Published online Jun 7, 2015. doi: 10.3748/wjg.v21.i21.6582
Peer-review started: November 3, 2014
First decision: November 26, 2014
Revised: December 18, 2015
Accepted: February 11, 2015
Article in press: February 11, 2015
Published online: June 7, 2015
Processing time: 221 Days and 13.1 Hours
AIM: To investigate the effects of transplantation of insulin-producing cells (IPCs) in the treatment of diabetic rats after 90% pancreatectomy.
METHODS: Human umbilical cord mesenchymal stem cells (UCMSCs) were isolated and induced into IPCs using differentiation medium. Differentiated cells were examined by dithizone (DTZ) staining, reverse transcription-polymerase chain reaction (RT-PCR), and real-time RT-PCR. C-peptide release, both spontaneously and after glucose challenge, was measured by ELISA. IPCs were then transplanted into Sprague-Dawley rats after 90% pancreatectomy and blood glucose levels and body weight were measured.
RESULTS: The differentiated cells were positive for DTZ staining and expressed pancreatic β-cell related genes. C-peptide release by the differentiated cells increased after glucose challenge (380.6 ± 15.32 pmol/L vs 272.4 ± 15.32 pmol/L, P < 0.05). Further, in the cell transplantation group, blood sugar levels were significantly lower than in the sham group 2 wk after transplantation (18.7 ± 2.5 mmol/L vs 25.8 ± 1.25 mmol/L, P < 0.05). Glucose tolerance tests showed that 45 min after intraperitoneal glucose injection, blood glucose levels were significantly lower on day 56 after transplantation of IPCs (12.5 ± 4.7 mmol/L vs 42.2 ± 9.3 mmol/L, P < 0.05).
CONCLUSION: Our results show that UCMSCs can differentiate into islet-like cells in vitro under certain conditions, which can function as IPCs both in vivo and in vitro.
Core tip: It is well known that islet transplantation can decrease the morbidity related to diabetes mellitus (DM) after total pancreatectomy (TP); however, islet shortage limits its application. To solve the islet shortage problem, we tried to induce mesenchymal stem cells isolated from human Wharton’s jelly to differentiate into insulin-producing cells (IPCs) in this study. To imitate the pathophysiological status of diabetic patients after TP, we used Sprague-Dawley rats after 90% pancreatectomy as a model of type 1 DM. We for the first time tested the possible curative effects of transplanting IPCs differentiated from umbilical cord mesenchymal stem cells into the model rats.
- Citation: Yu YB, Bian JM, Gu DH. Transplantation of insulin-producing cells to treat diabetic rats after 90% pancreatectomy. World J Gastroenterol 2015; 21(21): 6582-6590
- URL: https://www.wjgnet.com/1007-9327/full/v21/i21/6582.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i21.6582
Pancreatic adenocarcinoma is a highly malignant and invasive disease. It has the worst prognosis of all gastrointestinal malignancies[1]. Pancreatic resection plays an essential role in the treatment of pancreatic adenocarcinoma and is the only treatment with the potential to achieve long-term survival[2]. The most common operation for pancreatic neoplasia is pancreaticoduodenectomy. The Achilles heel of this operation is the pancreatic anastomosis, with an incidence of pancreatic leak ranging from 2%-29%[3]. The prognosis remains poor even in patients undergoing radical resection, and there is a median overall survival period of about 20 mo due to the high rate of relapse[4]. Theoretically, elimination of high risk pancreatic anastomosis by performing total pancreatectomy (TP) could reduce perioperative morbidity. Thus, TP has attracted more attention.
TP for pancreatic adenocarcinoma was first reported in 1943 and the patient died in the perioperative period because of a bile duct leak[5]. It was abandoned for a long time because of associated brittle diabetes. In the past decades, TP has shown a definite effect, since remarkable improvements have been achieved in both surgery and postoperative management of endocrine insufficiency of the apancreatic patient[6,7]. It is well known that islet transplantation can decrease the morbidity related to diabetes mellitus (DM) after TP. However, the problem of the worldwide lack of donor has yet to be resolved. Moreover, there still exist the seemingly inevitable problems which doctor and patient need to face: immune rejection and recurrent attacks against islets by the underlying autoimmunity[8,9]. Therefore, there is an urgent need for developing alternative cellular therapy strategies for DM.
In the search of new cell sources for transplantation, stem cells represent a critical alternative and would provide a potentially countless source of islet cells for transplantation. Mesenchymal stem cells (MSCs) isolated from human umbilical cord Wharton’s jelly are more primitive MSCs than those isolated from bone marrow or adipose tissue and do not express the major histocompatibility complex (MHC) class II (HLA-DR) antigens[10,11]. Some groups have isolated MSCs from human umbilical cord Wharton’s jelly and induced them to differentiate into islet-like cell clusters[6,12,13].
To solve the islet shortage problem, we tried to induce MSCs isolated from human Wharton’s jelly to differentiate into IPCs in this study. To imitate the pathophysiological status of diabetic patients after TP, we used Sprague-Dawley (SD) rats after 90% pancreatectomy as a model of type 1 DM. We for the first time examined the possible curative effects of transplanting IPCs differentiated from UCMSCs into SD rats after 90% pancreatectomy.
This study involving the use of SD rats was conducted in strict accordance with provisions and general recommendation of Chinese Experimental Animals Administration Legislation (Permit number SCXK-2010-0001). The cords were collected from obstetrical department of Huai’an First People’s Hospital with the informed consent of the tissue donor, and following the ethical and institutional guidelines.
With consent from the parents, fresh cords were obtained after birth and collected in normal saline (NS) at 4 °C. Following disinfection in 75% ethanol for 30 s, the umbilical cord vessels were torn off in NS. After the removal of the supernatant fraction, the mesenchymal tissue was diced into cubes and then crushed using serrated thumb forceps. The mince structure was washed with DMEM (Gibco, Carlsbad, CA, United States) and centrifuged at 200 ×g for 5 min. The mesenchymal tissue was digested with collagenase II (Gibco) at 37 °C for 1 h and further treated with 0.25% trypsin (Gibco) at 37 °C for 30 min. To neutralize the excess trypsin, fetal bovine serum (FBS, Gibco) was added to the mesenchymal tissue. The cells from the two enzymatic digestion steps were combined and counted using a hemocytometer. The mesenchymal cells were then used directly for cultures, and the medium was changed every third day.
After the third passages, UCMSCs were released by trypsinization. Mouse anti-human antigens CD13, CD34, CD45, CD90, CD105 and HLA-DR were acquired from BD Sciences (Shanghai, CHINA). A total of 1 × 106 cells were incubated with PE- or FITC-conjugated antibodies for 20 min at room temperature. Mouse IgG-PE and mouse IgG-FITC were used as isotype controls. The fluorescence intensity of the cells was analyzed using a flow cytometer (FACScan; BD Sciences), and the data were further analyzed using CELLQUEST Pro software (BD Sciences).
For pancreatic differentiation, UCMSCs from fourth passage reaching 80%-90% confluence were induced to differentiate into IPCs. The pancreatic inductive procedure for UCMSCs was performed according to a previous study[6]. Cells were cultured in DMEM/F12 (Gibco) medium containing 10% FBS, 10 mmol/L nicotinamide (Sigma-Aldrich, St.Louis, MO, United States), 4 nmol/L activin-A (Sigma-Aldrich) and 25 ng/mL epidermal growth factor (EGF, PeproTech, Rochy Hill, NJ, United States) for 1 wk. Then the culture medium was changed to DMEM/F12 for another week. Finally, 10 mmol/L nicotinamide, 10 ng/mL of basic fibroblastic growth factor (bFGF, PeproTech) and insulin/transferrin/selenium (ITS, Gibco) were added, and incubation was continued for 2 wk.
DTZ (Sigma-Aldrich) solution was prepared as reported previously by dissolving 10 mg of DTZ in 1 mL dimethylsulfoxide (DMSO, Sigma-Aldrich) and reserved at -20 °C. The stock DTZ solution was filtered through a nylon filter before use, and for staining 10 μL of the solution was added to 1 mL of cell culture medium. The culture dishes were incubated for 30 min at 37 °C in DTZ-containing solution and then were washed three times with NS. An inverted light microscope was used to examine the color of the clusters and the dishes were then incubated with DMEM containing 10% FBS.
After 2, 3 and 4 wk of induction, cells were rinsed twice with NS and incubated in L-DMEM (5.5 mmol/L glucose, Gibco) for 2 h. C-peptide levels in the culture medium were measured using a C-peptide ELISA kit (Cusabio, Barksdale, DE, United States). TMB substrate was used with absorbance read at 450 nm.
After 4 wk of cultivation, the differentiated cells were washed twice with NS and incubated in L-DMEM for 2 h. The culture medium was collected and the cells were then washed twice with NS, incubated for 2 h in H-DMEM (25 mmol/L glucose, Gibco), and the culture medium was collected again. C-peptide levels were measured as above.
Total RNA was extracted from the cells using TRIzol reagent (Invitrogen, Grand Island, NY, United States) according to the manufacturer’s instructions. Gene expression levels of insulin, PDX1, Pax4, Glut2 and Ngn3 were determined by RT-PCR or real-time RT-PCR. The cDNA templates were obtained using oligo(dT) primers (Invitrogen) and PrimeScript RTase reverse transcriptase (Invitrogen). The specific primer pairs and conditions are showed in Table 1.
| Gene | Sequence | Fragment length (bp) | Annealing temperature (°C) |
| Insulin | F: 5’- ATC AAG CAG ATC ACT GTC CTT CT-3’ | 162 | 60 |
| R: 5’- GAG AGC TTC CAC CAG GTG TG-3’ | |||
| PDX1 | F: 5’- TCC CAT GGA TGA AGT CTA CC-3’ | 246 | 60 |
| R: 5’- TGT CCT CCT CCT TTT TCC AC-3’ | |||
| Ngn3 | F: 5’- CGC CGG TAG AAA GGA TGA C-3’ | 316 | 60 |
| R: 5’- GAG TTG AGG TTG TGC ATT CG-3’ | |||
| Glut2 | F: 5’- AGT ACA ATG ACA GAA GAT AAG GTC-3’ | 423 | 60 |
| R: 5’- AGC TCC AAC TAA TGA CAG AAT G-3’ | |||
| Pax4 | F: 5’- ATC CTT AAG GTA TCT AAT GGC TG-3’ | 461 | 60 |
| R: 5’- GCC ACT GAA TCA GGA TAC TGC-3’ | |||
| GAPDH | F: 5’-AGA AGG CTG GGG CTC ATT TG-3’ | 258 | 52 |
| R: 5’-AGG GCC ATC CAC AGT CTT C-3’ |
Male SD rats at 6 wk age weighing 150-160 g were used. The rats were raised on a 12-h dark and 12-h light cycle (darks at 19:00) and fed ad libitum. Rats underwent surgery to either resect 90% of the pancreas (Px) or to have a sham procedure, as reported previously[14]. After the rats of the Px group were anesthetized with 10% chloral hydrate, a middle abdominal incision was made from the xyphoid to hip level. The gastric, splenic and duodenal regions of the pancreas were removed by using cotton tip applicators, leaving all major vessels intact to not compromise the surrounding organs. The area extending from the duct to the first part of the duodenum was classified as the residual pancreas (about 10% of the original pancreatic mass). Animals undergoing surgical spleen removal were put in a sham group. Fasting blood glucose concentrations were measured through the angular vein 4 times (at 08:00) in the first week. Thereafter, measurements were taken twice a week. Body weight was also measured twice a week at 08:00.
After two days of surgery, the rats were restrained and 1 × 107 IPCs were injected through the portal vein. The sham control group underwent the injection of only NS. Blood glucose and body weight were measured weekly. On day 56, intraperitoneal glucose tolerance was tested with 10% glucose (2 g of glucose/kg of body weight) administered intraperitoneally.
Data from the experiment are expressed as the mean ± standard deviation (SD). Group comparisons were made by Student’s t-test. All statistical analyses were conducted using SPSS 17.0 software program. P < 0.05 was adopted to show statistical significance.
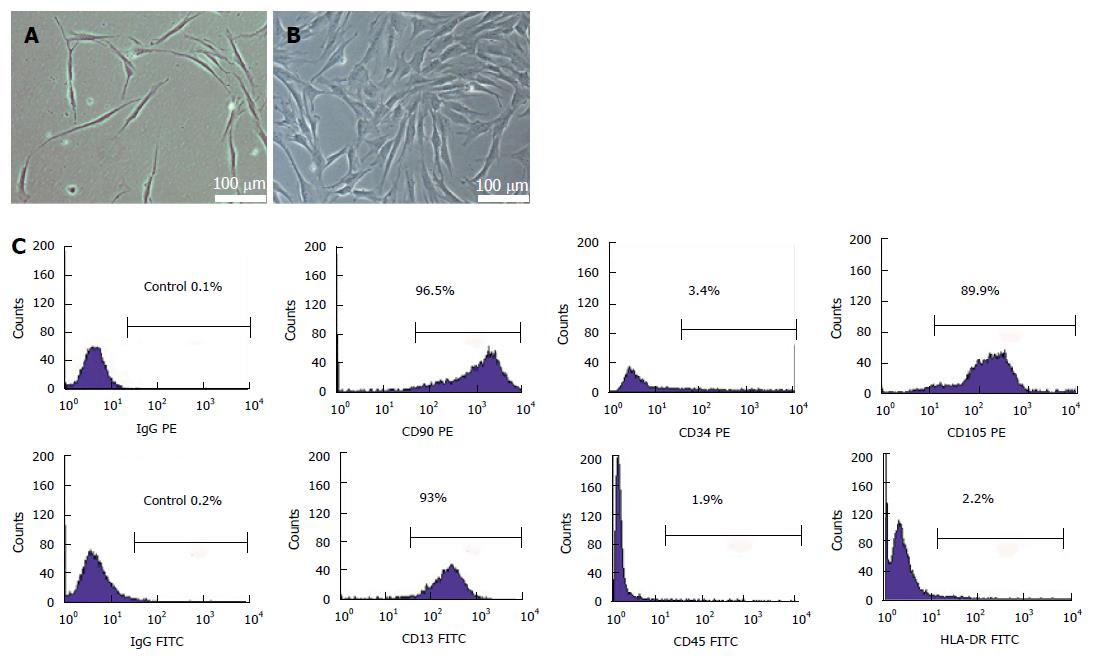
The isolated umbilical cord cells showed heterogeneity during the first 5 d of cultivation. The cells were elongated, adherent and spindle-shaped, and the individual colonies displayed fibroblast-like morphology after 48 h of plating (Figure 1A). Many cells then clonally grew and spread at the bottom of culture dish within the 12th d (Figure 1B). Flow cytometric analysis showed that the isolated cells at passage 4 strongly expressed the surface markers of MSCs, such as CD90, CD105 and CD13, but almost no markers of hematopoietic stem cells, such as CD34, CD45 and HLA-DR (Figure 1C).
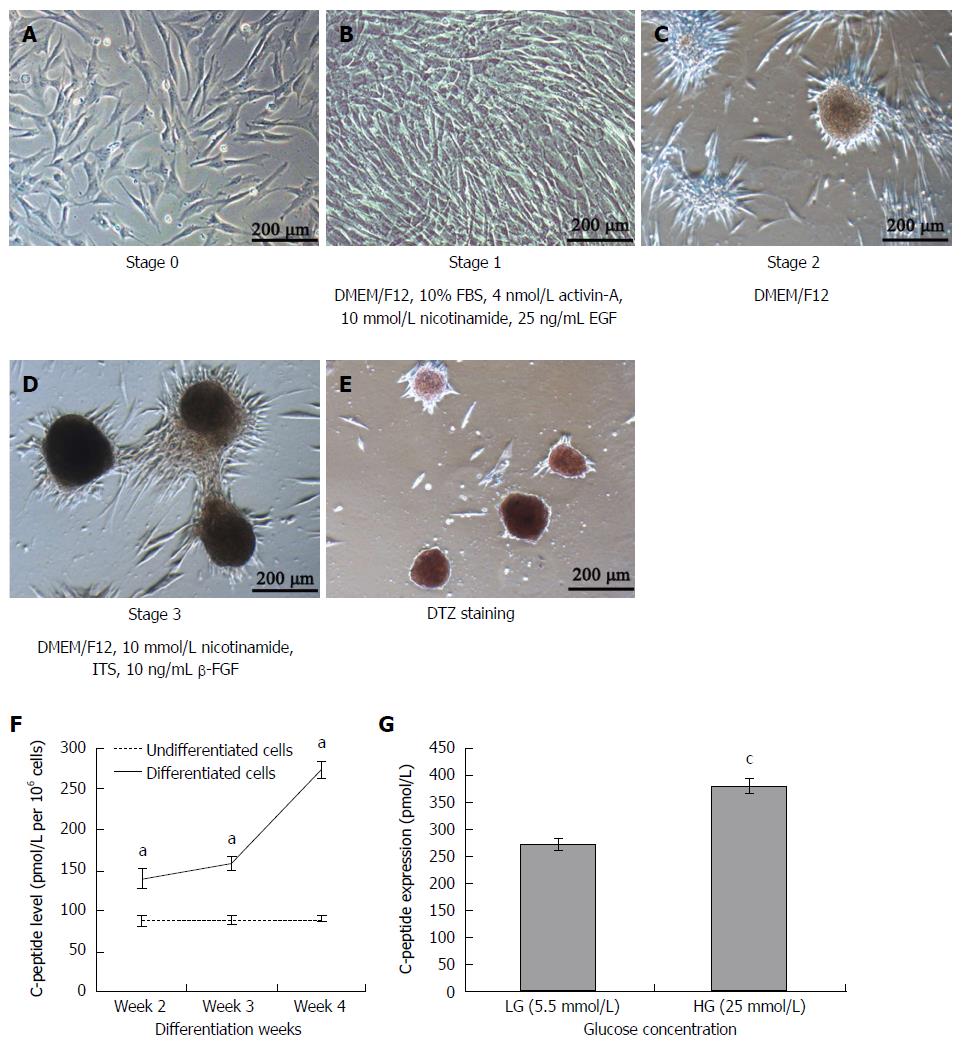
UCMSCs at passage 5 were induced to differentiate into islet-like cells (Figure 2A) by a 3-stage, 4-wk protocol. Upon exposure to serum-free media for an initial 7 d of induction, the adherent spindle-like cells acquired a round shape and assembled together (Figure 2B, stage 1). During stage 2, the round cells became aggregates and some new islet-like clusters began to appear (Figure 2C). The culture of clusters continued to mature and further differentiated into β-like cells (Figure 2D). At the end of stage 3, differentiation of UCMSCs into IPCs was evaluated through DTZ staining. DTZ specifically binds to zinc ion in insulin molecules, which allowed for the identification of clusters with IPCs, whereas undifferentiated cells were negative (Figure 2E).
As a marker of residual insulin production, C-peptide was used to represent insulin production by our differentiated cells[15]. The levels of C-peptide in the culture media of differentiated cells on weeks 2, 3 and 4 showed that these cells produced more C-peptide than the pre-treatment cells (Figure 2F). To examine whether these IPCs could respond to glucose stimulation, C-peptide level in 4-wk differentiated cells was measured. The result showed that differentiated cells secreted C-peptide in a low glucose concentration (5.5 mmol/L) medium and secreted approximately twice as much in a high glucose concentration (25 mmol/L) medium (Figure 2G). Thus, these differentiated MSCs could respond to changes in glucose concentration by altering their C-peptide production and, presumably, insulin production.
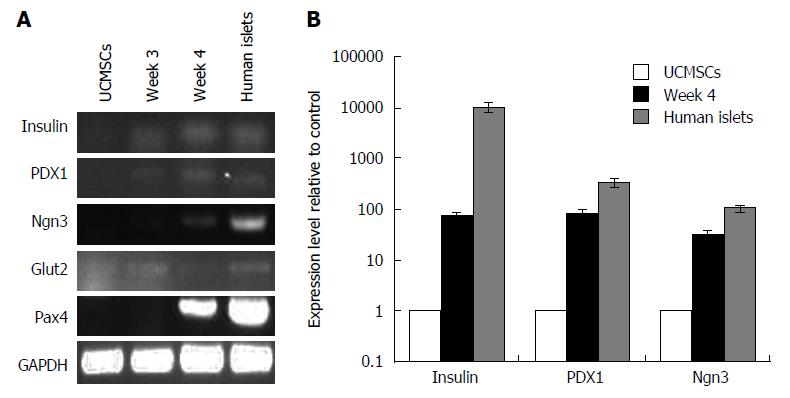
To determine whether the UCMSCs had been induced to differentiate into pancreatic endocrine cells, gene expression profiles for pancreatic β-cell differentiation markers and hormones were assayed by RT-PCR and real-time RT-PCR. As illustrated in Figure 3, the inductive cells expressed genes of pancreatic β-cell markers PDX1, Pax4, Glut2, Ngn3 and insulin. However, the expression of PDX1, Ngn3, and insulin in differentiated IPCs was significantly greater than that in undifferentiated MSCs (Figure 3).
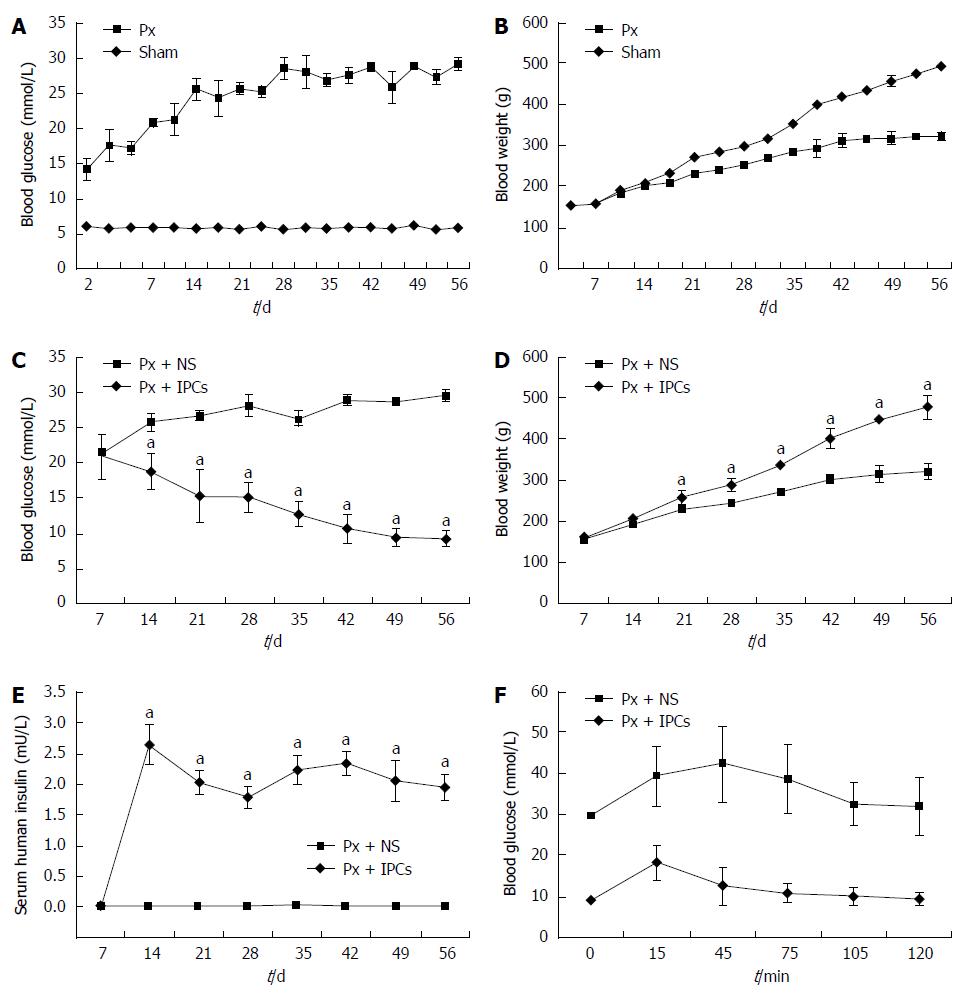
The blood glucose concentrations and body weight of Px rats and sham rats over the 8 wk of study are shown in Figure 4A and B. The blood glucose levels of Px rats elevated two days after surgery (P < 0.05) and the values kept elevating throughout the experimental period (Figure 4A). Body weight of Px rats was significantly lower than that of sham rats by day 17 of the protocol and remained significantly less than sham rats throughout the rest of the experimental period (Figure 4B).
We sought to determine if the differentiated IPCs had any effects when transplanted into SD rats. In the cell transplantation group, blood sugar levels were significantly lower than in the sham group 2 wk after transplantation (Figure 4C) and body weights were significantly higher at 3 wk after transplantation (Figure 4D). The insulin levels in Px rats transplanted with IPCs for two weeks were increased and maintained to week 8 after transplantation (Figure 4E).
To further estimate the function of the transplanted MSCs, glucose tolerance tests were done on day 56 after transplantation. After intraperitoneal glucose injection, blood glucose values in rats that had been transplanted with IPCs were significantly lower compared to rats that received NS (Figure 4F). In contrast, blood glucose levels remained elevated in rats that received NS. Collectively, these results indicated that transplantation of IPCs led to superior glucose control and tolerance as compared to controls.
Stem cell regeneration is a promising therapy for patients with insulin dependent DM after TP. Since multipotent mesenchymal stromal cells are free of ethical and immunological complications, they could provide unprecedented opportunity as starting material to derive insulin secreting cells. Many studies have demonstrated the low immunogenicity and pluripotency of UCMSCs[10,11]. Thus, these cells are expected to be an ideal source for transplantation. The phenotypic characteristic of the UCMSCs isolated in our study was in accordance with previous studies[16]. Indeed, the UCMSCs were positive for surface antigens CD13, CD90, and CD105 and negative for surface antigens CD34, CD45 and HLA-DR.
Our results illustrate that UCMSCs could be differentiated into IPCs under specific conditions. The pancreatic inductive protocol for UCMSCs was performed according to a previous study[6]. The adherent spindle-like cells acquired a round shape and assembled together after 1 wk of induction. Some new islet-like clusters began to appear after 2 wk of induction. DTZ staining is a method to identify IPCs, as reported previously[17,18]. DTZ is a substance that can bind to zinc. Compared with other cells, pancreatic islets have higher zinc contents. They become crimson red after DTZ staining[19]. As control cells were negative for DTZ staining, our results demonstrated that insulin has been produced by differentiated cells. We used human C-peptide to represent insulin secretion by differentiated cells due to the controversy surrounding insulin uptake by cells from medium supplements[20,21]. As the precursor of insulin, proinsulin is comprised of three segments: A-chain, B-chain, and C-peptide. However, unlike the A- and B-chains, cells cannot take up C-peptide, so C-peptide levels can be regarded as a marker of insulin production. In our studies, differentiated cells secreted more C-peptide than control cells after 2, 3 and 4 wk of induction. The islet-like clusters were found to be responsive to glucose challenge as evidenced by an around 1.5-fold increase in C-peptide secretion over basal stimulation. It indicated the ability of islet generated to synthesize, store and release insulin in response to glucose challenge. In our in vitro studies, RT-PCR and real time RT-PCR were used to examine the expression of β-cell development-related genes before and after induction of differentiation. After 3 wk of differentiation, the IPCs expressed the genes of pancreatic β-cell marker s PDX1, Pax4, Glut2, Ngn3 and insulin. Real-time RT-PCR showed that the expression of PDX1, Ngn3, and insulin was significantly greater in differentiated IPCs than in undifferentiated MSCs.
According to a previous study[14], we created the experimental diabetic rat models by removing 90% of the pancreas. We then transplanted the differentiated cells into diabetic rats in order to examine the function of IPCs. The portal vein[22], renal subcapsularspace[23], and tail lateral vein[24] have been previously reported as stem cell transplant locations in the rat. We transplanted the cells into the rat liver through the portal vein as this method is faster and easier to perform. In this study, after transplanting IPCs into diabetic rats, blood glucose levels decreased, suggesting that the transplanted cells secreted functional insulin. Thus, after transplantation, IPC grafts could reduce hyperglycemia in SD rats. At two weeks after transplantation, blood glucose levels decreased to about 18.7 mmol/L compared to 25.8 mmol/L in the control SD rats. The body weight of the transplanted diabetic rats increased rapidly, whereas the body weight of the untreated rats increased slowly. In vivo glucose tolerance test results showed that blood glucose levels were decreased in the cell transplantation group.
Taken together, our results show that UCMSCs can differentiate into islet-like cells in vitro under certain conditions and function as IPCs both in vivo and in vitro. These results suggest that UCMSCs are a promising stem cell source for β-cell regeneration. However, there are many questions that remain unresolved, such as how to push these cells to become mature β-cells, will the antibodies destroy the insulin-producing cells, and whether tumor formation will happen. Further work is required to address these problems.
The currently published literature on early clinical studies of autologous hematopoietic stem cells, MSCs, conditioned lymphocytes, mononuclear cells, and a combination of these cells in patients with DM has shown that these cell-based therapies are safe and feasible with some evidence of efficacy. Taking into account all the currently available results, we can expect DM to be treated successfully using stem cell regeneration therapy in the near future. However, questions such as the survival of the cells after grafting and improvements in the viability and maintenance of cellular function after transplantation remain to be answered.
Human umbilical cord mesenchymal stem cells (UCMSCs) were isolated and induced into IPCs using differentiation medium. The most common operation for pancreatic neoplasia is pancreaticoduodenectomy. The prognosis remains poor even in patients undergoing radical resection, and there is a median overall survival period of about 20 mo due to the high rate of relapse. Theoretically, elimination of high risk pancreatic anastomosis by performing total pancreatectomy (TP) could reduce perioperative morbidity. It was abandoned for a long time because of associated brittle diabetes. It is well known that islet transplantation can decrease the morbidity related to diabetes mellitus (DM) after TP. However, the problem of the worldwide lack of donor has yet to be resolved. Therefore, developing alternative cellular therapy strategies for DM is an urgent task. This study investigated whether UCMSCs could differentiate into IPCs and the effects of portal vein injection of these cells in the treatment of diabetic rats after 90% pancreatectomy.
In the search of new cell sources for transplantation, stem cells represent a critical alternative and would provide a potentially countless source of islet cells for transplantation. Some groups have isolated MSCs from human umbilical cord Wharton’s jelly and induced them to differentiate into islet-like cell clusters. To solve the islet shortage problem, we tried to induce MSCs isolated from human Wharton’s jelly to IPCs in this study. To imitate the pathophysiological status of diabetic patients after TP, we used SD rats after 90% pancreatectomy as a model of type 1 DM.
The authors for the first time examined the possible curative effects of transplanting IPCs differentiated from UCMSCs in SD rats after 90% pancreatectomy.
Based on the effect of reducing blood sugar levels of IPCs differentiated from UCMSCs, this study suggests the therapeutic potential of insulin producing cells in diabetic mellitus.
UCMSCs are a kind of stem cells isolated from human Wharton’s jelly. TP is an operation containing resection of all pancreatic tissue.
The manuscript discusses a very interesting topic. In addition, the manuscript is well written. It presents the results in a clear and well explained manner both in text and in figures.
P- Reviewer: Cecka F, Edwards MJ, Kang CM S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Wang CH
| 1. | Rocha-Lima CM. New directions in the management of advanced pancreatic cancer: a review. Anticancer Drugs. 2008;19:435-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 2. | Hartwig W, Werner J, Jäger D, Debus J, Büchler MW. Improvement of surgical results for pancreatic cancer. Lancet Oncol. 2013;14:e476-e485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 277] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 3. | Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, Neoptolemos J, Sarr M, Traverso W, Buchler M. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3282] [Cited by in RCA: 3514] [Article Influence: 175.7] [Reference Citation Analysis (34)] |
| 4. | Ueno H, Kosuge T, Matsuyama Y, Yamamoto J, Nakao A, Egawa S, Doi R, Monden M, Hatori T, Tanaka M. A randomised phase III trial comparing gemcitabine with surgery-only in patients with resected pancreatic cancer: Japanese Study Group of Adjuvant Therapy for Pancreatic Cancer. Br J Cancer. 2009;101:908-915. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 348] [Cited by in RCA: 321] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 6. | Wang HS, Shyu JF, Shen WS, Hsu HC, Chi TC, Chen CP, Huang SW, Shyr YM, Tang KT, Chen TH. Transplantation of insulin-producing cells derived from umbilical cord stromal mesenchymal stem cells to treat NOD mice. Cell Transplant. 2011;20:455-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 7. | Müller MW, Friess H, Kleeff J, Dahmen R, Wagner M, Hinz U, Breisch-Girbig D, Ceyhan GO, Büchler MW. Is there still a role for total pancreatectomy? Ann Surg. 2007;246:966-974; discussion 974-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 182] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 8. | Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3996] [Cited by in RCA: 3837] [Article Influence: 153.5] [Reference Citation Analysis (0)] |
| 9. | Gunnarsson R, Klintmalm G, Lundgren G, Wilczek H, Ostman J, Groth CG. Deterioration in glucose metabolism in pancreatic transplant recipients given cyclosporin. Lancet. 1983;2:571-572. [PubMed] |
| 10. | Weiss ML, Medicetty S, Bledsoe AR, Rachakatla RS, Choi M, Merchav S, Luo Y, Rao MS, Velagaleti G, Troyer D. Human umbilical cord matrix stem cells: preliminary characterization and effect of transplantation in a rodent model of Parkinson’s disease. Stem Cells. 2006;24:781-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 476] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 11. | Medicetty S, Bledsoe AR, Fahrenholtz CB, Troyer D, Weiss ML. Transplantation of pig stem cells into rat brain: proliferation during the first 8 weeks. Exp Neurol. 2004;190:32-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Chao KC, Chao KF, Fu YS, Liu SH. Islet-like clusters derived from mesenchymal stem cells in Wharton’s Jelly of the human umbilical cord for transplantation to control type 1 diabetes. PLoS One. 2008;3:e1451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 226] [Cited by in RCA: 224] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 13. | Kadam SS, Bhonde RR. Islet neogenesis from the constitutively nestin expressing human umbilical cord matrix derived mesenchymal stem cells. Islets. 2010;2:112-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Bonner-Weir S, Trent DF, Weir GC. Partial pancreatectomy in the rat and subsequent defect in glucose-induced insulin release. J Clin Invest. 1983;71:1544-1553. [PubMed] |
| 15. | Gjessing HJ, Matzen LE, Faber OK, Frøland A. Sensitivity and reproducibility of urinary C-peptide as estimate of islet B-cell function in insulin-treated diabetes. Diabet Med. 1989;6:329-333. [PubMed] |
| 16. | Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11055] [Cited by in RCA: 12693] [Article Influence: 705.2] [Reference Citation Analysis (2)] |
| 17. | Bonner-Weir S, Taneja M, Weir GC, Tatarkiewicz K, Song KH, Sharma A, O’Neil JJ. In vitro cultivation of human islets from expanded ductal tissue. Proc Natl Acad Sci USA. 2000;97:7999-8004. [PubMed] |
| 18. | Shiroi A, Yoshikawa M, Yokota H, Fukui H, Ishizaka S, Tatsumi K, Takahashi Y. Identification of insulin-producing cells derived from embryonic stem cells by zinc-chelating dithizone. Stem Cells. 2002;20:284-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 156] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 19. | Latif ZA, Noel J, Alejandro R. A simple method of staining fresh and cultured islets. Transplantation. 1988;45:827-830. [PubMed] |
| 20. | Hansson M, Tonning A, Frandsen U, Petri A, Rajagopal J, Englund MC, Heller RS, Håkansson J, Fleckner J, Sköld HN. Artifactual insulin release from differentiated embryonic stem cells. Diabetes. 2004;53:2603-2609. [PubMed] |
| 21. | Rajagopal J, Anderson WJ, Kume S, Martinez OI, Melton DA. Insulin staining of ES cell progeny from insulin uptake. Science. 2003;299:363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 571] [Cited by in RCA: 298] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 22. | Lopez-Talavera JC, Garcia-Ocaña A, Sipula I, Takane KK, Cozar-Castellano I, Stewart AF. Hepatocyte growth factor gene therapy for pancreatic islets in diabetes: reducing the minimal islet transplant mass required in a glucocorticoid-free rat model of allogeneic portal vein islet transplantation. Endocrinology. 2004;145:467-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 94] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 23. | Suen PM, Li K, Chan JC, Leung PS. In vivo treatment with glucagon-like peptide 1 promotes the graft function of fetal islet-like cell clusters in transplanted mice. Int J Biochem Cell Biol. 2006;38:951-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Banerjee M, Kumar A, Bhonde RR. Reversal of experimental diabetes by multiple bone marrow transplantation. Biochem Biophys Res Commun. 2005;328:318-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 76] [Article Influence: 3.8] [Reference Citation Analysis (0)] |