Published online Jun 28, 2015. doi: 10.3748/wjg.v21.i24.7598
Peer-review started: December 9, 2014
First decision: January 8, 2015
Revised: January 29, 2015
Accepted: February 11, 2015
Article in press: February 11, 2015
Published online: June 28, 2015
Processing time: 203 Days and 3.3 Hours
Plasmablastic lymphoma (PBL) is a rare form of non-Hodgkin’s lymphoma that is associated with human immunodeficiency virus (HIV) infection. Although PBL is most commonly observed in the oral cavity of HIV-positive patients, it can also be observed at extra-oral sites in HIV-negative patients. This report represents an unusual case of HIV-negative PBL that occurred in the sigmoid colon. This patient had a history of systemic lupus erythematosus and an underlying immunosuppressive state from long term steroid therapy. The lymphoma cells were positive for CD138, kappa light chain restriction and Epstein-Barr virus and negative for CD20/L26, CD3, CD79a, UCHL1 (CD45RO) and cytokeratin (AE1/AE3). The patient died approximately 2 mo after the operation. In the present paper, we review cases of PBL of the colon in HIV-negative patients.
Core tip: Plasmablastic lymphoma (PBL) is a rare form of non-Hodgkin’s lymphoma that is associated with human immunodeficiency virus (HIV) infection. Although PBL is most commonly observed in the oral cavity of HIV-positive patients, it can also be observed at extra-oral sites in HIV-negative patients with an underlying immunosuppressive state. The gastrointestinal tract and skin are the most commonly involved extra-oral organ systems and cases of PBL in the colon are unusual. We report a case of HIV-negative PBL that occurred in the sigmoid colon.
- Citation: Haramura T, Haraguchi M, Irie J, Ito S, Tokai H, Noda K, Kitajima M, Minami S, Inoue K, Sasaki Y, Oshima K, Eguchi S. Case of plasmablastic lymphoma of the sigmoid colon and literature review. World J Gastroenterol 2015; 21(24): 7598-7603
- URL: https://www.wjgnet.com/1007-9327/full/v21/i24/7598.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i24.7598
Considered as a diffuse large B-cell non-Hodgkin’s lymphoma variant, plasmablastic lymphoma (PBL) is an aggressive and rapidly growing lymphoma characterized by weak/absent expression of conventional B-cell markers and by strong expression of plasma cell markers. PBL was described as a new disease entity in the 4th WHO classification[1]. PBL was first reported in the oral cavity in the setting of human immunodeficiency virus (HIV) in 1997 by Delecluse et al[2], originally described in 16 cases of a variant of diffuse large B-cell lymphoma (DLBCL). Although PBL is strongly associated with HIV infection, an increasing number of cases have recently been recognized in a non-HIV population, as in our case[3]. These cases often occur in patients with an underlying immunosuppressive state, such as from solid organ and bone marrow transplantation, and from lymphoproliferative or autoimmune disorders. A characteristic feature of PBL is its rapidly progressive clinical course. However, the overall survival is better in HIV-positive patients treated with highly active antiretroviral therapy (HAART) and appropriate chemotherapy than in HIV-negative patients[4].
Several reports have described the occurrence of PBL in extra-oral sites, including the skin, stomach, small intestine, anal mucosa or perianal area, lung, liver, retroperitoneum and other regions[5-10].
We describe a case of PBL of the sigmoid colon in a HIV-negative but Epstein-Barr virus (EBV)-positive patient who presented with gastrointestinal bleeding.
According to our research, before our case, there have only been four cases of PBL of the colon in an HIV-negative patient. Herein, we review the literature of rare cases of PBL of the colon.
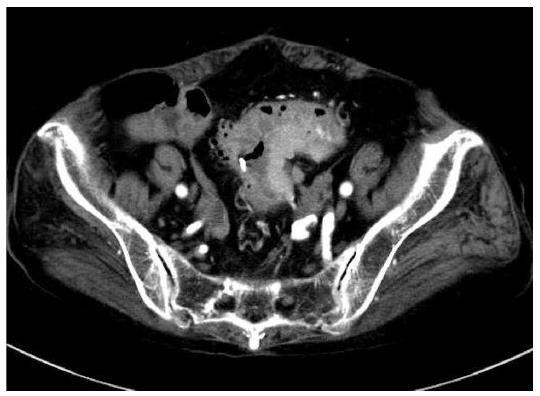
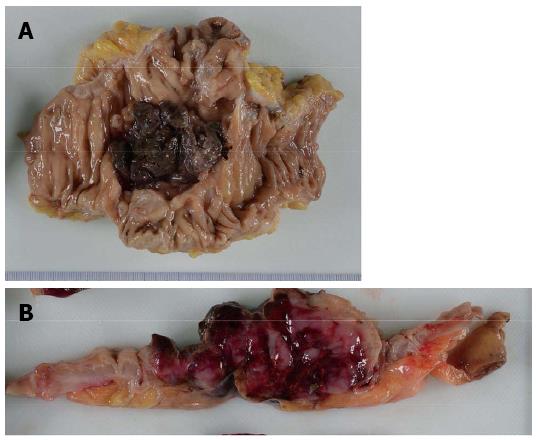
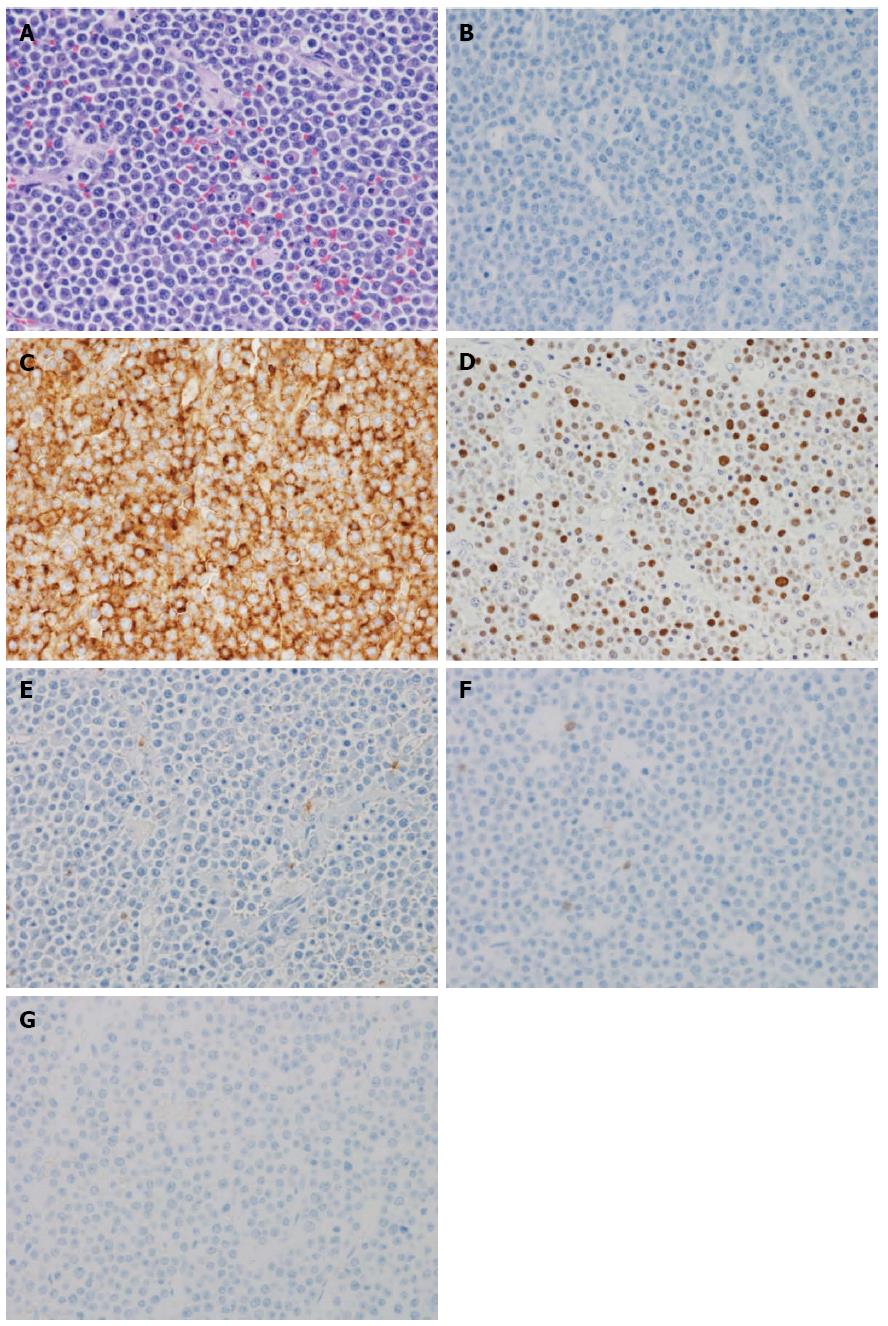
An 86-year-old female was admitted to our hospital’s emergency department because of bloody stool. On physical examination, she had no abdominal pain and no tenderness with guarding or rebound. At presentation, laboratory data revealed the following results: white blood count at 13100 cells/mm2, hemoglobin at 13.2 mg/dL, C-reactive protein at 2.1 mg/dL, and negative status for hepatitis B and C and for human T-cell lymphotropic virus 1. The patient was known to have systemic lupus erythematosus (SLE) and type 2 diabetes mellitus. The patient was receiving steroidal treatment for SLE. A computed tomography scan of her abdomen revealed a mass measuring 5 cm, multiple diverticula in the sigmoid colon and enlarged lymph nodes in the sigmoid mesentery (Figure 1). Free air was not detected in the abdominal cavity. Colonoscopy was performed and a tumor with bleeding in the sigmoid colon was revealed. Because bleeding due to sigmoid colon cancer was suspected, sigmoidectomy with Hartmann’s procedure was urgently performed. The excised material revealed the tumor to be tender, 5 cm × 4.5 cm in size, and displaying the diverticulosis (Figure 2A). On the cut surface, the tumor was soft, consistent with hemorrhage (Figure 2B). Microscopically, the tumor cells had large hyperchromatic nuclei with prominent nucleoli. Some tumor cells showed plasmacytic differentiation (Figure 3A). There was no epithelial component. Therefore, malignant lymphoma or diffuse lymphoma was suspected.
Immunohistochemical examination revealed that the tumor cells were negative for CD20/L26 (Figure 3B), CD3, CD79a, UCHL1 (CD45RO) and cytokeratin (AE1/AE3), but positive for CD138 (Figure 3C), kappa light chain restriction and EBV (Figure 3D). The patient’s HIV negative status was proven after the surgery. Based on these results, our final diagnosis was PBL. The patient continued to deteriorate clinically and developed multiorgan failure. The patient died approximately 2 mo after surgery after refusing chemotherapy and radiotherapy. Autopsy was not performed.
PBL has been recognized as a distinct entity, a subtype of diffuse large B-cell lymphoma, by the WHO classification of lymphoproliferative disorders[11]. PBL accounts for 1.5% of all nodal non-Hodgkin’s lymphomas and has a strong predilection for immunodeficiency, particularly HIV. In patients with PBL in HIV infection, the median age was 38 years with a male predominance of 7:1. The prognosis remains poorer than that of other DLBCL[12]. The risk of developing non-Hodgkin’s lymphoma is 200 times higher in HIV-positive patients than in otherwise healthy persons. However, an increasing number of PBL cases have recently been recognized in patients without HIV infection. In HIV-negative individuals, PBL cases have been reported after solid organ transplantation, in association with steroid therapy for autoimmune disease, and some other types of immunosuppression[13,14]. About one-third of PBL-diagnosed HIV negative patients have presented with an underlying immunosuppressive state[15]. The present patient had a diagnosis of SLE and a history of steroidal treatment for 35 years before developing lymphoma. This steroidal therapy probably led to the iatrogenic immunocompromised state. There was no family history of any hematological malignancies. Except for the steroidal therapy, our patient had never received any other treatment which could lead to an iatrogenic immunocompromised state.
The present case is HIV-negative but EBV-positive. EBV plays an important role in the tumorigenesis of HIV-associated PBL[16]. HIV infection creates a favorable environment for chronic EBV infection, with a subsequent latency that predisposes EBV-transformed B-cells to become malignant. It has been reported that EBV infection was detected in 72% of PBL cases[17]. However, EBV infection has been detected in only 17% of HIV-negative PBL cases, which suggests that PBL pathogenesis is not specific to EBV infection[5].
The histological appearance of PBL is usually monomorphic with a diffuse lymphoid infiltrate and cohesive growth pattern[18]. The main differential diagnosis of PBL includes other forms of DLBCL, plasmacytoma/myeloma, Burkitt’s lymphomas, poorly differentiated carcinoma and malignant melanoma; for such a differential diagnosis, the help of morphological characteristics and behavior are often effective[16]. Unlike PBL, DLBCL always expresses CD20, CD45-RA and CD79a. Plasmacytoma typically consists of mature plasma cells without a high rate of mitotic activity. Burkitt’s lymphomas express a membrane-bound IgM heavy chain isotype. PBL expresses immunoreactivity for plasma cell markers (CD38, CD138) and is weakly positive or negative for CD45 and CD20. CD79a is positive in approximately 50%-85% of all PBL cases[16]. CD138 is a highly specific and sensitive marker of plasmacytic differentiation within the spectrum of hematological malignancy. CD138 reactivity has been reported with variable frequency in immunoblastic diffuse large B-cell lymphoma. CD56 and cyclin D1 are usually negative. However, a differential diagnosis between PBL and plasmacytoma is difficult without histological examination.
The most notable feature of PBL is its predilection for the oral cavity. Most patients with PBL present with a primary oral lesion, often complaining of a toothache or tooth abscess[19]. PBL can be observed at extra-oral sites in HIV-negative patients. As for extra-oral organ systems, the gastrointestinal tract and skin are the most commonly involved[19] but only four PBL cases of the colon in HIV-negative patients have been reported before our case[4,20,21] (Table 1).
The general prognosis of PBL is very poor, with a rapidly progressive clinical course[19]. However, clinicopathological characteristics of PBL patients differ between HIV-positive and HIV-negative status, where HIV-positive patients have better response to chemotherapy and longer survival[5]. For HIV-positive PBL patients, recent reports have noted improved survival when treating with both HAART and appropriate chemotherapy, such as cyclophosphamide, doxorubicin, vincristine and prednisone, with similar outcomes for HIV-infected patients to other non-Hodgkin’s lymphomas[19]. Several patients have been documented to have survived for more than 3 years from the time of initial diagnosis of PBL, usually with a combination of HAART plus chemotherapy[19].
Extra-oral PBL can often occur in HIV-negative patients and is highly aggressive, with a poor prognosis. Although identifying extra-oral PBL requires familiarity with lymphoma variants and related differential diagnosis procedures, PBL should be suspected if the patient is immunosuppressed. A high index of suspicion by the clinician and pathologist might lead to initiating appropriate treatments and account for better outcomes.
An 86-year-old female was admitted to our hospital’s emergency department because of bloody stool.
The physical sign of our case was bloody stool; on physical examination, she had no abdominal pain and no tenderness with guarding or rebound.
Diffuse large B-cell lymphoma, plasmacytoma/myeloma, Burkitt’s lymphomas, poorly differentiated carcinoma and malignant melanoma.
Laboratory data revealed the following results: white blood count at 13,100 cells/mm2, hemoglobin at 13.2 mg/dL, C-reactive protein at 2.1 mg/dL, and negative status for hepatitis B and C and for human T-cell lymphotropic virus 1.
A computed tomography scan of our patient’s abdomen revealed a mass measuring 5 cm, multiple diverticula in the sigmoid colon and enlarged lymph nodes in the sigmoid mesentery.
Microscopically, the tumor cells had large hyperchromatic nuclei with prominent nucleoli and some tumor cells showed plasmacytic differentiation, while immunohistochemical examination revealed that the tumor cells were negative for CD20/L26, CD3, CD79a, UCHL1 (CD45RO) and cytokeratin (AE1/AE3), but positive for CD138, kappa light chain restriction and Epstein-Barr virus (EBV).
Sigmoidectomy with Hartmann’s procedure was performed.
Considered as a diffuse large B-cell non-Hodgkin’s lymphoma variant, plasmablastic lymphoma (PBL) was described as a new disease entity in the 4th WHO classification.
Extra-oral PBL can often occur in human immunodeficiency virus (HIV)-negative patients and is highly aggressive with a poor prognosis. However, a high index of suspicion by the clinician and pathologist might lead to initiating appropriate treatments and account for better outcomes.
This is a case report of PBL which is a rare form of non-Hodgkin’s lymphoma that is associated with HIV infection. The author of this manuscript should give more information about this kind of lymphoma, including the epidemiology and distribution of the disease.
P- Reviewer: Harmanci O, Yao HR S- Editor: Ma YJ L- Editor: Roemmele A E- Editor: Zhang DN
| 1. | Swerdlow SH. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC press 2008; Available from: http://www.uptodate.com/contents/classification-of-the-hematopoietic-neoplasms. |
| 2. | Delecluse HJ, Anagnostopoulos I, Dallenbach F, Hummel M, Marafioti T, Schneider U, Huhn D, Schmidt-Westhausen A, Reichart PA, Gross U. Plasmablastic lymphomas of the oral cavity: a new entity associated with the human immunodeficiency virus infection. Blood. 1997;89:1413-1420. [PubMed] |
| 3. | Choi SY, Cho YA, Hong SD, Lee JI, Hong SP, Yoon HJ. Plasmablastic lymphoma of the oral cavity in a human immunodeficiency virus-negative patient: a case report with literature review. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;117:e115-e120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Mansoor M, Alani FS, Aslam MB, Kumar SN, Sahasrabudhe N, Khan D. A case report of cecal plasmablastic lymphoma in a HIV-negative patient. Eur J Gastroenterol Hepatol. 2012;24:332-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Wang HW, Yang W, Sun JZ, Lu JY, Li M, Sun L. Plasmablastic lymphoma of the small intestine: case report and literature review. World J Gastroenterol. 2012;18:6677-6681. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Tani J, Miyoshi H, Nomura T, Yoneyama H, Kobara H, Mori H, Morishita A, Himoto T, Masaki T. A case of plasmablastic lymphoma of the liver without human immunodeficiency virus infection. World J Gastroenterol. 2013;19:6299-6303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Mani D, Guinee DG, Aboulafia DM. AIDS-associated plasmablastic lymphoma presenting as a poorly differentiated esophageal tumor: a diagnostic dilemma. World J Gastroenterol. 2008;14:4395-4399. [PubMed] |
| 8. | Jiang P, Liu M, Liu B, Liu B, Zhou Y, Dong L. Human immunodeficiency virus-negative plasmablastic lymphoma in the neck: a rare case report and literature review. Eur J Med Res. 2014;19:64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Marques I, Lagos A, Costa-Neves B. Gastric plasmablastic lymphoma in HIV-negative patient. Rev Esp Enferm Dig. 2013;105:166-167. [PubMed] |
| 10. | Saraceni C, Agostino N, Cornfield DB, Gupta R. Plasmablastic lymphoma of the maxillary sinus in an HIV-negative patient: a case report and literature review. Springerplus. 2013;2:142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Jaffe ES, Harris NL, Diebold J, Muller-Hermelink HK. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues. A progress report. Am J Clin Pathol. 1999;111:S8-12. [PubMed] |
| 12. | Castillo J, Pantanowitz L, Dezube BJ. HIV-associated plasmablastic lymphoma: lessons learned from 112 published cases. Am J Hematol. 2008;83:804-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 206] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 13. | Folk GS, Abbondanzo SL, Childers EL, Foss RD. Plasmablastic lymphoma: a clinicopathologic correlation. Ann Diagn Pathol. 2006;10:8-12. [PubMed] |
| 14. | Hernandez C, Cetner AS, Wiley EL. Cutaneous presentation of plasmablastic post-transplant lymphoproliferative disorder in a 14-month-old. Pediatr Dermatol. 2009;26:713-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Liu JJ, Zhang L, Ayala E, Field T, Ochoa-Bayona JL, Perez L, Bello CM, Chervenick PA, Bruno S, Cultrera JL. Human immunodeficiency virus (HIV)-negative plasmablastic lymphoma: a single institutional experience and literature review. Leuk Res. 2011;35:1571-1577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 16. | Koike M, Masuda A, Ichikawa K, Shigemitu A, Komatus N. Plasmablastic lasmablastic lymphoma of the duodenal and jejunum. Int J Clin Exp Pathol. 2014;7:4479-4483. [PubMed] |
| 17. | Castillo JJ, Winer ES, Stachurski D, Perez K, Jabbour M, Milani C, Colvin G, Butera JN. Clinical and pathological differences between human immunodeficiency virus-positive and human immunodeficiency virus-negative patients with plasmablastic lymphoma. Leuk Lymphoma. 2010;51:2047-2053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 139] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 18. | Carbone A, Gaidano G, Gloghini A, Ferlito A, Rinaldo A, Stein H. AIDS-related plasmablastic lymphomas of the oral cavity and jaws: a diagnostic dilemma. Ann Otol Rhinol Laryngol. 1999;108:95-99. [PubMed] |
| 19. | Riedel DJ, Gonzalez-Cuyar LF, Zhao XF, Redfield RR, Gilliam BL. Plasmablastic lymphoma of the oral cavity: a rapidly progressive lymphoma associated with HIV infection. Lancet Infect Dis. 2008;8:261-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Teruya-Feldstein J, Chiao E, Filippa DA, Lin O, Comenzo R, Coleman M, Portlock C, Noy A. CD20-negative large-cell lymphoma with plasmablastic features: a clinically heterogenous spectrum in both HIV-positive and -negative patients. Ann Oncol. 2004;15:1673-1679. [PubMed] |
| 21. | Hatanaka K, Nakamura N, Kishimoto K, Sugino K, Uekusa T. Plasmablastic lymphoma of the cecum: report of a case with cytologic findings. Diagn Cytopathol. 2011;39:297-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |