Published online Jul 21, 2015. doi: 10.3748/wjg.v21.i27.8340
Peer-review started: January 16, 2015
First decision: April 13, 2015
Revised: April 20, 2015
Accepted: May 7, 2015
Article in press: May 7, 2015
Published online: July 21, 2015
Processing time: 188 Days and 4.9 Hours
AIM: To investigate the effects of Clostridium butyricum (C. butyricum) on experimental gastric ulcers (GUs) induced by alcohol, restraint cold stress, or pyloric ligation in mice, respectively.
METHODS: One hundred and twenty mice were randomly allocated into three types of gastric ulcer models (n = 40 each), induced by alcohol, restraint cold stress, or pyloric ligation. In each GU model, 40 mice were allocated into four groups (n = 10 each): the sham control group; model group (GU induction without pretreatment); C. butyricum group (GU induction with C. butyricum pretreatment); and Omeprazole group (GU induction with Omeprazole pretreatment). The effects of C. butyricum were evaluated by examining the histological changes in the gastric mucosal erosion area, the activities of superoxide dismutase (SOD) and catalase (CAT), the level of malondialdehyde (MDA), and the contents of interleukin (IL)-1β, tumor necrosis factor (TNF)-α, leukotriene B4 (LTB4) and 6-keto-PGF-1α (degradation product of PGI2) in the gastric tissue.
RESULTS: Our data showed that C. butyricum significantly reduced the gastric mucosal injury area and ameliorated the pathological conditions of the gastric mucosa. C. butyricum not only minimized the decreases in activity of SOD and CAT, but also reduced the level of MDA in all three GU models used in this study. The accumulation of IL1-β, TNF-α and LBT4 decreased, while 6-keto-PGF-1α increased with pretreatment by C. butyricum in all three GU models.
CONCLUSION: Our data demonstrated the protective effects of pretreatment with C. butyricum on anti-oxidation and anti-inflammation in different types of GU models in mice. Further studies are needed to explore its potential clinical benefits.
Core tip: In this study, we reported that the probiotic Clostridium butyricum (C. butyricum) pretreatment obviously attenuated gastric mucosal lesions induced by different stimulations. The oxidative stress- and inflammation-related parameters detected in this study showed that anti-oxidation and anti-inflammation participate in the underlying mechanism of C. butyricum protective effect on gastric mucosa. Our findings provide a potential protective method for the gastric mucosa and a novel application for C. butyricum in the clinic.
-
Citation: Wang FY, Liu JM, Luo HH, Liu AH, Jiang Y. Potential protective effects of
Clostridium butyricum on experimental gastric ulcers in mice. World J Gastroenterol 2015; 21(27): 8340-8351 - URL: https://www.wjgnet.com/1007-9327/full/v21/i27/8340.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i27.8340
The stomach is an important organ for food digestion. In some cases, however, its digestive function may become too aggressive, resulting in self-digestion and gastric ulcers (GU)[1]. GU is commonly caused by an exposure to excessive amounts of various endogenous or exogenous factors, including excess secretion of gastric acids and pepsin, reactive oxygen species (ROS), stress, alcohol, non-steroidal anti-inflammatory drugs (NSAIDs), Helicobacter pylori (H. pylori) infection and smoking[2]. Although an estimated 10% of population in the world suffers from this disease[3], its severity is often not recognized, possibly because of its quick clinical recovery if timely treatment is started. Despite considerable progress with extensive application of gastroprotective agents, such as proton-pump inhibitor (PPI) and eradication therapy for H. pylori, some issues remain in the current therapy of GU. First, drug resistance of H. pylori may require changes in the treatment regimens[3]. Furthermore, antibiotics administered orally alter intestinal flora[4,5] and may cause drug resistance in some bacteria, leading to unexpected difficulties in the treatment of a variety infectious diseases. Second, the recurrence rate of GU is high with current therapies[6]. While the causes for GU relapses are complicated, infection by H. pylori may be an important contributor[5,7]. The weakened defensive barrier may also be a key factor contributing to recurrence[6]. However, only a few drugs are available that promote the repair of gastric mucosa. Third, knowledge of adverse effects of current drugs used to treat ulcers is accumulating[8]. For example, extended use of PPIs appears to cause an increased risk of fractures[9,10], hepatitis[11,12] and bacterial pneumonia[13,14]. Therefore, it is necessary to explore new therapies to prevent and treat GU.
Clostridium butyricum (C. butyricum) has been used as a probiotic to treat and prevent non-antimicrobial-induced or antimicrobial-associated diarrhea[15,16]. Its in vivo inhibition on H. pylori was reported by Takahashi et al[17]. The direct protective effects of C. butyricum on GU, however, are not clear.
This study was designed to test the hypothesis that pretreatment with C. butyricum could prevent lesions in the gastric mucosa. Clinical GU cases are often induced by alcohol overuse, stress, and excess of gastric acid and pepsin; therefore, we developed and used three GU models in mice induced by alcohol, cold stress or pylorus ligation, in this study. In each model, the pretreatment effects were evaluated by comparisons with no pretreatment and standard pretreatment with Omeprazole.
C. butyricum was purchased from Miyarisan Pharmaceutical Co., Ltd (Nagano, Japan). Trypticase-phytone-yeast extract (TPY) liquid culture medium (Table 1) was obtained from Hope Bio-Technology Co., Ltd (Qingdao, China). Omeprazole was from Aosaikang Pharmaceutical Co., Ltd (Jiangsu, China). Detection kits for superoxide dismutase (SOD) and catalase (CAT) were purchased from Jiancheng Bioengineering Institute (Nanjing, China). The kits for malondialdehyde (MDA) detection and bicinchoninic acid (BCA) protein assay were bought from Beyotime Institute of Biotechnology (Shanghai, China). Enzyme-linked immunosorbent assay (ELISA) kits for IL-1β, TNF-α, leukotriene B4 (LTB4) and 6-keto-PGF-1α were obtained from Westang Bio-tech Co., Ltd (Shanghai, China).
| Components | Weight (g/L) |
| Casein hydrolysate | 1.0 |
| Soya bean peptone | 5.0 |
| Yeast powder | 2.0 |
| Glucose | 5.0 |
| L-Cysteine | 0.5 |
| K2HPO4 | 2.0 |
| MgCl2 | 0.5 |
| ZnSO4 | 0.25 |
| CaCl2 | 0.15 |
| FeCl3 | 0.000001 |
| Tween-80 | 1.0 |
| pH | 6.5 ± 0.1 |
Male Institute for Cancer Research (ICR) mice, weighing 25-30 g, were provided by the Experimental Animal Center of Wenzhou Medical University (Wenzhou, China). All the animal procedures carried out in the present study were in accordance with the guidelines of the Animal Ethics Committee of Wenzhou Medical University.
Animals were given free access to tap water and food in air-conditioned animal quarters under a 12 h light-12 h dark cycle. 120 mice were randomly allocated into three types of gastric ulcer models (n = 40 each), induced by alcohol, restraint cold stress or pyloric ligation. All the models are classical and stable for the study of molecular mechanisms and screening of therapeutic or preventive drugs of GU[18-20].
Following the indicated pretreatments (see next paragraph), in the alcohol-induced GU model, mice were orally administered with pure alcohol 0.01 mL/g body weight (bw) and sacrificed by cervical dislocation 1 h later before the stomach tissues were collected for analysis[18].
In the cold-restraint stress-induced GU model, mice were lightly anesthetized by pentobarbital anesthesia (30 mg/kg bw, intraperitoneally) and fixed solidly to immobilization the mice. The bodies of mice under xiphoid were subjected to cold water (21 °C) for 20 h to induce a cold stress[19].
In the pylorus ligation-induced GU model[20], the abdomen of mouse was opened under light anesthesia with pentobarbital and the stomach was taken out under sterile conditions. The pyloric end was then isolated and ligated, followed by suture of the abdominal wall. The animals were sacrificed after the ligation for 20 h.
In each GU model and before induction, 40 mice were allocated into four groups (n = 10 each): sham control group; GU model group (GU induction without pretreatment); C. butyricum group (GU induction with C. butyricum pretreatment); and Omeprazole group (GU induction with Omeprazole pretreatment). The sham control group animals received only the culture medium, and the GU group animals received the culture medium and alcohol administration, restraint cold stress or pylorus ligation, without any pretreatment.
In the C. butyricum group, the animals received C. butyricum pretreatment before GU model preparation. C. butyricum was cultured with TPY liquid culture medium in an anaerobic incubator. Ten hours later, C. butyricum at the logarithmic phase was used for intragastric administration of mice in C. butyricum group (dose = 5 × 108 CFU/mouse) for 5 d via an orogastric tube.
In the Omeprazole group, the animals were intraperitoneally administered with Omeprazole (13 mg/kg bw), 30 min before replication of GU models. The mice were sacrificed by cervical dislocation followed by isolation of the stomach from surrounding organs without any substantial connective tissues or vessels. The gastric specimens were used for lesion assessment, pathological observation and determination of parameters of oxidative stress and inflammation. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by Laboratory Animal Ethics Committee of Wenzhou Medical University (Permit number: wydw2012-0109). All surgery was performed under sodium pentobarbital anesthesia, and all efforts were made to minimize suffering.
The stomachs of mice were fixed by injection of 1 mL 10% formalin solution, and digital images were taken by photo scanning. The ratio of gastric hemorrhagic ulcer area and total gastric area was calculated by the Image-Pro Plus (IPP) 6.0 software from Media Cybernetics (Bethesda, CA, United States). The specimens were embedded in paraffin, sectioned and stained with hematoxylin and eosin (HE). Pathological changes were identified under a Nikon Eclipse SOi light microscope (Nikon Co., Japan).
The stomachs of mice were washed mildly to remove food debris and then weighed using an electronic balance. Ice-cold normal saline with a volume corresponding to 9 × the tissue weight was added. As extensive lesions of the gastric mucosa existed in the acute GU models, the whole stomach was homogenized with a Potter-Elvehjem glass homogenizers, and centrifuged at 2400 ×g for 15 min. The supernatant was collected for the assays of SOD, CAT and MDA.
The activity of total SOD of gastric tissue was measured from gastric homogenate by the method of xanthine oxidase[21], with absorbance at 550 nm using a 722N spectrophotometer from the Scientific Instrument Co., Ltd (Shanghai, China). The activity of SOD in per mg of tissue protein was calculated according to the formula supplied by the manufacturer.
The activity of CAT was determined at 405 nm with a 722N spectrophotometer from the Scientific Instrument Co., Ltd (Shanghai, China), through the ammonium molybdate colorimetric method[22], according to the formula provided by the manufacturer.
Lipid peroxidation in stomach samples was determined by measuring thiobarbituric acid reactive substances, as described by Ohkawa et al[23]. The absorbance at 532 nm was measured with an ELx800 absorbance microplate reader (BioTek Instruments, Inc., Winooski, VT, United States). The concentration of thiobarbituric acid reactive substances was measured using a standard curve of malondialdehyde, and the results were expressed as μmol MDA /g protein.
IL-1β, TNF-α, LTB4 and 6-keto-PGF-1α of the gastric tissue were quantitated by the ELISA method. Briefly, 100 μL of homogenate was added to a 96-well plate that was coated with a corresponding primary antibody, and incubated for 3 h. The plate was washed twice with Phosphate Buffered Saline Tween-20 (PBST) and 100 μL of biotin conjugate was added to the plate, followed by an incubation of 45 min at room temperature. After washing to remove the non-specific binding, 100 μL of streptavidin-HRP was added and incubated for 45 min. Then the plate was washed with PBST and 100 μL of Chromogen was added to each well. Twenty minutes later, the reaction was stopped with stop solution. The absorbance was measured at 450 nm using an ELx800 absorbance microplate reader (BioTek Instruments). The levels of IL-1β, TNF-α, LTB4 and 6-keto-PGF-1α were calculated using standard curves, according to the instructions from the manufacturer.
The protein contents of stomach samples were detected by the bicinchoninic acid assay (BCA) method. The absorbance at 562 nm was detected by BioTek ELx800 Absorbance Microplate Reader (BioTek Instruments) according to the manufacturer’s instruction. The protein concentration of the samples was calculated using a protein standard curve, and used to normalize the parameters of oxidative stress or inflammation in the stomach tissue.
All values were expressed as the mean ± SD. Statistical differences were evaluated by Fisher’s Least Significant Difference (LSD) test followed by a Dunnett’s T3 multiple comparison test. A P value less than 0.05 was considered statistically significant.
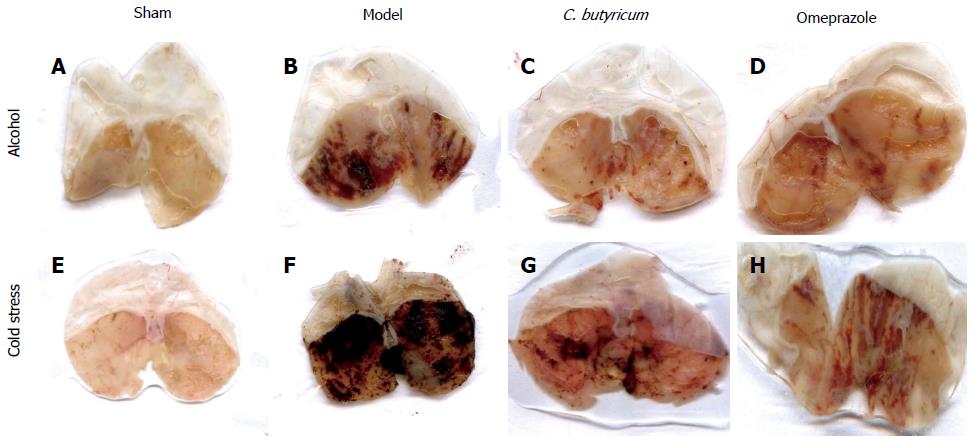
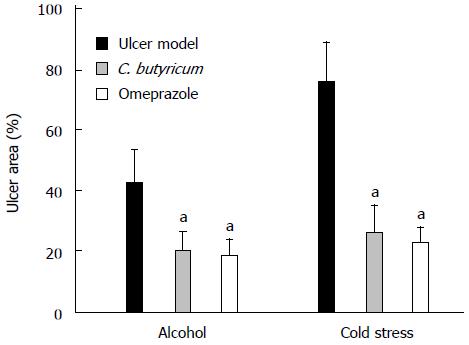
The effects of pretreatment with C. butyricum were compared macroscopically to non-treated (Model group) and those pretreated with Omeprazole (Omeprazole group) (Figure 1). Quantitative comparisons of the gross morphology (Figure 1) were determined using the ratio of hemorrhagic area to total gastric area by the IPP 6.0 software (Figure 2). Alcohol overuse induced substantial gastric mucosal lesions, including many hemorrhagic injuries along the vessels of gastric mucosa. In some mice, shrinkage of the gastric wall was also observed. Restraint cold stress, also caused severe pathological changes within a larger hemorrhagic area, with a dark red appearance and excessive shrinkage. Pretreatment with C. butyricum pretreatment significantly reduced gastric lesions in both alcohol and cold induced GU models (Figure 2, P < 0.01), with a comparable protective effect to Omeprazole, a standard treatment drug (Figure 2). The pylorus ligation model was not included in the comparison because the mucosal lesions were diffused in the group, which made it difficult to evaluate extent of injury and the effects of pretreatment.
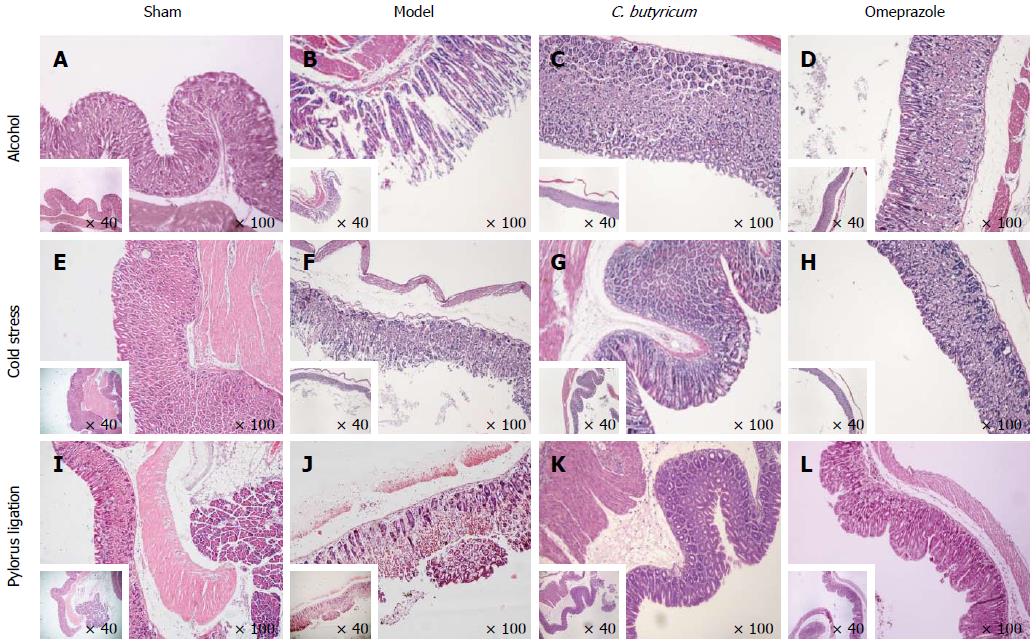
To further examine the morphological lesions, we performed the conventional HE staining. Histological examination showed that the epithelial lining was intact, and the glandular cavity was clear and without any inflammatory cell infiltration in the gastric mucosa in sham control mice (Figure 3A, 3E and 3I). In the three GU models without pretreatments, marked histological changes were observed, including infiltration of neutrophils, lymphocytes and many erythrocytes into the mucosa. Some secretory glands were broken and many deciduous cells were observed in the glandular cavities (Figure 3B, 3F and 3J). Even mucosal desquamation was found in some gastric tissues from GU mice. Among the three GU models without pretreatments, the histological lesions in cold stress ulcer model were the worst (Figure 3F). Pretreatment with C. butyricum alleviated the pathological changes (Figure 3C, 3G and 3K), with comparable results to pretreatment with Omeprazole (Figure 3D, 3H and 3L). All the results were consistent with the macroscopical changes described above.
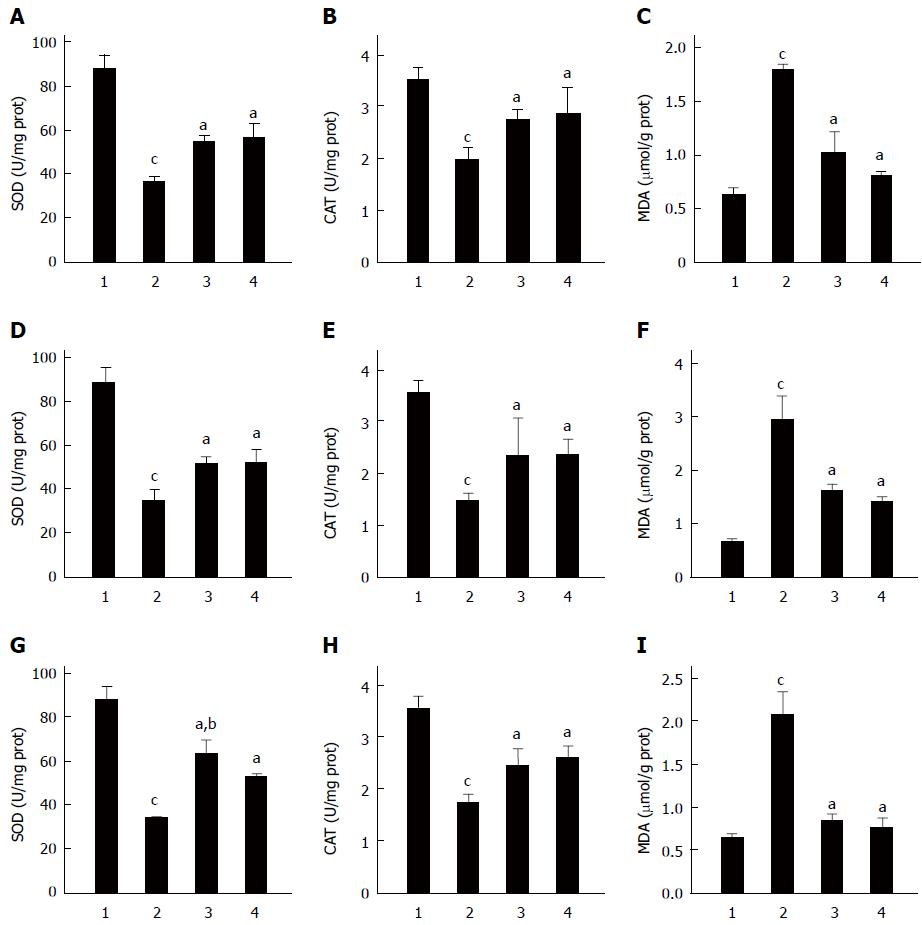
Oxidative stress is a main contributor to the development of gastric mucosa lesions[24]. Figure 4 shows the changes in the activities of SOD and CAT, and the content of MDA, in gastric tissues, which were used to evaluate the extent of oxidative stress. The data showed that in all three GU models, the activities of SOD and CAT decreased and the level of MDA increased (P < 0.01). Pretreatment with C. butyricum attenuated those changes (Figure 4, P < 0.01), indicating that pretreatment with C. butyricum reduced oxidative stress in GU mice. With pretreatment of Omeprazole, changes in the oxidative parameters were similar to those from pretreatment of C. butyricum, although the activity of SOD in C. butyricum group was higher than that of the Omeprazole group in the pylorus ligation model (P < 0.01, Figure 4G).
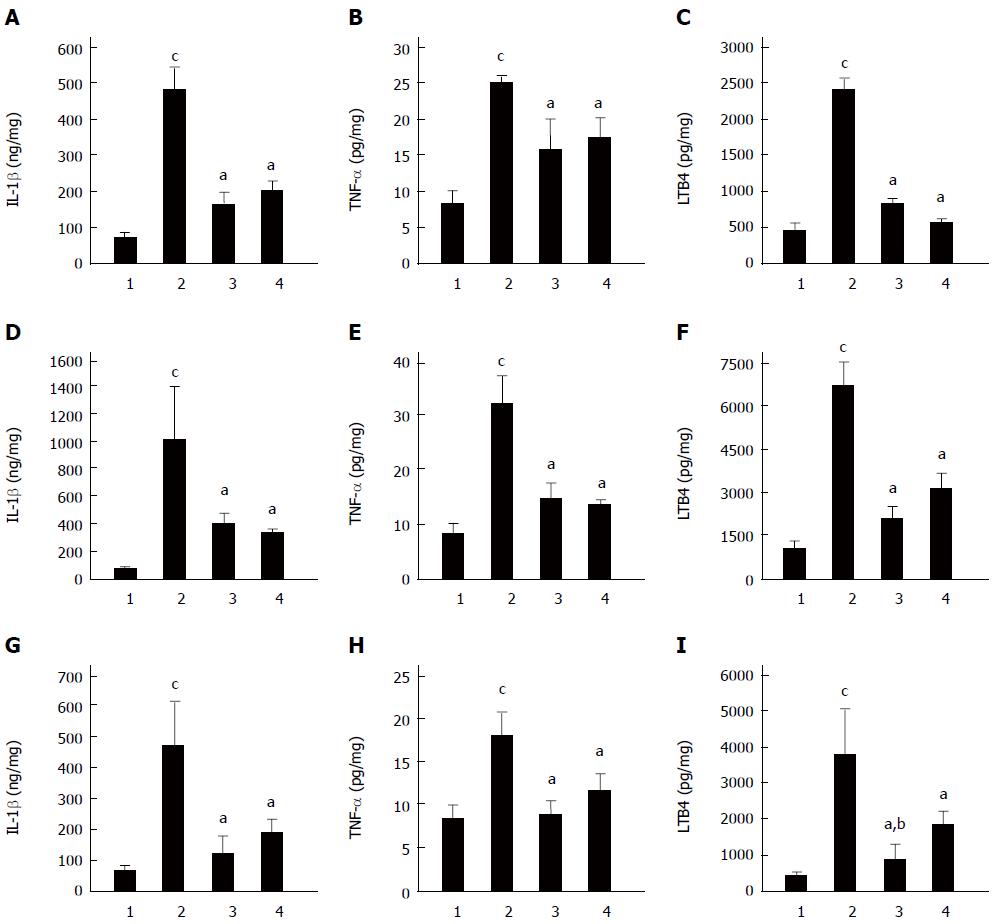
Excessive inflammation plays an important role in the development of gastric mucosal lesions induced by various proinflammatory stimuli; therefore, we performed cytokine detection to investigate the underlying mechanism of the protective effects of C. butyricum on gastric mucosa. Changes of IL-1β, TNF-α and LTB4 in gastric tissues in three models with different pretreatments are shown in Figure 5. The levels of IL-1β, TNF-α and LTB4 were significantly elevated, by different magnitudes, in all three GU models. Pretreatment with C. butyricum attenuated this elevation of proinflammatory factors (P < 0.01) (Figure 5). Overall, the effects of C. butyricum pretreatment were similar to those of Omeprazole, despite a lower level of LTB4 in C. butyricum group in the pylorus ligation GU mice (P < 0.01) (Figure 5). These data indicated that C. butyricum ameliorated inflammatory reactions in the development of gastric mucosal lesions induced by different stresses in mice.
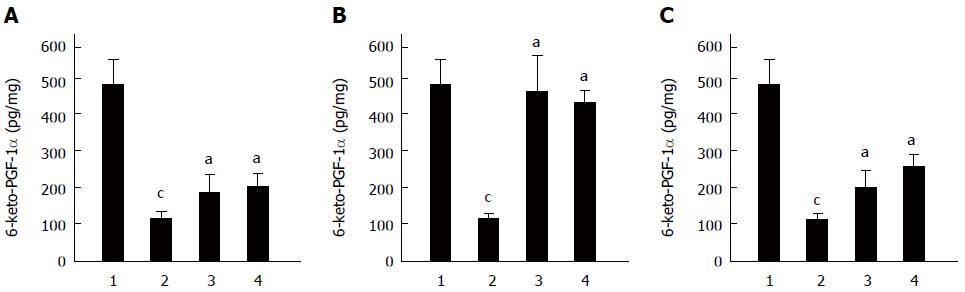
As PGE2 and PGI2 are not stable under physiological conditions; therefore, the stable metabolite of PGI2, 6-keto-PGF-1α, was used to reflect changes in proinflammatory factors. Figure 6 shows that 6-keto-PGF-1α was initially at a high level under normal conditions whereas the different ulcer-inducing stress factors significantly reduced the level of 6-keto-PGF-1α in the gastric mucosa. Pretreatment with C. butyricum markedly attenuated the decrease 6-keto-PGF-1α induced by stressful stimulations (P < 0.01), with similar effects to Omeprazole (Figure 6).
C. butyricum was discovered by Dr. Miyairi in 1933[16]. It has been reported to have therapeutical effects on colitis in rats[25], inhibitory cytotoxic effects from Clostridium difficile in vitro[16], and suppression of colonization by H. pylori in vivo[17]. C. butyricum has been used clinically to treat antibiotic associated diarrhea caused by H. pylori eradication therapy[26], possibly through the recovery of intestinal microbiota balance. To investigate whether C. butyricum has a potential protective effect from gastric mucosal lesions, we assessed the effects of C. butyricum pretreatments on gastric mucosa from injuries induced by alcohol, stress or excessive gastric acid and digestive enzymes. Our data showed that C. butyricum pretreatment effectively attenuated the oxidative stress and inflammatory responses, as well as reduced severity of gastric lesions from three experimental GU models in mice.
Previous studies demonstrated that the injury of gastric mucosa is to a large extent attributed to oxidative stress[24]. Accordingly, we wanted to find out whether C. butyricum could protect gastric mucosa from lesions by alleviating oxidative stress. The antioxidases, such as SOD and CAT, are important protective factors in oxidative damage[24]. MDA, which is a lipid peroxidation product, is stable and is used as a typical parameter to reflect oxidative injuries[27]. Our data showed that pretreatment with C. butyricum alleviated the level of MDA and improved the activities of SOD and CAT in different GU models of mice. On the other hand, inflammation is an important factor involved in gastric injury. In the current study, IL-1β, TNF-α and LTB4 were increased in GUs induced by alcohol, cold stress and pylorus ligation. This is consistent with previous reports[28-30]. Administration of C. butyricum dramatically inhibited the elevation of IL-1β, TNF-α and LTB4, indicating that C. butyricum exerted potential protective effects on gastric mucosa by restraining excessive inflammation in GUs.
Prostaglandins (PGs) are important proinflammatory factors with complicated functions in the body. However, some PGs, such as PGE2 and PGI2, are critical in the mucosal defense of the stomach[1,31] and play a key role in protecting gastric mucosa from injury in the development of GUs[32-34]. NSAIDs cause gastric mucosal lesions, which may be explained by inhibiting the activity of cyclooxygenases (COXs) and thus reducing the production of PGs. Since PGI2 is the main subtype of PGs in gastric tissue, we quantified the contents of 6-keto-PGF-1α, a stable degradation product of PGI2[35,36] in the current study. Our results showed that the 6-keto-PGF-1α level was reduced in three different GU models. Pretreatment with C. butyricum significantly attenuated the decrease of 6-keto-PGF-1α, suggesting possible protective effects of C. butyricum. Therefore, it is possible that C. butyricum provides protection for gastric mucosa against injuries by reducing oxidative stresses, attenuating pro-inflammatory cytokines and increasing the level of PGI2. Further studies are needed to test this hypothesis.
To further assess the effects of C. butyricum, the general conditions and behavior of mice in different GU models were observed and compared. We found that the behavior of alcohol-induced GU mice was improved by the pretreatment with C. butyricum, while the mice without pretreatment lay at the bottom of cage. In the cold-induced GU mice, pretreatment of C. butyricum alleviated the depression induced by cold stress. Similarly, in the pylorus ligation GU mice, pretreatment with C. butyricum eliminated the odor of gastric juice in these mice.
Previous studies have shown that C. butyricum prevents inflammation-related diseases via its metabolites[25,37]. It is reasonable to speculate that the metabolites of C. butyricum may play a role in protection of the gastric mucosa. Butyrate and hydrogen are the two major metabolic products of C. butyricum. As a primary energy source of colonic epithelial cells, butyrate promotes water and sodium absorption, tightens the junctions between epithelial cells and accelerates the repair of damaged epithelial cells[38,39] in vitro. Butyrate has been reported to ameliorate inflammation in diversion colitis[40-42], and attenuate ROS production[43] in intestinal epithelial Caco-2 cells stimulated by lipopolysaccharide and colonic mucosa from patients with Crohn’s disease. In addition, butyrate was shown to increase the expression and activity of COXs in human monocytes[44] and ROS 17/2.8 osteoblastic cells[45], as well as human colon tumor tissue[46]. In this study, we showed that C. butyricum reduced oxidative stress and inflammatory responses in different GU models. Furthermore, our other study (unpublished data) demonstrated that butyrate exerted protective effects on gastric mucosa challenged with ulcer-inducing stimuli, which provides an explanation for the anti-GU effect of C. butyricum.
C. butyricum is capable of producing a large amount of hydrogen; therefore, it has been used as a clean energy resource of hydrogen[47-49]. Recently, hydrogen was used in therapeutics of various diseases as an anti-inflammatory gas[50,51], which opens an exciting approach for the treatment of inflammatory diseases. For example, a recent study showed that hydrogen could alleviate injury in stress-induced GU in mice[52]. Thus, hydrogen might be a contributor to C. butyricum protective effects against GU. However, the metabolites of bacteria are highly dependent on survival environments. Unlike butyrate, which has a relatively stable turnover, hydrogen production is sensitive to the culture conditions. Although C. butyricum can survive and colonize in the stomach, the metabolites of C. butyricum may be different from those under optimized conditions. Therefore, further studies are required to identify whether butyrate and hydrogen are the crucial products of C. butyricum for the gastric mucosa protective effects.
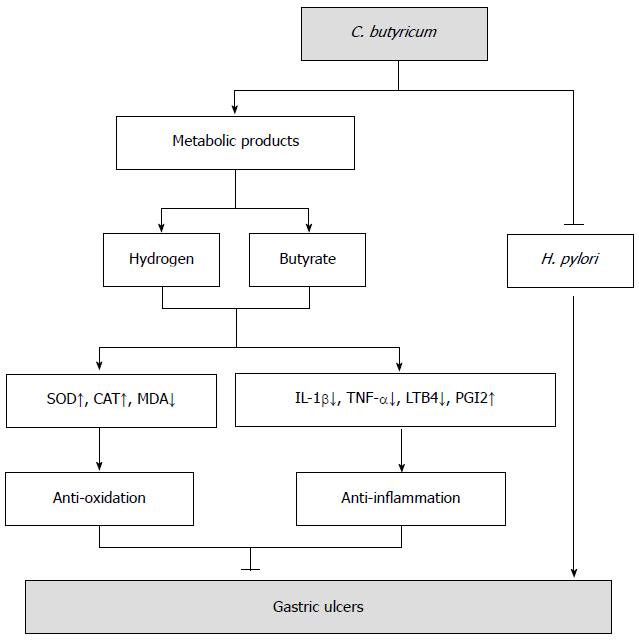
C. butyricum might be a good choice in the future for pretreatment of GUs, especially with H. pylori infection. From the findings of this study, we designed a theoretical working model for C. butyricum (Figure 7), which includes three possible benefits of C. butyricum in clinical practice. First, C. butyricum directly inhibits H. pylori growth through antagonistic interaction, eliminating the most important cause of the occurrence and reoccurrence of GU. Furthermore, C. butyricum is suitable for patients suffering from GU but not sensitive to the therapy of eradication H. pylori. Second, C. butyricum attenuates gastric mucosal lesions independently, which provides direct evidence for therapeutical administration. Third, C. butyricum colonization of the stomach provides a long-lasting protection that prevents the occurrence and recurrence of ulcers. The main metabolic products, including butyrate and hydrogen, play crucial roles in the protective effects of C. butyricum. In addition, the probiotic C. butyricum can be used as a nutrition annexing agent for those sensitive to gastric mucosal lesions or infected by Helicobacter pylori, which is a prominent advantage lacking in the current clinical practice of GU treatment. Nonetheless, future investigations are needed to test these speculations.
In summary, pretreatment with C. butyricum reduced oxidative stress, inflammatory responses, and injury severity in mice with 3 different GU models in this study. These data may suggest a potential protective effect of C. butyricum on GU and support further effort to explore its possible clinical application.
We thank Dr. W.Z. Martini from US Army Institute of Surgical Research at Sam Houston, TX, United States, for critical reading and editing of this manuscript. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Gastric ulcer (GU), which has a high incidence, can cause perforation, loss of blood and cancerization; therefore it is very important to prevent GU. However, the drugs currently used for GU in clinic are poor at preventing GU occurrence and relapse. Meanwhile, the resistance of Helicobacter pylori (H. pylori) to antibiotics requires constant changes the therapeutic strategies. Thus, there is an urgent need to find new methods to prevent GU. The probiotic Clostridium butyricum (C. butyricum) can inhibit H. pylori in vivo, but a direct protective effect of C. butyricum on gastric mucosa has not been reported.
C. butyricum is widely used in clinics to resist pathogenic bacteria, especially in infection related diseases of intestine. The current study aimed to test whether C. butyricum could prevent the gastric mucosa from developing lesions induced alcohol, stress and excessive gastric juice.
There are some problems in the current therapy of GU: (1) H. pylori resistance to antibiotics is difficult to resolve; (2) the recurrence rate of GU is high with current therapies. H. pylori infection and weakened defensive barrier are the most important contributors; and (3) there are no effective agents among the current drugs that promote gastric mucosa repair.
C. butyricum, which was reported to inhibit H. pylori colonization in the stomach, can produce butyrate and hydrogen, which are anti-inflammatory and anti-oxidative substances. In particular, butyrate is clinically applied for colitis to repair intestinal mucosa. Thus, C. butyricum may be an ideal drug to resolve H. pylori resistance and repair gastric mucosa.
The study results suggest that C. butyricum is a potentially protective probiotic that could be used to prevent GU induced by different factors.
GU is caused by excessive amounts of various factors, including excess secretion of gastric acids and pepsin, stress, alcohol, non-steroidal anti-inflammatory drugs, H. pylori infection and smoking. Over-excessive inflammation and oxidative stress play critical roles in the pathogenesis of GU. C. butyricum with its acid fast character can colonize the stomach.
This is a well-written study in which the authors analyzed the preventive effect of C. butyricum on GU induced by different stimulations in mice. The results suggest that C. butyricum is a potential protective probiotic that could be used to prevent different causes of GU.
P- Reviewer: Ozturk O, Rocha JBT, Rodrigo L S- Editor: Yu J L- Editor: Stewart G E- Editor: Liu XM
| 1. | Wallace JL. Prostaglandins, NSAIDs, and gastric mucosal protection: why doesn’t the stomach digest itself? Physiol Rev. 2008;88:1547-1565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 434] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 2. | Zapata-Colindres JC, Zepeda-Gómez S, Montaño-Loza A, Vázquez-Ballesteros E, de Jesús Villalobos J, Valdovinos-Andraca F. The association of Helicobacter pylori infection and nonsteroidal anti-inflammatory drugs in peptic ulcer disease. Can J Gastroenterol. 2006;20:277-280. [PubMed] |
| 3. | den Hollander WJ, Kuipers EJ. Current pharmacotherapy options for gastritis. Expert Opin Pharmacother. 2012;13:2625-2636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Armuzzi A, Cremonini F, Bartolozzi F, Canducci F, Candelli M, Ojetti V, Cammarota G, Anti M, De Lorenzo A, Pola P. The effect of oral administration of Lactobacillus GG on antibiotic-associated gastrointestinal side-effects during Helicobacter pylori eradication therapy. Aliment Pharmacol Ther. 2001;15:163-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 190] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 5. | Cremonini F, Di Caro S, Covino M, Armuzzi A, Gabrielli M, Santarelli L, Nista EC, Cammarota G, Gasbarrini G, Gasbarrini A. Effect of different probiotic preparations on anti-helicobacter pylori therapy-related side effects: a parallel group, triple blind, placebo-controlled study. Am J Gastroenterol. 2002;97:2744-2749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 214] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 6. | Ogoshi K. Gastric Ulcer Relapse. Digestive Endoscopy. 1994;6:219-223. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 7. | Befrits R, Sjöstedt S, Tour R, Leijonmarck CE, Hedenborg L, Backman M. Long-term effects of eradication of Helicobacter pylori on relapse and histology in gastric ulcer patients: a two-year follow-up study. Scand J Gastroenterol. 2004;39:1066-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Chubineh S, Birk J. Proton pump inhibitors: the good, the bad, and the unwanted. South Med J. 2012;105:613-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | Yu EW, Bauer SR, Bain PA, Bauer DC. Proton pump inhibitors and risk of fractures: a meta-analysis of 11 international studies. Am J Med. 2011;124:519-526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 233] [Cited by in RCA: 214] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 10. | Khalili H, Huang ES, Jacobson BC, Camargo CA, Feskanich D, Chan AT. Use of proton pump inhibitors and risk of hip fracture in relation to dietary and lifestyle factors: a prospective cohort study. BMJ. 2012;344:e372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 148] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 11. | Darabi K. Proton-pump-inhibitor-induced hepatitis. South Med J. 2005;98:844-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | El-Matary W, Dalzell M. Omeprazole-induced hepatitis. Pediatr Emerg Care. 2005;21:529-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Canani RB, Cirillo P, Roggero P, Romano C, Malamisura B, Terrin G, Passariello A, Manguso F, Morelli L, Guarino A. Therapy with gastric acidity inhibitors increases the risk of acute gastroenteritis and community-acquired pneumonia in children. Pediatrics. 2006;117:e817-e820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 264] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 14. | Eom CS, Jeon CY, Lim JW, Cho EG, Park SM, Lee KS. Use of acid-suppressive drugs and risk of pneumonia: a systematic review and meta-analysis. CMAJ. 2011;183:310-319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 305] [Cited by in RCA: 277] [Article Influence: 19.8] [Reference Citation Analysis (1)] |
| 15. | Sato R, Tanaka M. Intestinal distribution and intraluminal localization of orally administered Clostridium butyricum in rats. Microbiol Immunol. 1997;41:665-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Woo TD, Oka K, Takahashi M, Hojo F, Osaki T, Hanawa T, Kurata S, Yonezawa H, Kamiya S. Inhibition of the cytotoxic effect of Clostridium difficile in vitro by Clostridium butyricum MIYAIRI 588 strain. J Med Microbiol. 2011;60:1617-1625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 17. | Takahashi M, Taguchi H, Yamaguchi H, Osaki T, Kamiya S. Studies of the effect of Clostridium butyricum on Helicobacter pylori in several test models including gnotobiotic mice. J Med Microbiol. 2000;49:635-642. [PubMed] |
| 18. | Swarnakar S, Mishra A, Ganguly K, Sharma AV. Matrix metalloproteinase-9 activity and expression is reduced by melatonin during prevention of ethanol-induced gastric ulcer in mice. J Pineal Res. 2007;43:56-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 62] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 19. | Sutanto W, de Kloet ER. The use of various animal models in the study of stress and stress-related phenomena. Lab Anim. 1994;28:293-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 65] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Diniz LR, Vieira CF, Santos EC, Lima GC, Aragão KK, Vasconcelos RP, Araújo PC, Vasconcelos Yde A, Oliveira AC, Oliveira HD. Gastroprotective effects of the essential oil of Hyptis crenata Pohl ex Benth. on gastric ulcer models. J Ethnopharmacol. 2013;149:694-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170-3175. [PubMed] |
| 22. | Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15211] [Cited by in RCA: 15197] [Article Influence: 370.7] [Reference Citation Analysis (0)] |
| 23. | Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17627] [Cited by in RCA: 18802] [Article Influence: 408.7] [Reference Citation Analysis (0)] |
| 24. | Graziani G, D’Argenio G, Tuccillo C, Loguercio C, Ritieni A, Morisco F, Del Vecchio Blanco C, Fogliano V, Romano M. Apple polyphenol extracts prevent damage to human gastric epithelial cells in vitro and to rat gastric mucosa in vivo. Gut. 2005;54:193-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 119] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 25. | Zhang HQ, Ding TT, Zhao JS, Yang X, Zhang HX, Zhang JJ, Cui YL. Therapeutic effects of Clostridium butyricum on experimental colitis induced by oxazolone in rats. World J Gastroenterol. 2009;15:1821-1828. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 26. | Imase K, Takahashi M, Tanaka A, Tokunaga K, Sugano H, Tanaka M, Ishida H, Kamiya S, Takahashi S. Efficacy of Clostridium butyricum preparation concomitantly with Helicobacter pylori eradication therapy in relation to changes in the intestinal microbiota. Microbiol Immunol. 2008;52:156-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 27. | Al Batran R, Al-Bayaty F, Jamil Al-Obaidi MM, Abdualkader AM, Hadi HA, Ali HM, Abdulla MA. In vivo antioxidant and antiulcer activity of Parkia speciosa ethanolic leaf extract against ethanol-induced gastric ulcer in rats. PLoS One. 2013;8:e64751. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 102] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 28. | Ahmad A, Husain A, Mujeeb M, Khan SA, Najmi AK, Siddique NA, Damanhouri ZA, Anwar F. A review on therapeutic potential of Nigella sativa: A miracle herb. Asian Pac J Trop Biomed. 2013;3:337-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 566] [Cited by in RCA: 623] [Article Influence: 62.3] [Reference Citation Analysis (0)] |
| 29. | Gyires K. Neuropeptides and gastric mucosal homeostasis. Curr Top Med Chem. 2004;4:63-73. [RCA] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 30. | Tariq M, Ageel AM. Gastric antiulcer and cytoprotective effects of dipyridamole in rats. J Pharmacol Exp Ther. 1990;253:944-949. [PubMed] |
| 31. | Konturek SJ, Piastucki I, Brzozowski T, Radecki T, Dembińska-Kieć A, Zmuda A, Gryglewski R. Role of prostaglandins in the formation of aspirin-induced gastric ulcers. Gastroenterology. 1981;80:4-9. [PubMed] |
| 32. | Kapui Z, Boér K, Rózsa I, Blaskó G, Hermecz I. Investigations of indomethacin-induced gastric ulcer in rats. Arzneimittelforschung. 1993;43:767-771. [PubMed] |
| 33. | Abdel Salam OM, Mózsik G, Szolcsányi J. Studies on the effect of intragastric capsaicin on gastric ulcer and on the prostacyclin-induced cytoprotection in rats. Pharmacol Res. 1995;32:209-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 34. | Materia A, Jaffe BM, Money SR, Rossi P, De Marco M, Basso N. Prostaglandins in commercial milk preparations. Their effect in the prevention of stress-induced gastric ulcer. Arch Surg. 1984;119:290-292. [PubMed] |
| 35. | Schildknecht S, Bachschmid M, Baumann A, Ullrich V. COX-2 inhibitors selectively block prostacyclin synthesis in endotoxin-exposed vascular smooth muscle cells. FASEB J. 2004;18:757-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 36. | Poggi A, Niewiarowski S, Stewart GJ, Sobel E, Smith JB. Human platelet secreted proteins and prostacyclin production by bovine aortic endothelial cells. Proc Soc Exp Biol Med. 1983;172:543-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 37. | Qadis AQ, Goya S, Yatsu M, Kimura A, Ichijo T, Sato S. Immune-stimulatory effects of a bacteria-based probiotic on peripheral leukocyte subpopulations and cytokine mRNA expression levels in scouring holstein calves. J Vet Med Sci. 2014;76:677-684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 38. | Scheppach W, Weiler F. The butyrate story: old wine in new bottles? Curr Opin Clin Nutr Metab Care. 2004;7:563-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 180] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 39. | Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ. Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther. 2008;27:104-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1657] [Cited by in RCA: 1850] [Article Influence: 108.8] [Reference Citation Analysis (0)] |
| 40. | Hallert C, Björck I, Nyman M, Pousette A, Grännö C, Svensson H. Increasing fecal butyrate in ulcerative colitis patients by diet: controlled pilot study. Inflamm Bowel Dis. 2003;9:116-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 154] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 41. | Scheppach W, Sommer H, Kirchner T, Paganelli GM, Bartram P, Christl S, Richter F, Dusel G, Kasper H. Effect of butyrate enemas on the colonic mucosa in distal ulcerative colitis. Gastroenterology. 1992;103:51-56. [PubMed] |
| 42. | Vernia P, Cittadini M, Caprilli R, Torsoli A. Topical treatment of refractory distal ulcerative colitis with 5-ASA and sodium butyrate. Dig Dis Sci. 1995;40:305-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 43. | Russo I, Luciani A, De Cicco P, Troncone E, Ciacci C. Butyrate attenuates lipopolysaccharide-induced inflammation in intestinal cells and Crohn’s mucosa through modulation of antioxidant defense machinery. PLoS One. 2012;7:e32841. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 87] [Article Influence: 6.7] [Reference Citation Analysis (1)] |
| 44. | Kovarik JJ, Hölzl MA, Hofer J, Waidhofer-Söllner P, Sobanov Y, Koeffel R, Saemann MD, Mechtcheriakova D, Zlabinger GJ. Eicosanoid modulation by the short-chain fatty acid n-butyrate in human monocytes. Immunology. 2013;139:395-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 45. | Iida T, Kawato T, Tanaka H, Tanabe N, Nakai K, Zhao N, Suzuki N, Ochiai K, Maeno M. Sodium butyrate induces the production of cyclooxygenases and prostaglandin E2 in ROS 17/2.8 osteoblastic cells. Arch Oral Biol. 2011;56:678-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 46. | Jahns F, Wilhelm A, Jablonowski N, Mothes H, Radeva M, Wölfert A, Greulich KO, Glei M. Butyrate suppresses mRNA increase of osteopontin and cyclooxygenase-2 in human colon tumor tissue. Carcinogenesis. 2011;32:913-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 47. | Ferreira AF, Ortigueira J, Alves L, Gouveia L, Moura P, Silva C. Biohydrogen production from microalgal biomass: energy requirement, CO2 emissions and scale-up scenarios. Bioresour Technol. 2013;144:156-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 48. | Jo JH, Lee DS, Park D, Park JM. Biological hydrogen production by immobilized cells of Clostridium tyrobutyricum JM1 isolated from a food waste treatment process. Bioresour Technol. 2008;99:6666-6672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 49. | Sun YQ, Xiu ZL, Li XH, Zhang DJ. Stoichiometric analysis of biological hydrogen production by fermentative bacteria. Int J Hydrogen Energy. 2006;31:539-549. [DOI] [Full Text] |
| 50. | Cardinal JS, Zhan J, Wang Y, Sugimoto R, Tsung A, McCurry KR, Billiar TR, Nakao A. Oral hydrogen water prevents chronic allograft nephropathy in rats. Kidney Int. 2010;77:101-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 136] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 51. | Buchholz BM, Kaczorowski DJ, Sugimoto R, Yang R, Wang Y, Billiar TR, McCurry KR, Bauer AJ, Nakao A. Hydrogen inhalation ameliorates oxidative stress in transplantation induced intestinal graft injury. Am J Transplant. 2008;8:2015-2024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 239] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 52. | Liu X, Chen Z, Mao N, Xie Y. The protective of hydrogen on stress-induced gastric ulceration. Int Immunopharmacol. 2012;13:197-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |