 2015
Processing time: 212 Days and 20.6 Hours
Endoscopic ultrasonography-guided fine needle aspiration (EUS-FNA) is a useful and relatively safe tool for the diagnosis and staging of pancreatic cancer. However, there have recently been several reports of tumor seeding after EUS-FNA of adenocarcinomas. A 78-year-old man was admitted to our hospital due to upper gastric pain. Examinations revealed a 20 mm mass in the pancreatic body, for which EUS-FNA was performed. The cytology of the lesion was adenocarcinoma, and the stage of the cancer was T3N0M0. The patient underwent surgery with curative intent, followed by adjuvant chemotherapy with S-1. An enlarging gastric submucosal tumor was found on gastroscopy at 28 mo after surgery accompanied by a rising level of CA19-9. Biopsy result was adenocarcinoma, consistent with a pancreatic primary tumor. Tumor seeding after EUS-FNA was strongly suspected. The patient underwent surgical resection of the gastric tumor with curative intent. The pathological result of the resected gastric specimen was adenocarcinoma with a perfectly matched mucin special stain result with the previously resected pancreatic cancer. This is the first case report of tumor seeding after EUS-FNA which was surgically resected and inspected pathologically.
Core tip: This manuscript is the first case report about tumor seeding after endoscopic ultrasonography-guided fine needle aspiration (EUS-FNA) which was surgically resected and inspected pathologically. EUS-FNA is a widely performed procedure; however, there are no clear guidelines indicating the selection of treatments in cases of tumor seeding after the procedure, as this adverse event is relatively rare. This manuscript may help in the selection of patients undergoing EUS-FNA, and clarifies the points we should be careful about after the procedure.
- Citation: Tomonari A, Katanuma A, Matsumori T, Yamazaki H, Sano I, Minami R, Sen-yo M, Ikarashi S, Kin T, Yane K, Takahashi K, Shinohara T, Maguchi H. Resected tumor seeding in stomach wall due to endoscopic ultrasonography-guided fine needle aspiration of pancreatic adenocarcinoma. World J Gastroenterol 2015; 21(27): 8458-8461
- URL: https://www.wjgnet.com/1007-9327/full/v21/i27/8458.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i27.8458
Endoscopic ultrasonography-guided fine needle aspiration (EUS-FNA) is a useful and relatively safe tool for the diagnosis and staging of pancreatic cancer. The reported complication rates of EUS-FNA are low (< 1% in large centers[1]), and the risks of tumor seeding are lower compared to transcutaneous methods[2,3]. However, there have recently been several reports of tumor seeding after EUS-FNA of non-cystic solid adenocarcinomas[4,5]. The previously reported cases were all diagnosed at unresectable stages when the tumor seeding lesions were found. This is the first case report of tumor seeding after EUS-FNA which was surgically resected and inspected pathologically.
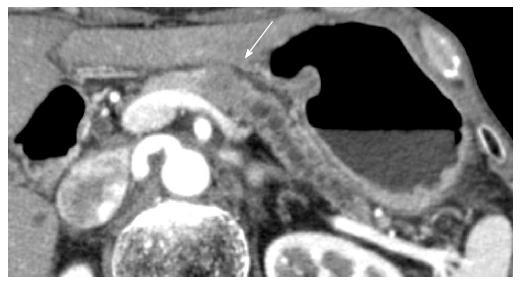
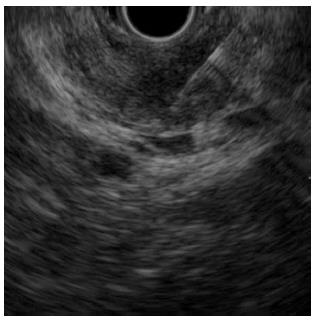
A 78-year-old man with persistent upper gastric pain was referred to our center for detailed examination. Computed tomography (CT) revealed a 20-mm-diameter hypodense mass lesion in the pancreatic body, with an upstream dilatation of the main pancreatic duct (Figure 1). CA19-9 was abnormal with a level of 162.4 kU/mL (normal range, 0-37.0 kU/mL). At EUS, the patient had a 21-mm-diameter hypoechoic mass in the pancreatic body. EUS-FNA of the mass was performed using a 22-G needle (Expect, Boston Scientific, Natick, MA) (Figure 2). Suction was applied at each pass, and a total of two passes were made. Cytology of the lesion revealed an adenocarcinoma. No metastatic disease was found and the patient underwent surgery with curative intent.
There were no liver metastases, ascites, or peritoneal implantation at surgery. An intra-operative frozen section diagnosis of a lymph node around the abdominal aorta was performed, which revealed to be negative for metastasis. A 20-mm palpable mass was identified, with evidence of inflammation in the distal pancreas. Distal pancreatectomy and splenectomy were performed. Histology of the specimen revealed a 25 mm × 25 mm mass with no lymphatic or vascular involvement, and the resection margins were negative for malignancy. There were no abnormalities in the spleen. The final diagnosis was T3N0M0 with an extension of moderately differentiated adenocarcinoma beyond the pancreas in the ventral direction.
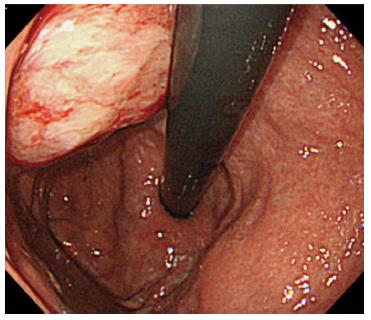
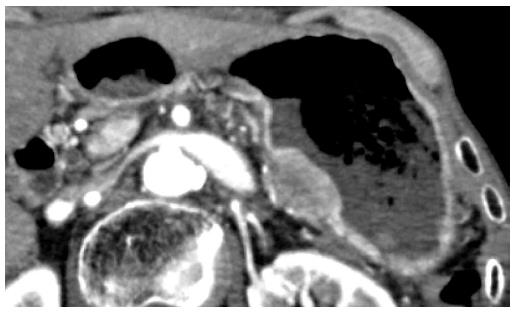
The patient underwent adjuvant chemotherapy with S-1 starting at 2 mo after surgery. At 9 mo after surgery, a periodical follow-up gastroscopy performed at a private clinic revealed a 5-mm-diameter submucosal tumor at the posterior wall of the gastric body. The patient had no symptoms at that time. The private clinician who had performed the exam made the decision to follow the submucosal tumor, and gastroscopy was repeated after 2 mo, which showed no changes in the shape or the size of the submucosal tumor. At 8 mo after surgery, CA19-9 level gradually started to rise. Gastroscopy performed at 28 mo after surgery revealed an increase in the size of the submucosal tumor, and at this point, the patient was referred to our center for further examinations (Figure 3). Histology of the lesion indicated adenocarcinoma, consistent with a pancreatic primary tumor. CT showed a 28-mm submucosal tumor adjacent to the pancreatic resection site (Figure 4). There were no apparent metastatic lesions, and the patient underwent surgery with curative intent.
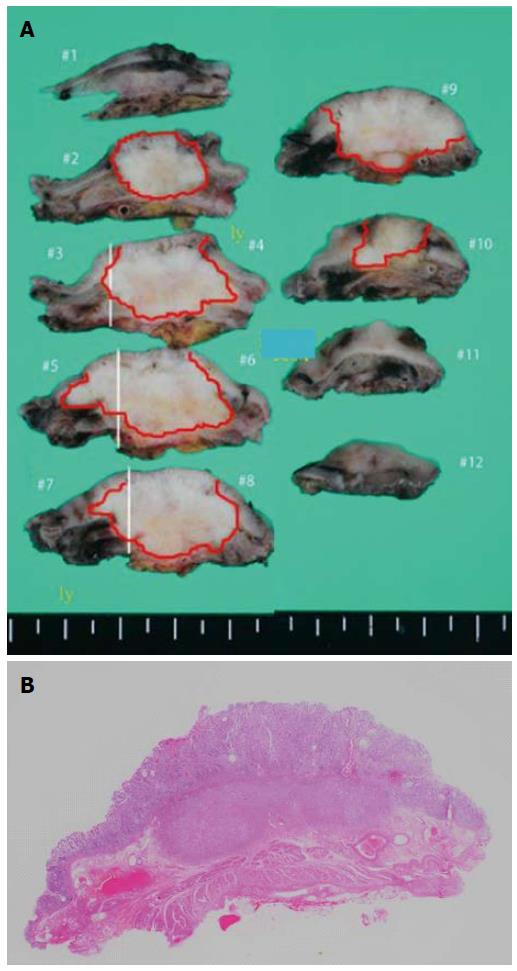
Subtotal gastrectomy was performed. Histology of the specimen revealed a 32 mm × 30 mm submucosal tumor, consisting of moderately to well differentiated adenocarcinoma (Figure 5). The specimen was positive for vascular and lymphatic involvement. Mucin special stain results for the resected stomach specimen were MUC1 positive, MUC2 negative, MUC5AC positive, and MUC6 negative. Mucin special stains for the previously resected specimen of the pancreas were additionally performed. The results were MUC1 positive, MUC2 negative, MUC5AC positive, and MUC6 negative, which were perfectly compatible with the resected stomach lesion.
Although gastric recurrence of adenocarcinoma may occur by direct invasion, the resected specimen in this case strongly suggests tumor seeding after EUS-FNA due to the facts that its origin is submucosal, and the mucin special stains were perfectly matched to the original pancreatic cancer. There has been a retrospective study which reported that there is no association with an increased rate of gastric or peritoneal cancer recurrence following EUS-FNA[6]. However, it is also true that there have been several reports of tumor seeding after the procedure. This may be due to the rate of tumor seeding after EUS-FNA being so low that it is statistically difficult to establish an association between the procedure and the recurrence. Also, unresected recurrences are difficult to distinguish between direct invasion and tract seeding, and this may contribute to the fact that the reported incidence rate of seeding is so low.
EUS-FNA is an important procedure usually performed pre-operatively to determine the indication of surgical resection of the tumor. The reported incidences of tumor seeding are so low that the advantage of performing EUS-FNA before surgery may not outweigh the risk of needle tract seeding. However, as the number of EUS-FNA is expected to continue to increase, the possibility of post-procedural EUS-FNA needle tract seeding should always be minded. In cases when surgical resection will not include the needle tract (e.g., resection of the tail/body of the pancreas), EUS-FNA should be either avoided, or the number of needle passes limited. When EUS-FNA is indispensable for the diagnosis and treatment of the disease, excision of the needle tract path may be necessary for consideration. When this is not possible, close periodical examinations including gastroscopy should be scheduled for patients who undergo EUS-FNA without needle tract resection.
A case of tumor seeding of a pancreatic adenocarcinoma in the stomach wall at the endoscopic ultrasonography-guided fine needle aspiration (EUS-FNA) puncture site, was treated surgically and inspected pathologically.
A submucosal tumor of the stomach wall found at a periodical post-operative endoscopy was suspected as a seeding tumor recurrence of the resected pancreatic adenocarcinoma.
Pancreatic adenocarcinoma metastasis or a new completely separate submucosal lesion such as a GIST.
Periodical tumor marker follow-ups were performed, showing a gradual CA19-9 increase.
Periodical gastroscopy revealed a lesion in a submucosal tumor form at the puncture site of the EUS-FNA which was performed preoperatively.
Endoscopically performed biopsy revealed an adenocarcinoma, leading to the suspicion of a tumor recurrence as a seeding after EUS-FNA.
Subtotal gastrectomy was performed, and mucin special stain results for the resected stomach specimen were perfectly compatible with the previously resected pancreatic adenocarcinoma.
When EUS-FNA is indispensable for the diagnosis and treatment of the disease, excision of the needle tract path may be necessary for consideration. When this is not possible, close periodical examinations including gastroscopy should be scheduled for patients who undergo EUS-FNA without needle tract resection.
This is a rare case of tumor recurrence of a pancreatic adenocarcinoma as seeding after EUS-FNA, treated surgically and inspected pathologically.
P- Reviewer: Altonbary AY, Hirahara N S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Liu XM
| 1. | Carrara S, Arcidiacono PG, Mezzi G, Petrone MC, Boemo C, Testoni PA. Pancreatic endoscopic ultrasound-guided fine needle aspiration: complication rate and clinical course in a single centre. Dig Liver Dis. 2010;42:520-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 2. | Smith EH. Complications of percutaneous abdominal fine-needle biopsy. Review. Radiology. 1991;178:253-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 357] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 3. | Micames C, Jowell PS, White R, Paulson E, Nelson R, Morse M, Hurwitz H, Pappas T, Tyler D, McGrath K. Lower frequency of peritoneal carcinomatosis in patients with pancreatic cancer diagnosed by EUS-guided FNA vs. percutaneous FNA. Gastrointest Endosc. 2003;58:690-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 278] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 4. | Paquin SC, Gariépy G, Lepanto L, Bourdages R, Raymond G, Sahai AV. A first report of tumor seeding because of EUS-guided FNA of a pancreatic adenocarcinoma. Gastrointest Endosc. 2005;61:610-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 163] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 5. | Katanuma A, Maguchi H, Hashigo S, Kaneko M, Kin T, Yane K, Kato R, Kato S, Harada R, Osanai M. Tumor seeding after endoscopic ultrasound-guided fine-needle aspiration of cancer in the body of the pancreas. Endoscopy. 2012;44 Suppl 2 UCTN:E160-E161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 6. | Ngamruengphong S, Xu C, Woodward TA, Raimondo M, Stauffer JA, Asbun HJ, Wallace MB. Risk of gastric or peritoneal recurrence, and long-term outcomes, following pancreatic cancer resection with preoperative endosonographically guided fine needle aspiration. Endoscopy. 2013;45:619-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |