Published online Jul 21, 2015. doi: 10.3748/wjg.v21.i27.8373
Peer-review started: February 6, 2015
First decision: March 10, 2015
Revised: March 25, 2015
Accepted: May 4, 2015
Article in press: May 4, 2015
Published online: July 21, 2015
Processing time: 166 Days and 21.2 Hours
AIM: To establish a clinical scoring model to predict risk of acute-on-chronic liver failure (ACLF) in chronic hepatitis B (CHB) patients.
METHODS: This was a retrospective study of 1457 patients hospitalized for CHB between October 2008 and October 2013 at the Beijing Ditan Hospital, Capital Medical University, China. The patients were divided into two groups: severe acute exacerbation (SAE) group (n = 382) and non-SAE group (n = 1075). The SAE group was classified as the high-risk group based on the higher incidence of ACLF in this group than in the non-SAE group (13.6% vs 0.4%). Two-thirds of SAE patients were randomly assigned to risk-model derivation and the other one-third to model validation. Univariate risk factors associated with the outcome were entered into a multivariate logistic regression model for screening independent risk factors. Each variable was assigned an integer value based on the regression coefficients, and the final score was the sum of these values in the derivation set. Model discrimination and calibration were assessed using area under the receiver operating characteristic curve and the Hosmer-Lemeshow test.
RESULTS: The risk prediction scoring model included the following four factors: age ≥ 40 years, total bilirubin ≥ 171 μmol/L, prothrombin activity 40%-60%, and hepatitis B virus DNA > 107 copies/mL. The sum risk score ranged from 0 to 7; 0-3 identified patients with lower risk of ACLF, whereas 4-7 identified patients with higher risk. The Kaplan-Meier analysis showed the cumulative risk for ACLF and ACLF-related death in the two risk groups (0-3 and 4-7 scores) of the primary cohort over 56 d, and log-rank test revealed a significant difference (2.0% vs 33.8% and 0.8% vs 9.4%, respectively; both P < 0.0001). In the derivation and validation data sets, the model had good discrimination (C index = 0.857, 95% confidence interval: 0.800-0.913 and C index = 0.889, 95% confidence interval: 0.820-0.957, respectively) and calibration demonstrated by the Hosmer-Lemeshow test (χ2 = 4.516, P = 0.808 and χ2 = 1.959, P = 0.923, respectively).
CONCLUSION: Using the scoring model, clinicians can easily identify patients (total score ≥ 4) at high risk of ACLF and ACLF-related death early during SAE.
Core tip: Acute-on-chronic liver failure (ACLF) is a severe life-threatening clinical syndrome characterized by liver failure and associated with an extremely high mortality if liver transplantation or artificial liver support is not available. Early diagnosis might allow for effective patient risk stratification and more appropriate medical care. However, previous studies on severe acute exacerbation of ACLF have focused on the clinical outcomes, and global uniform standards and correlation studies for predicting ACLF occurrence are lacking. We developed a clinical scoring model to predict the risk of ACLF early in patients with chronic hepatitis B.
- Citation: Gao FY, Liu Y, Li XS, Ye XQ, Sun L, Geng MF, Wang R, Liu HM, Zhou XB, Gu LL, Liu YM, Wan G, Wang XB. Score model for predicting acute-on-chronic liver failure risk in chronic hepatitis B. World J Gastroenterol 2015; 21(27): 8373-8381
- URL: https://www.wjgnet.com/1007-9327/full/v21/i27/8373.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i27.8373
Chronic hepatitis B (CHB) is a significant global health threat with more than 350 million people infected[1]. In the natural history of chronic hepatitis B virus (HBV) infection, severe acute exacerbation (SAE) is not uncommon. It is characterized by high serum alanine aminotransferase (ALT) levels, jaundice, and coagulopathy, and has a cumulative incidence of 10%-30% annually[2-5]. The exacerbations can be mild, but some patients may progress to acute-on-chronic liver failure (ACLF) and even death[6,7].
ACLF is a severe, life-threatening clinical syndrome characterized by liver failure and is associated with multiple end-organ failure[8]. Liver transplantation is the only definitive therapy available to salvage this group of patients, however, this is not readily available or feasible in many parts of the world where HBV is highly endemic. Therefore, for early diagnosis and treatment, it is necessary to determine the risk factors and assess the risk of ACLF. However, global uniform standards and correlation studies for predicting ACLF occurrence are lacking.
In the present study, we analyzed the clinical data of patients hospitalized for CHB at the Beijing Ditan Hospital, Capital Medical University, China over a five-year period (from October 2008 to October 2013). The aims of this study were to identify the independent risk factors for ACLF, to develop a risk prediction model to identify patients at an increased risk of ACLF, and to facilitate implementation of preventive strategies.
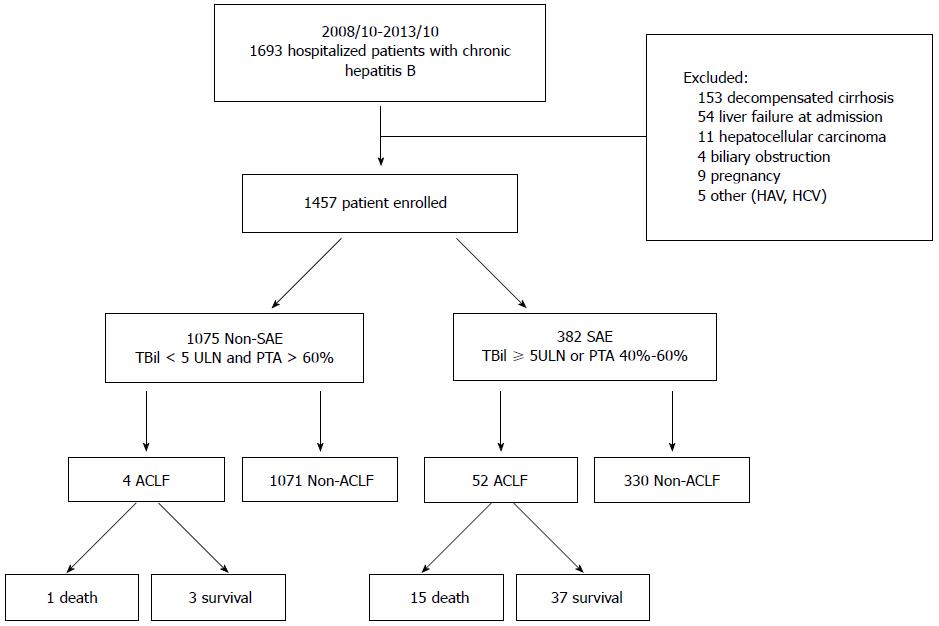
Patients with CHB hospitalized at the Beijing Ditan Hospital, Capital Medical University, China, between October 2008 and October 2013, were retrospectively studied. The preliminary screening identified 1693 patients with ALT levels elevated to > 5 times the upper limit of normal (ULN; 40 IU/L) with complete clinical, laboratory, imaging, and follow-up data. Patients who did not meet the research standards were excluded (n = 236). A total of 1457 patients were enrolled in the study and were subsequently divided into either the SAE or non-SAE group (Figure 1).
Patients enrolled in the study had no coinfection with hepatitis A, C, D, or E viruses or other viruses including HIV, cytomegalovirus, and Epstein-Barr virus, hepatocellular carcinoma (HCC), liver decompensated cirrhosis, alcoholic liver disease, drug-induced hepatitis, autoimmune hepatitis, Wilson disease, jaundice caused by biliary obstruction, prolonged prothrombin time (PT) induced by blood system disease, or serious diseases involving other organ systems, and were not pregnant.
The inclusion criteria for the present study were based on those proposed by Fujiwara et al[9] and Wang et al[10] and the consensus recommendations of the Asian Pacific Association for the Study of the Liver (APASL 2008; India)[11], with minor modifications. The criteria for SAE of CHB were as follows: (1) presence of hepatitis B surface antigen for > 6 mo before hospitalization; (2) ALT > 5 × ULN (200 IU/L); and (3) total bilirubin (TBil) ≥ 5 × ULN (85 μmol/L) or prothrombin activity (PTA) 40%-60%. ACLF was defined as the recent development of jaundice (TBil ≥ 5 × ULN) and coagulopathy [PTA < 40% or international normalized ratio (INR) of PT ≥ 1.5], complicated within 4 wk by ascites and/or encephalopathy in a patient with previously diagnosed or undiagnosed chronic liver disease.
All patients received standard medical treatment except for liver transplantation, including bed rest, liver-protective treatment, energy supplements and vitamins, intravenous infusion of plasma and albumin, maintaining water-electrolyte and acid-base equilibrium, and preventing and treating complications. Antiviral therapy, including lamivudine, adefovir dipivoxil, entecavir, or telbivudine, was administered according to HBV replication levels, financial condition, and the willingness of the patient.
Retrospectively collected data included patient demographics; clinical and laboratory variables including PT, PTA, INR, white blood cell count, neutrophil count, lymphocyte count, serum potassium, sodium, creatinine, alpha-fetoprotein, aspartate transaminase, ALT, TBil, albumin, cholinesterase, hepatitis B e antigen, and HBV DNA levels; and imaging findings. Baseline data were the data obtained at the first diagnosis from computerized and paper medical records. In addition, the severity of liver disease was assessed using the Model for End-Stage Liver Disease (MELD) score and the Child-Turcotte-Pugh (CTP) score. The MELD score was calculated according to the following formula: MELD score = 3.78 × ln[TBil (mg/dL)] + 11.2 × ln[INR] + 9.57 × ln[Cr (mg/dL)] + 6.43 × (constant for liver disease etiology = 0 if cholestatic or alcoholic, otherwise = 1)[12]. The modified CTP score included five parameters: TBil level, albumin level, PT, and the presence and severity of ascites and encephalopathy[13].
Statistical analysis to identify risk factors was performed using SPSS version 19.0 (IBM Corp., Armonk, NY, United States). Patient characteristics were compared between patients with and without ACLF and between the derivation and validation samples using the χ2 or Fisher’s exact tests for categorical variables and the t test or Mann-Whitney U test for variables with an abnormal distribution. Percentages were reported to describe categorical variables, mean ± SD were reported to describe the normally distributed continuous variables, and medians with interquartile ranges were reported for continuous variables with skewed distributions. Logistic regression analysis was used for multivariate analysis. In the development of risk prediction models for ACLF, the regression coefficients of the predictors were converted to integer risk scores by rounding the quotient from dividing the regression coefficients, and the final score was the sum of these values. Model discrimination and calibration were assessed using the area under the receiver operating characteristic (ROC) curve (C statistic) and the Hosmer-Lemeshow test. The cutoff sensitivity and specificity of the risk score were calculated, and the patients were divided into high-risk and low-risk groups. The Kaplan-Meier method was used to compare the cumulative risk for ACLF and death in the two groups, and the significance of difference was tested with the log-rank test. P < 0.05 was considered statistically significant.
The statistical methods of this study were reviewed by Gang Wan from the Statistics Section of Beijing Ditan Hospital, Capital Medical University.
Of the 1693 patients who were seropositive for hepatitis B surface antigen at the time of study entry, the following were excluded by imaging (abdominal ultrasound, CT, and magnetic resonance imaging): 153 with liver decompensated cirrhosis, 54 with liver failure at admission, 11 with HCC, four with biliary obstruction, and nine who were pregnant. Patients coinfected with hepatitis A virus (n = 2), and hepatitis C virus (n = 3) were excluded using serologic assays. A total of 1457 patients were enrolled in the study. Patients were divided into SAE (n = 382) and non-SAE (n = 1075) groups. In the non-SAE group, 4/1075 (0.37%) patients progressed to ACLF after admission to the hospital. However, in the SAE group, 52/382 (13.61%) patients developed ACLF (Figure 1). Additionally, the mean number of days between hospital admission and development of ACLF was 9 d (range, 2-34 d), and the rate of ACLF-related death (n = 15) was 28.8% in the SAE group. The ACLF incidence was significantly higher in the SAE group than in the non-SAE group (P < 0.001) (Figure 1).
Given the low incidence of ACLF in the non-SAE group, we mainly focused on the SAE group. Using univariate analysis, we compared patients with ACLF to those without ACLF in the SAE group and found that they were older, had worse underlying diseases, had lower PTA and albumin and sodium levels, and higher TBil and HBV DNA levels (Table 1). With multivariate analysis of the primary cohort, only age ≥ 40 years, TBil ≥ 171 μmol/L (10 × ULN), PTA 40%-60%, and HBV DNA > 107 copies/mL remained independent risk factors for ACLF (Table 2).
| Variables | Total | Non-ACLF | ACLF | P value |
| (n = 382) | (n = 330) | (n = 52) | ||
| Demographics | ||||
| Sex | ||||
| Female | 60 (15.7) | 49 (14.8) | 11 (21.2) | 0.2452 |
| Male | 322 (84.3) | 281 (85.2) | 41 (78.8) | |
| Age (yr) | ||||
| < 40 | 226 (59.2) | 207 (62.7) | 19 (36.5) | < 0.0012 |
| ≥ 40 | 156 (40.8) | 123 (37.3) | 33 (63.5) | |
| Underlying disease | ||||
| HBV carriers | 98 (25.7) | 93 (28.2) | 5 (9.6) | 0.0012 |
| CHB | 255 (58.9) | 217 (65.8) | 38 (73.1) | |
| Compensatory cirrhosis | 29 (7.6) | 20 (6.0) | 9 (17.3) | |
| Biochemical indicators | ||||
| ALT (U/L) | 746.9 (338.1, 1229.0) | 754.2 (361.6, 1215.7) | 644.4 (324.9, 1430.6) | 0.9243 |
| AST (U/L) | 448.0 (217.7, 836.1) | 432.5 (216.1, 806.9) | 529.4 (273.8, 960.9) | 0.0913 |
| TBil (μmol/L) | ||||
| < 171 | 234 (61.3) | 210 (63.6) | 24 (46.2) | 0.0162 |
| ≥ 171 | 148 (38.7) | 120 (36.4) | 28 (53.8) | |
| PTA (%) | ||||
| > 60 | 184 (48.1) | 181 (54.8) | 3 (5.8) | < 0.0012 |
| 50.1-60 | 124 (32.5) | 100 (30.3) | 24 (46.1) | |
| 40-50 | 74 (19.4) | 49 (14.9) | 25 (48.1) | |
| ALB4 (g/L) | 37.2 ± 4.8 | 37.4 ± 4.6 | 36.0 ± 5.3 | 0.0431 |
| CHE (U/L) | 4801.8 ± 1645.5 | 4864.2 ± 1559.1 | 4666.2 ± 1840.4 | 0.4201 |
| K (mmol/L) | 3.91 ± 0.44 | 3.92 ± 0.44 | 3.90 ± 0.41 | 0.8261 |
| Na (mmol/L) | 138.7 ± 2.9 | 138.8 ± 2.9 | 137.6 ± 2.8 | 0.0041 |
| Cr5 (μmol/L) | 67.6 ± 14.5 | 67.6 ± 12.7 | 67.1 ± 22.7 | 0.8131 |
| Virology indicators | ||||
| HBeAg6 (IU/mL) | ||||
| Negative | 88 (23.0) | 73 (22.1) | 15 (28.8) | 0.3682 |
| Positive | 280 (73.3) | 243 (73.6) | 37 (71.2) | |
| HBV DNA (copies/mL) | ||||
| < 106 | 153 (40.1) | 144 (43.6) | 9 (17.3) | < 0.0012 |
| 106-107 | 104 (27.2) | 93 (28.2) | 11 (21.2) | |
| > 107 | 125 (32.7) | 93 (28.2) | 32 (61.5) |
| Variables | Odds ratio | 95%CI | P value | |
| Lower | Upper | |||
| Age (yr) | ||||
| < 40 | Reference | |||
| ≥ 40 | 2.859 | 1.399 | 5.840 | 0.004 |
| TBil (μmol/L) | ||||
| < 171 | Reference | |||
| ≥ 171 | 2.894 | 1.408 | 5.946 | 0.004 |
| PTA (%) | ||||
| > 60 | Reference | |||
| 50.1-60 | 10.19 | 2.894 | 35.882 | < 0.001 |
| 40-50 | 26.285 | 7.311 | 94.499 | < 0.001 |
| HBV DNA (copies/mL) | ||||
| < 106 | Reference | |||
| 106-107 | 1.929 | 0.691 | 5.389 | 0.210 |
| > 107 | 7.15 | 2.918 | 17.523 | < 0.001 |
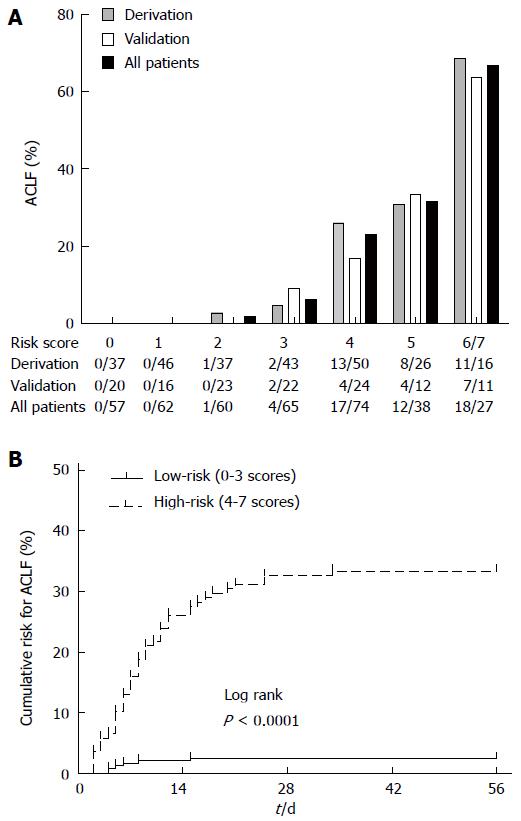
All patients with SAE were randomly allocated into the model derivation and validation sets at a 2:1 ratio. ACLF risk predictors at study entry were comparable between the model derivation and validation sets (P > 0.05). All the risk predictors included in the risk prediction model were significantly associated with outcome on forward logistic regression analyses (P < 0.05). The regression coefficients of the predictors in the risk prediction model were converted into integer risk scores (Table 3). We arbitrarily assigned the regression coefficient of 1.128 associated with age ≥ 40 years as equivalent to one risk point, and divided each regression coefficient associated with the different risk factor levels by 1.128 to determine the number of risk points (rounded to one digit). The risk score was the sum of the risk points from each of the four risk factors in the range of 0 to 7. At the cutoff point of 4 determined by ROC analysis, sensitivity and specificity for ACLF occurrence were 88.6% and 72.2%, respectively, at the maximum Youden index. The sum risk scores of 0-3 identified patients at lower risk of ACLF (0%-4.7%), whereas the sum risk scores of 4-7 identified patients at higher risk (26.0%-68.8%). The score distributions in the derivation, validation, and all patient populations were examined. Scores 6 and 7 were collapsed into a single category because of the small number of patients in the score category of 7 (Figure 2A). The Kaplan-Meier analysis showed the cumulative risk for ACLF in the two risk groups (0-3 and 4-7) of the primary cohort over 56 d, and log-rank test revealed a significant difference (2.0% vs 33.8%, P < 0.0001) (Figure 2B).
| ACLF predictors | Regression coefficient | P value | Risk score |
| Age ≥ 40 (yr) | 1.128 | 0.010 | 1 |
| TBil ≥ 171 (μmol/L) | 1.237 | 0.007 | 1 |
| PTA (%) | |||
| 50.1-60 | 2.158 | 0.001 | 2 |
| 40-50 | 2.868 | < 0.001 | 3 |
| HBV DNA > 107 (copies/mL) | 1.946 | < 0.001 | 2 |
| Overall risk level | ACLF rate | Total score | |
| Low risk | 0%-4.7% | 0-3 | |
| High risk | 26.0%-68.8% | 4-7 |
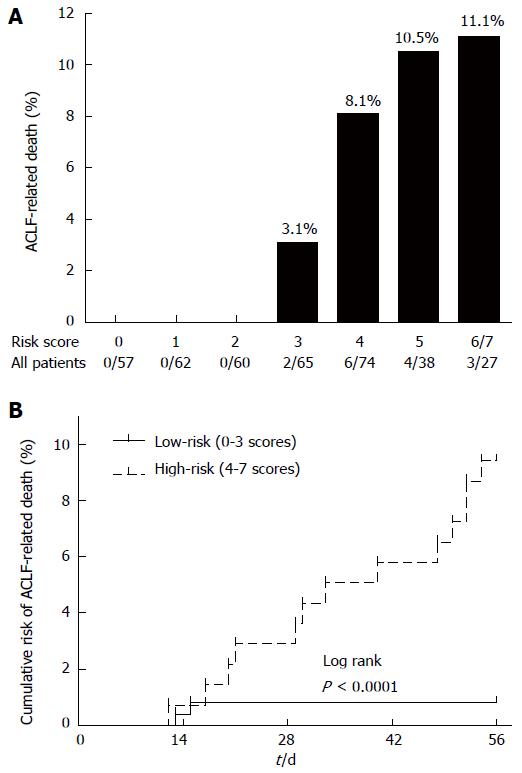
The relationship of the score model and ACLF-related death was also evaluated. We observed that the mortality rate gradually increased with increasing score, from 0% in scores 0-2, 3.1% in score 3, 8.1% in score 4, and 10.5% in score 5, to 11.1% in scores 6 and 7, in the primary cohort (Figure 3A). The cumulative risk of ACLF-related death was also significantly higher in the high-risk group (4-7) than that in the low-risk group (0-3) (P < 0.0001) (Figure 3B).
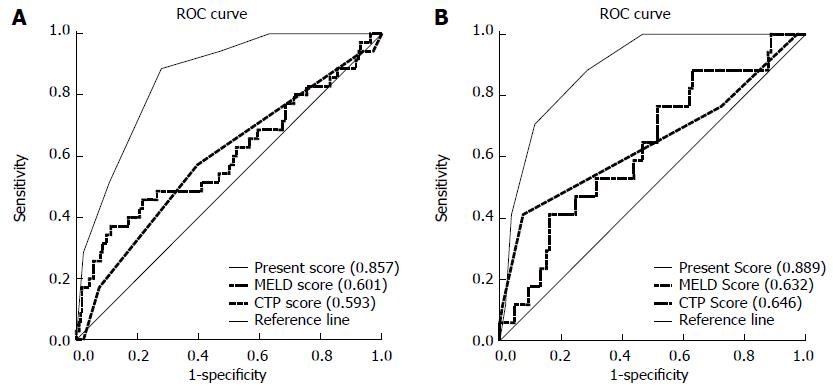
The prediction model discriminated patients who developed ACLF from those who did not using the C index of 0.857 in the derivation cohort [95% confidence interval (CI): 0.800-0.913] and 0.889 in the validation cohort (95%CI: 0.820-0.957). Model calibration was also good in both groups, as demonstrated by the Hosmer-Lemeshow test (χ2 = 4.516, P = 0.808, and χ2 = 1.959, P = 0.923, respectively). Moreover, we used the area under the ROC curve to compare the predictive value of this score model with MELD and CTP scores. As shown in Figure 4A, the C index was 0.857 for our score model in the derivation cohort, 0.601 for MELD, and 0.593 for CTP. In the validation cohort, the C index was 0.889 for our score model, 0.632 for MELD, and 0.646 for CTP (Figure 4B). This indicates that this score model was superior to MELD or CTP score in estimating the risk of ACLF.
There is increasing interest in predicting the probability of adverse events for various medical purposes. Accurately predicting the probability of adverse events allows for effective patient risk stratification, thus permitting more appropriate medical care. It is well established that SAE of CHB may lead to liver failure with potential severe and even lethal consequences[6,7]. An estimated 40%-50% of hepatitis B e-antigen-positive patients can undergo the immune clearance phase and partly develop SAE[14]. A three-month mortality has been reported in > 50% of cases of HBV-ACLF without liver transplantation[15,16]. The majority of the research on SAE or ACLF focuses on the clinical outcomes; epidemiologic data regarding the progression of SAE to ACLF, for use in predicting the ACLF risk, are poorly documented.
This study is believed to be the first to focus on the occurrence and prediction of ACLF. Our data showed that 13.6% (52/382) of SAE patients progressed to ACLF and that 28.8% (15/52) of those died of ACLF-related causes within 56 d. In patients hospitalized for SAE receiving early intervention, the ACLF mortality rate in the present study decreased by > 14.5% compared to the ACLF mortality rate (43.3%) in our previous study[17]. Univariate and multivariate analyses identified the following four early warning and monitoring indicators: age ≥ 40 years, TBil ≥ 171 μmol/L (10 × ULN), PTA 40%-60%, and HBV DNA > 107 copies/mL. We developed a risk score prediction model after ascribing different weights to each factor. Using these routinely available clinical data, the model facilitated identification of patients (total score ≥ 4) at high risk for ACLF and ACLF-related death early in the course of SAE.
Several prognostic models of liver failure outcome include age as an important epidemiologic risk factor[18]. The most widely used prognostic model, the King’s College Hospital criteria, includes age ≥ 40 years as an indicator of adverse outcome in patients with a non-acetaminophen etiology[19]. To date, how the liver is affected by increasing age has not been fully elucidated[20]; however, some studies have confirmed that liver volume and hepatic blood flow decrease[21] and hepatic metabolism and hepatocyte morphology change[22,23] with aging. In our study, we observed that patients ≥ 40 years of age were more likely to progress to ACLF.
The main mechanisms of hepatocellular jaundice are the decreased ability of cytosolic unconjugated bilirubin-binding proteins (e.g., ligandin) and UDP-glucuronic acid transferase to take up and bind cytosolic unconjugated bilirubin. In addition, other processes such as hepatocellular and hepatic lobule damage that cause exudative and edema-like lesions, could reduce the excretion of conjugated bilirubin[24]. As such, serum bilirubin level is an important indicator of the extent of hepatocellular damage and necrosis. The results of this study demonstrate that patients with higher TBil levels (≥ 171 μmol/L; 10 × ULN) had a higher relative risk of ACLF onset.
PTA is recognized as a reliable marker of the protein synthetic function of the liver and, thus, a marker of the hepatic functional reserve. It is included in many authorized criteria for the diagnosis of liver failure and is used as an indication for liver transplantation worldwide. However, it is rarely applied to predict ACLF risk[12,25,26]. In classifying the PTA of SAE patients, we found that a PTA of 40%-60% was an important independent risk factor for ACLF. In particular, the risk score of 40%-50% PTA was triple that of other independent risk factors, including age and TBil level.
Pretreatment HBV DNA level was closely related to cirrhosis and HCC[27-30], and could also be attributed to the enhanced immune response in SAE, which led to massive hepatocellular necrosis and clearance of the majority of HBV. The results of aggravated liver injury and decreased liver reservation could increase the chance of mortality. Jeng et al[31] found that serum HBV DNA level was the only significant risk factor for predicting hepatic decompensation in patients with acute exacerbation of CHB. Hsu et al[32] observed that pretreatment HBV DNA level stratified the risk of death in patients with SAE of CHB before the manifestation of overt liver failure. Consistent with these previous studies, our analysis identified that patients in the ACLF group tended to have a higher viral load; the risk score of HBV DNA ≥ 107 copies/mL was double that of other independent risk factors including age and TBil level, highlighting the significance of pretreatment HBV DNA level in predicting ACLF risk.
A potential limitation of this study was that it was a retrospective, single-center study. Stratification of continuous variables as well as conversion of the regression coefficients to score point values, although necessary for clinical practicality, could be assumed to have resulted in a loss of information and decreased model accuracy. Therefore, larger, prospective, randomized studies are required to confirm the results.
In conclusion, a risk score model using routinely collected clinical data to predict the risk of ACLF and ACLF-related death in patients hospitalized with SAE of CHB has excellent prediction accuracy and discriminatory ability. This model might be useful as a tool in identifying those patients at the highest risk of ACLF and in guiding monitoring and treatment decisions.
Acute-on-chronic liver failure (ACLF) is a severe life-threatening clinical syndrome characterized by liver failure and is associated with high mortality if liver transplantation or artificial liver support is not available. Therefore, for early diagnosis and treatment, it is necessary to determine the risk factors and assess the risk of ACLF.
The previous research on the severe acute exacerbation (SAE) or ACLF focuses on the clinical outcomes; epidemiologic data regarding the progression of SAE to ACLF, for use in predicting the ACLF risk, are poorly documented.
This study developed a risk score model using routinely collected clinical data to predict the risk of ACLF and ACLF-related death in patients hospitalized with SAE of chronic hepatitis B that was superior to the Model for End-stage Liver Disease and Child-Turcotte-Pugh scores.
The risk score model might be useful as a tool in identifying those patients (total score ≥ 4) at the highest risk of ACLF and in guiding monitoring and treatment decisions early in the course of SAE.
According to the diagnostic criteria of ACLF recommended by the Asian Pacific Association for the Study of the Liver: acute hepatic insult manifesting as jaundice (serum bilirubin > 85 μmol/L) and coagulopathy (prothrombin activity < 40% or international normalized ratio of prothrombin time ≥ 1.5), complicated within 4 wk by ascites and/or encephalopathy in patients with previously diagnosed or undiagnosed chronic liver diseases.
This study gives a clinical score model that can easily be applied to predict the risk of ACLF in patients with chronic hepatitis B. It is important for predicting the risk of ACLF and ACLF-related death early in the course of SAE. The sample size is adequate and statistical methods are accurate. The quality of the manuscript’s presentation and readability are good.
P- Reviewer: Wu HC, Wang L S- Editor: Yu J L- Editor: AmEditor E- Editor: Zhang DN
| 1. | Liaw YF, Chu CM. Hepatitis B virus infection. Lancet. 2009;373:582-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 929] [Cited by in RCA: 993] [Article Influence: 62.1] [Reference Citation Analysis (1)] |
| 2. | Seeff LB, Koff RS. Evolving concepts of the clinical and serologic consequences of hepatitis B virus infection. Semin Liver Dis. 1986;6:11-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Lok AS, Lai CL. Acute exacerbations in Chinese patients with chronic hepatitis B virus (HBV) infection. Incidence, predisposing factors and etiology. J Hepatol. 1990;10:29-34. [PubMed] |
| 4. | Liaw YF. Acute exacerbation and superinfection in patients with chronic viral hepatitis. J Formos Med Assoc. 1995;94:521-528. [PubMed] |
| 5. | Perrillo RP. Acute flares in chronic hepatitis B: the natural and unnatural history of an immunologically mediated liver disease. Gastroenterology. 2001;120:1009-1022. [PubMed] |
| 6. | Wong VW, Chan HL. Severe acute exacerbation of chronic hepatitis B: a unique presentation of a common disease. J Gastroenterol Hepatol. 2009;24:1179-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 7. | Liaw YF, Leung N, Kao JH, Piratvisuth T, Gane E, Han KH, Guan R, Lau GK, Locarnini S. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2008 update. Hepatol Int. 2008;2:263-283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 666] [Cited by in RCA: 743] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 8. | Garg H, Sarin SK, Kumar M, Garg V, Sharma BC, Kumar A. Tenofovir improves the outcome in patients with spontaneous reactivation of hepatitis B presenting as acute-on-chronic liver failure. Hepatology. 2011;53:774-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 205] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 9. | Fujiwara K, Yasui S, Yonemitsu Y, Fukai K, Arai M, Imazeki F, Suzuki A, Suzuki H, Sadahiro T, Oda S. Efficacy of combination therapy of antiviral and immunosuppressive drugs for the treatment of severe acute exacerbation of chronic hepatitis B. J Gastroenterol. 2008;43:711-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Wang K, Wu ZB, Ye YN, Liu J, Zhang GL, Su YJ, He HL, Zheng YB, Gao ZL. Plasma Interleukin-10: A Likely Predictive Marker for Hepatitis B Virus-Related Acute-on-Chronic Liver Failure. Hepat Mon. 2014;14:e19370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Sarin SK, Kumar A, Almeida JA, Chawla YK, Fan ST, Garg H, de Silva HJ, Hamid SS, Jalan R, Komolmit P. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the study of the liver (APASL). Hepatol Int. 2009;3:269-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 630] [Cited by in RCA: 643] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 12. | Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D’Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3462] [Cited by in RCA: 3676] [Article Influence: 153.2] [Reference Citation Analysis (0)] |
| 13. | Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646-649. [PubMed] |
| 14. | Sheen IS, Liaw YF, Tai DI, Chu CM. Hepatic decompensation associated with hepatitis B e antigen clearance in chronic type B hepatitis. Gastroenterology. 1985;89:732-735. [PubMed] |
| 15. | Sun QF, Ding JG, Xu DZ, Chen YP, Hong L, Ye ZY, Zheng MH, Fu RQ, Wu JG, Du QW. Prediction of the prognosis of patients with acute-on-chronic hepatitis B liver failure using the model for end-stage liver disease scoring system and a novel logistic regression model. J Viral Hepat. 2009;16:464-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 16. | Cui YL, Yan F, Wang YB, Song XQ, Liu L, Lei XZ, Zheng MH, Tang H, Feng P. Nucleoside analogue can improve the long-term prognosis of patients with hepatitis B virus infection-associated acute on chronic liver failure. Dig Dis Sci. 2010;55:2373-2380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 17. | Liu H, Zhang H, Wan G, Sang Y, Chang Y, Wang X, Zeng H. Neutrophil-lymphocyte ratio: a novel predictor for short-term prognosis in acute-on-chronic hepatitis B liver failure. J Viral Hepat. 2014;21:499-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 18. | Schiødt FV, Chung RT, Schilsky ML, Hay JE, Christensen E, Lee WM. Outcome of acute liver failure in the elderly. Liver Transpl. 2009;15:1481-1487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | O’Grady JG, Alexander GJ, Hayllar KM, Williams R. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology. 1989;97:439-445. [PubMed] |
| 20. | Zeeh J, Platt D. The aging liver: structural and functional changes and their consequences for drug treatment in old age. Gerontology. 2002;48:121-127. [PubMed] |
| 21. | Wynne HA, Cope LH, Mutch E, Rawlins MD, Woodhouse KW, James OF. The effect of age upon liver volume and apparent liver blood flow in healthy man. Hepatology. 1989;9:297-301. [PubMed] |
| 22. | Cotreau MM, von Moltke LL, Greenblatt DJ. The influence of age and sex on the clearance of cytochrome P450 3A substrates. Clin Pharmacokinet. 2005;44:33-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 208] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 23. | Schmucker DL. Hepatocyte fine structure during maturation and senescence. J Electron Microsc Tech. 1990;14:106-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 59] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Tiribelli C, Ostrow JD. New concepts in bilirubin and jaundice: report of the Third International Bilirubin Workshop, April 6-8, 1995, Trieste, Italy. Hepatology. 1996;24:1296-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Bernuau J, Rueff B, Benhamou JP. Fulminant and subfulminant liver failure: definitions and causes. Semin Liver Dis. 1986;6:97-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 398] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 27. | Iloeje UH, Yang HI, Su J, Jen CL, You SL, Chen CJ. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology. 2006;130:678-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1164] [Cited by in RCA: 1174] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 28. | Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, Huang GT, Iloeje UH. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2309] [Cited by in RCA: 2364] [Article Influence: 124.4] [Reference Citation Analysis (0)] |
| 29. | Yuen MF, Tanaka Y, Fong DY, Fung J, Wong DK, Yuen JC, But DY, Chan AO, Wong BC, Mizokami M. Independent risk factors and predictive score for the development of hepatocellular carcinoma in chronic hepatitis B. J Hepatol. 2009;50:80-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 486] [Cited by in RCA: 487] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 30. | Lee MH, Yang HI, Liu J, Batrla-Utermann R, Jen CL, Iloeje UH, Lu SN, You SL, Wang LY, Chen CJ. Prediction models of long-term cirrhosis and hepatocellular carcinoma risk in chronic hepatitis B patients: risk scores integrating host and virus profiles. Hepatology. 2013;58:546-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 256] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 31. | Jeng WJ, Sheen IS, Liaw YF. Hepatitis B virus DNA level predicts hepatic decompensation in patients with acute exacerbation of chronic hepatitis B. Clin Gastroenterol Hepatol. 2010;8:541-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 32. | Hsu YC, Wu CY, Chang CY, Tai CM, Tseng CH, Perng DS, Mo LR, Lin JT. Pretreatment viral DNA stratifies mortality risk in patients receiving antiviral therapy for severe acute exacerbation of chronic hepatitis B. Antivir Ther. 2013;18:221-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |