Published online Jun 7, 2015. doi: 10.3748/wjg.v21.i21.6561
Peer-review started: December 1, 2014
First decision: January 8, 2015
Revised: February 5, 2015
Accepted: March 12, 2015
Article in press: March 12, 2015
Published online: June 7, 2015
Processing time: 192 Days and 17.6 Hours
AIM: To determine the protective effect of triple viable probiotics on gastritis induced by Helicobacter pylori (H. pylori) and elucidate the possible mechanisms of protection.
METHODS: Colonization of BIFICO strains in the mouse stomach was determined by counting colony-forming units per gram of stomach tissue. After treatment with or without BIFICO, inflammation and H. pylori colonization in the mouse stomach were analyzed by hematoxylin and eosin and Giemsa staining, respectively. Cytokine levels were determined by enzyme-linked immunosorbent assay and Milliplex. The activation of nuclear factor (NF)-κB and MAPK signaling in human gastric epithelial cells was evaluated by Western blot analysis. Quantitative reverse transcription-polymerase chain reaction was used to quantify TLR2, TLR4 and MyD88 mRNA expression in the mouse stomach.
RESULTS: We demonstrated that BIFICO, which contains a mixture of Enterococcus faecalis, Bifidobacterium longum and Lactobacillus acidophilus, was tolerant to the mouse stomach environment and was able to survive both the 8-h and 3-d courses of administration. Although BIFICO treatment had no effect on the colonization of H. pylori in the mouse stomach, it ameliorated H. pylori-induced gastritis by significantly inhibiting the expression of cytokines and chemokines such as TNF-α, IL-1β, IL-10, IL-6, G-CSF and MIP-2 (P < 0.05). These results led us to hypothesize that BIFICO treatment would diminish the H. pylori-induced inflammatory response in gastric mucosal epithelial cells in vitro via the NF-κB and MAPK signaling pathways. Indeed, we observed a decrease in the expression of the NF-κB subunit p65 and in the phosphorylation of IκB-α, ERK and p38. Moreover, there was a significant decrease in the production of IL-8, TNF-α, G-CSF and GM-CSF (P < 0.05), and the increased expression of TLR2, TLR4 and MyD88 induced by H. pylori in the stomach was also significantly reduced following BIFICO treatment (P < 0.05).
CONCLUSION: Our results suggest that the probiotic cocktail BIFICO can ameliorate H. pylori-induced gastritis by inhibiting the inflammatory response in gastric epithelial cells.
Core tip: We investigated the effects of a traditional probiotic pharmaceutical cocktail in China, composed of the viable bacteria Enterococcus faecalis, Bifidobacterium longum and Lactobacillus acidophilus, on Helicobacter pylori (H. pylori)-induced gastritis in experimental mice and found that it could ameliorate H. pylori-induced gastritis by inhibiting the epithelial cell inflammatory response.
-
Citation: Yu HJ, Liu W, Chang Z, Shen H, He LJ, Wang SS, Liu L, Jiang YY, Xu GT, An MM, Zhang JD. Probiotic BIFICO cocktail ameliorates Hel
icobacter pylori induced gastritis. World J Gastroenterol 2015; 21(21): 6561-6571 - URL: https://www.wjgnet.com/1007-9327/full/v21/i21/6561.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i21.6561
Helicobacter pylori (H. pylori) is a common human pathogen that colonizes approximately 50% of the world’s population[1,2]. This bacterium can persist for decades in its preferred niche, the gastric mucosa, despite triggering vigorous host innate and adaptive immune responses[3]. H. pylori infection causes chronic gastritis, which is asymptomatic in the majority of carriers but is considered to be a major risk factor for the development of gastric and duodenal ulcers and gastric malignancies[2]. Between 10% and 15% of individuals suffering from H. pylori-induced gastritis develop peptic ulcer disease (PUD), and approximately 1% progress to gastric cancer (GC)[4]. Although successful eradication of H. pylori cures the majority of those diagnosed with gastritis and PUD, the prevalence of strains resistant to currently available antimicrobial agents has increased dramatically in recent years. Therefore, alternative treatment approaches, including novel methods to eradicate H. pylori or to reduce H. pylori-induced inflammation in the stomach, need to be investigated.
The gastric mucosa is the first barrier of defense against H. pylori infection. Direct interaction of H. pylori with gastric epithelial cells stimulates pattern recognition receptors (PRRs), such as toll-like receptors (TLRs) and downstream signaling pathways. The inflammatory cytokines released upon PRR activation recruit the innate immune cells residing in the gastric lamina propria under steady state conditions[3,5,6]. As a result, H. pylori can induce significant inflammation of the gastric mucosa.
The stomach is not a sterile organ and is estimated to support a community of up to 200 bacterial species[7]. However, when present, H. pylori is usually numerically dominant and readily visible in gastric biopsy tissue sections as helical rod-shaped organisms covering the gastric epithelium and surrounded in mucus. In a conducive environment, the stomach bacterial community forms hierarchies in which only a selected group of bacteria occupy the mucosal layer and epithelium, and non-selected bacteria are expelled from the mucosal surface. Competition within the bacterial community plays a pivotal role in the prevention of pathogenic bacterial invasion. Therefore, it is reasonable to hypothesize that supplementation with probiotic bacterial strains could inhibit the colonization of H. pylori and the resulting gastritis by preventing H. pylori access to the mucosal surface. Indeed, several probiotics including Lactobacillus spp., Saccharomyces spp., Bifidobacterium spp., and Bifidobacterium clausii have been studied for their impact on H. pylori infection[8-13].
BIFICO capsules, which contain a mixture of the viable bacteria Enterococcus faecalis (EF), Bifidobacterium longum (BL), and Lactobacillus acidophilus (L), were approved as an over-the-counter (OTC) drug product in October 2002 by the current Chinese regulatory authority, the State Food and Drug Administration (SFDA)[14]. This product is indicated for the treatment of disorders caused by an imbalance of normal intestinal flora. In this study, we investigated the effect of BIFICO capsules on an H. pylori SS1-infected mouse model and demonstrated that BIFICO treatment ameliorates H. pylori-induced gastritis by inhibiting TLR activation.
The animal protocol was designed to minimize pain or discomfort to the animals. The animals were acclimatized to laboratory conditions (23 °C, 12 h/12 h light/dark, 50% humidity, ad libitum access to food and water) for two weeks prior to experimentation. Intragastric gavage administration was carried out with conscious animals, using straight gavage needles appropriate for the animal size (15-17 g body weight: 22 gauge, 1 inch length, 1.25 mm ball diameter). All animals were euthanized by barbiturate overdose (intravenous injection, 150 mg/kg pentobarbital sodium) for tissue collection.
Antibodies against phospho-ERK (9101) and phospho-IκB-α (9246) were purchased from Cell Signaling Technology. Antibodies against p65 (sc8008), PCNA (proliferating cell nuclear antigen) (sc56), ERK (sc-154) and IκB-α (sc-371) were obtained from Santa Cruz Biotechnology.
C57BL/6 female mice (6-8-wk-old) were obtained from Shanghai Slac Laboratory Animal Co. LTD (China). All procedures in this study were carried out in compliance with National Institutes of Health guidelines for the Care and Use of Laboratory Animals, and the protocol was approved by the Animal Ethics Committee of Tongji University (Permit Number: TJmed-012-65). The mice were anesthetized with intraperitoneal pentobarbital sodium and sacrificed by cervical dislocation.
The H. pylori reference strain SS1 was used in this study. The bacteria were grown in a microaerobic humidified atmosphere at 37 °C on 10% lysed sheep blood Columbia agar. After 48-72 h, bacteria were harvested in PBS (pH 7.4) and re-suspended to a concentration of 6 × 108 colony-forming units (CFU)/mL using the McFarland standard kit. GES-1 cells were cultured in DMEM supplemented with 10% FBS (Hyclone) and 100 μg/mL penicillin/streptomycin.
Female C57BL/6N mice were housed at the animal facility of Tongji University under standard conditions in sterile cages. Mice were given drinking water containing 0.6 g/L of penicillin/streptomycin for 8 d prior to the experiments. The mice were then inoculated intragastrically with 109 CFU H. pylori every 48 h for a total of 6 times. Two weeks after infection, the mice were administered BIFICO (Lactobacillus acidophilus, 107 CFU; Bifidobacterium longum, 107 CFU; Enterococcus faecalis, 107 CFU) once a day for 7 d. Animals were sacrificed at 3, 4, and 5 wk post-infection. Age-matched uninfected mice were included as controls in all experiments.
The equivalent of 2.5 × 107 cells from a culture were washed and resuspended in 1 mL of PBS in a six-well plate. H. pylori was exposed to four doses of 100000 μjoules/cm2 in a CL-1000 UV crosslinker (UVP, Upland, California, United States) with agitation between each dose to treat cells evenly.
The equivalent of 2.5 × 107 cells from a culture were washed extensively to remove any traces of protein or polysaccharide. UV-inactivated H. pylori was exposed to confluent human gastric epithelial cells (GES-1) with or without BIFICO coculture at an MOI of 5. The cells were lysed after 15, 30, 45 and 60 min of stimulation to assay for inflammatory signal activation. After 24 h of stimulation, the cell supernatants were collected for cytokine assays.
Epithelial cells were serum-starved overnight, stimulated, and lysed in lysis buffer containing 150 mmol/L NaCl, 50 mmol/L HEPES pH 7.4, 1 mmol/L EDTA, 1% Nonidet P-40, and protease inhibitors to obtain cell lysate extracts or buffer containing 10 mmol/L HEPES pH 7.9, 1.5 mmol/L MgCl2, 10 mmol/L KCl, 0.5 mmol/L DTT, and 0.05% NP40 to obtain nuclear extracts. The nuclear extracts were prepared in extraction buffer (5 mmol/L HEPES, 1.5 mmol/L MgCl2, 0.2 mmol/L EDTA, 0.5 mmol/L DTT, 6% glycerol, pH 7.9). Cell lysate extracts and nuclear extracts were subjected to SDS-PAGE and then analyzed using the indicated antibodies.
The levels of TNF-α, IL-8, GM-CSF, and G-CSF in supernatants of H. pylori-stimulated GES-1 cells were measured with Ready-SET-GO enzyme-linked immunosorbent assay (ELISA) kits (eBioscience). All samples were measured in triplicate according to the manufacturer’s protocol.
Homogenized stomach tissue extracts were analyzed simultaneously for cytokines and chemokines using the Milliplex MAP mouse cytokine and chemokine magnetic bead panel (Millipore) according to the manufacturer’s instructions. Standard curves were generated for each cytokine and chemokine with the standards included in each kit. The median fluorescence intensity for each analysis was calculated with a four- or five-point logistic parameter curve. Cytokine and chemokine measurements below the detection level of the assay were calculated using a default value of 0 pg/mL for the particular analysis.
Total RNA was extracted from stomach tissue with 1 mL of TRIzol reagent according to the manufacturer’s instructions (Invitrogen). Next, 1 μg total RNA was reverse-transcribed using the ReverTra Ace qPCR RT Kit (FSQ-101) according to the manufacturer’s instructions (Toyobo). A LightCycler (LC480, Roche) and a SYBR reverse transcription-polymerase chain reaction (RT-PCR) kit (QPK-212, Toyobo) were used for quantitative real-time RT-PCR analysis. Expression levels were normalized to those obtained for the control gene GAPDH (glyceraldehyde phosphate dehydrogenase).
The statistical methods of this study were reviewed by Zi-Sheng Ai from Tongji University School of Medicine. At least two biological replicates were performed for all experiments unless otherwise indicated. Differences between groups were analyzed by Student’s t-test. P values less than 0.05 were considered statistically significant.
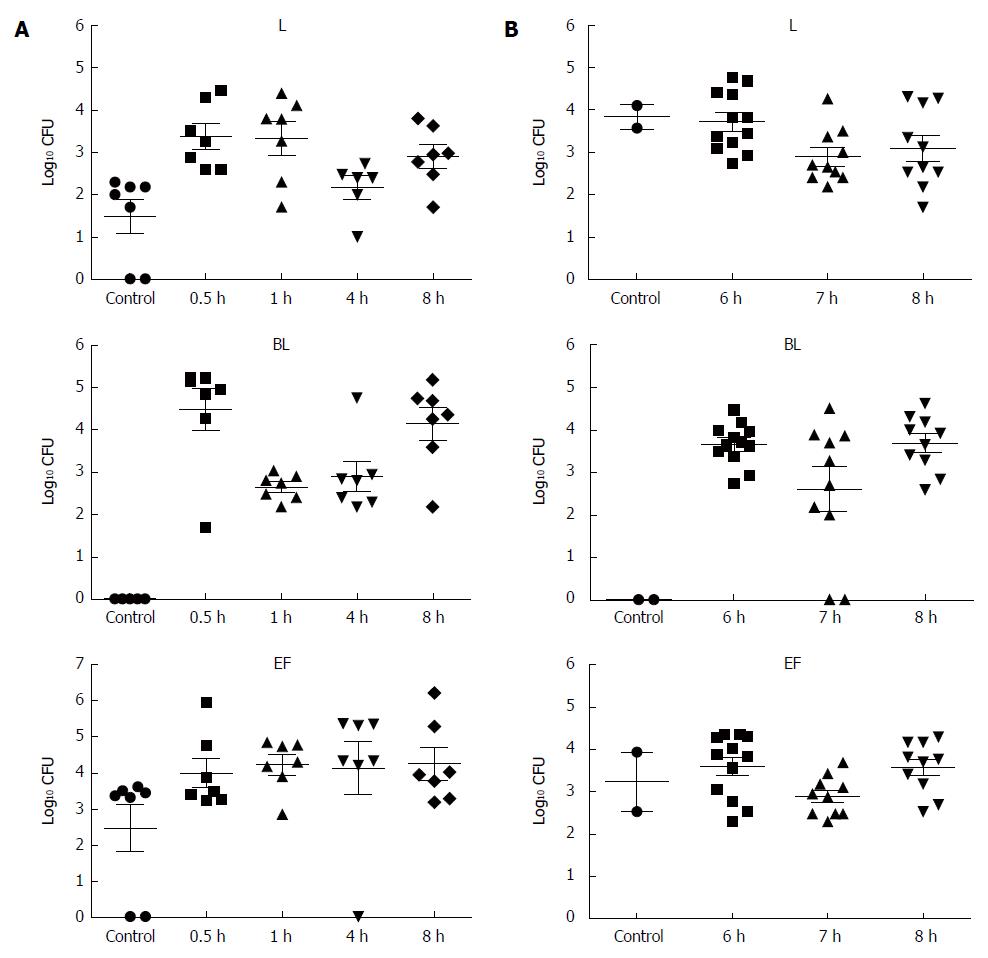
To determine whether BIFICO strains are able to colonize the mouse stomach, bacterial colonization analysis was carried out. Mice were infected intragastrically with 1 × 107 bacteria per mouse. Colonization was determined by counting CFU per gram (CFU/g) of stomach tissue. Two independent colonization experiments were performed; the first was an 8-h time point after a single-dose administration, and the second was a time course colonization experiment with CFU counts on days 6, 7, and 8 after consecutive administration of strains for 5 d. Data shown in Figure 1 suggest that BIFICO strains are tolerant to the stomach environment and are able to survive both the 8-h and 3-d courses of administration.
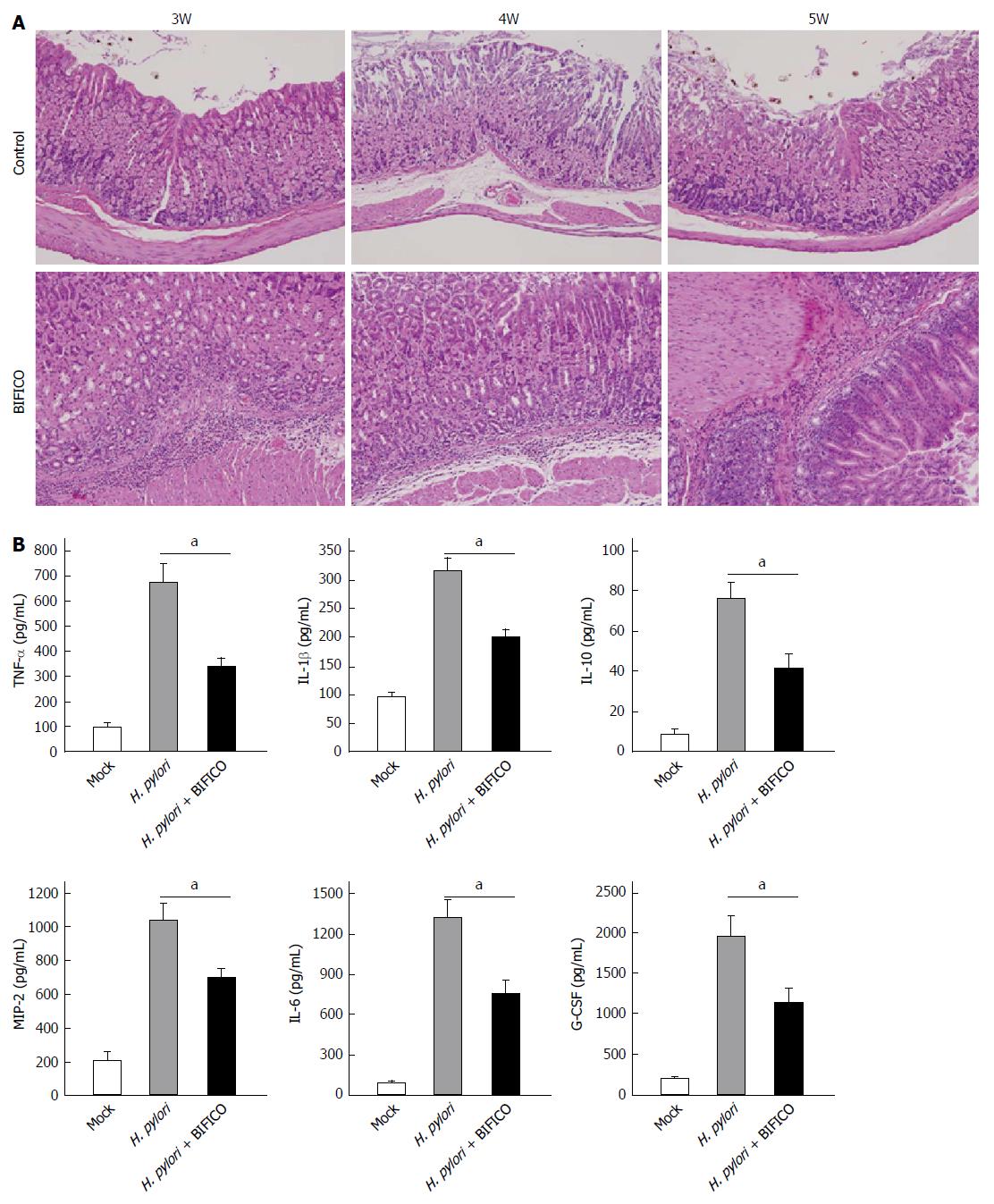
Gastric mucosa samples from control and BIFICO-treated mice were analyzed. For hematoxylin and eosin (HE) staining, inflammatory cell evasion was detected throughout the observation period. However, a significant decrease in mouse gastric mucosal inflammation was observed in the BIFICO-treated group at all time points (Figure 2A).
Analysis of cytokines and chemokines relevant to gastritis showed that BIFICO treatment significantly inhibited the H. pylori infection-induced up-regulation and secretion of TNF-α, IL-1β, IL-10, IL-6, G-CSF and MIP-2.
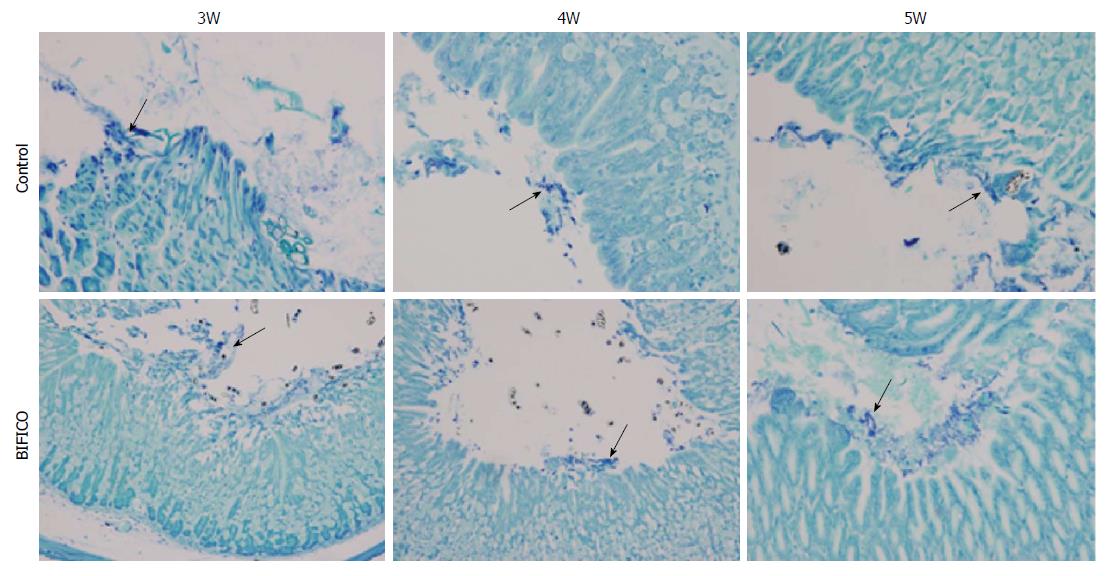
Because competition within the bacterial community plays a pivotal role in the prevention of pathogenic bacterial invasion, it is necessary to determine whether supplementation with BIFICO strains reduces the colonization of H. pylori in the mouse stomach. H. pylori colonization was detected in gastric samples by histopathologic evaluation with Giemsa staining at 3, 4 and 5 wk after the first administration. The difference in H. pylori populations between the gastric samples of mice in the BIFICO pretreatment group and the control group was not significant at the indicated times (Figure 3). These results suggest that BIFICO does not suppress the colonization of H. pylori in the mouse stomach.
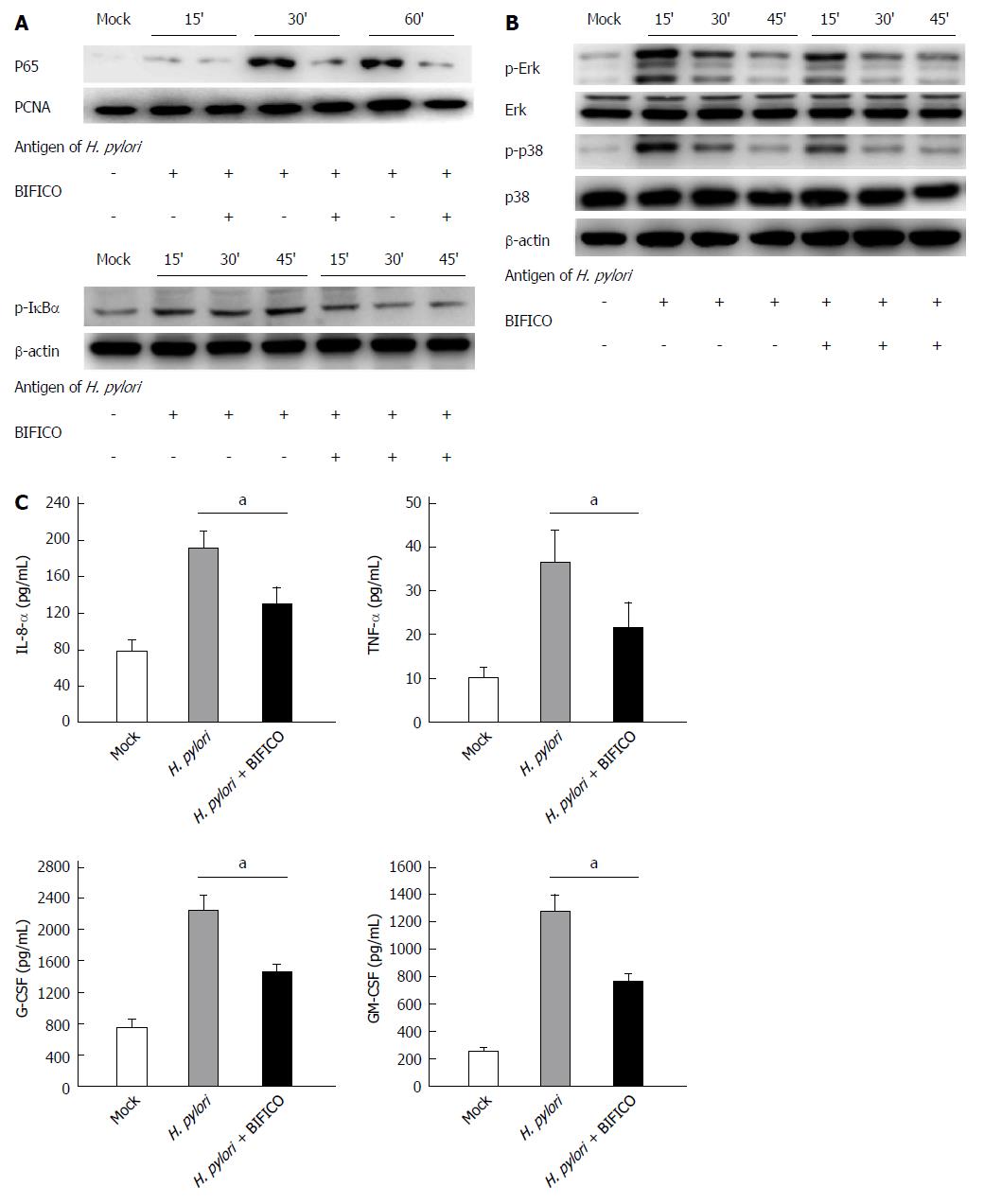
The NF-κB and MAPK pathways play a central role in H. pylori-induced gastritis. To investigate the mechanism underlying the inhibitory effect of BIFICO, we used inactivated H. pylori to activate the NF-κB and MAPK pathways. Western blot analysis showed that H. pylori infection stimulates the NF-κB and MAPK inflammatory cascade in GES-1 cells, resulting in a significant increase in p65 and phosphorylation of IκB-α, ERK and p38 (Figure 4A and B). All of these inflammatory responses to H. pylori were inhibited by the BIFICO strains (Figure 4A and B).
Similar results were observed in the inflammatory cytokine analysis. ELISAs were performed to measure IL-8, TNF-α, G-CSF and GM-CSF expression in the supernatants of GES-1 cells treated with or without BIFICO. Co-culture with BIFICO significantly inhibited the up-regulation of each of these cytokines and chemokines (Figure 4C). Taken together, these results suggest that BIFICO diminishes the in vitro inflammatory response stimulated by H. pylori via the NF-κB and MAPK signaling pathways.
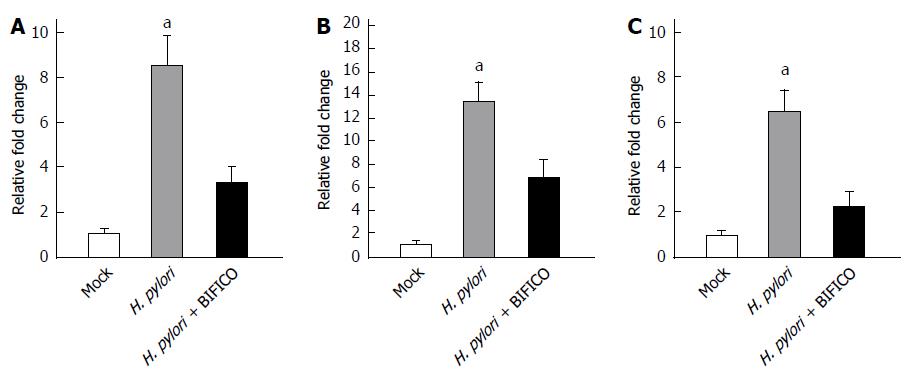
Because both commensal and pathogenic bacteria are recognized by a number of PRRs, we next investigated the PRRs involved in BIFICO-mediated suppression of inflammation. Quantitative RT-PCR analyses were carried out to measure the mRNA levels of TLR2 (Figure 5A), TLR4 (Figure 5B) and MyD88 (Figure 5C). The H. pylori-induced increases in the mRNA levels of TLR2 (Figure 5A), TLR4 (Figure 5B) and MyD88 were inhibited by BIFICO, suggesting that the TLR/MyD88 pathway is involved in the BIFICO-mediated down-regulation of the H. pylori-induced immune response in gastric mucosal epithelial cells.
In this study, we demonstrated that the probiotic BIFICO cocktail could ameliorate H. pylori-induced gastritis. Specifically, colonization with probiotic strains of BIFICO attenuated histopathological changes and gastritis in H. pylori SS1-infected mice. BIFICO treatment resulted in reduced levels of cytokines and chemokines relevant to gastritis, such as TNF-α, IL-1β, IL-10, IL-6, G-CSF and MIP-2. However, BIFICO did not suppress colonization of H. pylori in the stomach. BIFICO suppressed the inflammatory response stimulated by H. pylori in vitro via the NF-κB and MAPK signaling pathways. In addition, our results show that the TLR/MyD88 pathway is involved in BIFICO-mediated down-regulation of the H. pylori-induced immune response in gastric mucosal epithelial cells.
BIFICO capsules, which are composed of viable Enterococcus faecalis, Bifidobacterium longum and Lactobacillus acidophilus, were approved as a prescription drug by the Ministry of Health of China in April 1995 and were then approved as an OTC drug product in October 2002 by the current Chinese regulatory authority, the SFDA. This product is indicated for the treatment of acute and chronic diarrhea caused by an imbalance of normal intestinal flora, mild to moderate diarrhea, indigestion and abdominal distention. More than 700 million BIFICO capsules have been consumed by over 8000000 Chinese patients since 1995. These patients ranged in age from 1 mo to 75 years and received BIFICO capsules for the treatment of a range of clinical disorders including diarrhea, irritable bowel syndrome (IBS), ulcerative colitis (UC), newborn jaundice and others.
Because competition in the bacterial community plays a pivotal role in the prevention of H. pylori invasion, it is believed that supplementation with probiotic bacterial strains may inhibit the colonization of H. pylori and subsequent gastritis by preventing H. pylori access to the mucosal surface. In recent years, the application of probiotics in treating H. pylori infection has become an active area of research. Several probiotics, including Lactobacillus spp., Saccharomyces spp., Bifidobacterium spp. and Bifidobacterium clausii, have been studied for the treatment of H. pylori infection. However, it remains unclear whether probiotics can improve the eradication of H. pylori more effectively than standard triple therapy[8-10,15]. To evaluate the clinical efficacy of BIFICO capsules, a multi-center, positive parallel controlled clinical trial was carried out. A total of 85 H. pylori-positive patients were randomly assigned to a 7-d triple therapy based on rabeprazole (20 mg b.i.d.), clarithromycin (500 mg b.i.d.) and amoxicillin (1000 mg b.i.d.) (RCA group: 44 subjects) or to the same regimen supplemented with a 14-d therapy of BIFICO capsules (3 × 107 CFU of each strain, b.i.d.) before and after triple therapy (RCAB group: 41 subjects). Analysis of the outcome of this study was based on clinical improvement, and H. pylori eradication was confirmed based on the (13)C-urea breath test. However, these results were not consistent with those observed in the mouse model. Although colonization of H. pylori was not significantly suppressed in the in vivo study, eradication of H. pylori was improved in the clinical trial. In the RCA group, eradication was successful in 59.1% of patients (26 out of 44 patients), and in the RCAB group, the eradication rate increased to 82.9% (34 out of 41 patients). The frequency of side effects, including abdominal pain, abdominal distension, nausea and diarrhea, in the RCAB group was lower than that in the RCA group (data not shown).
Recent studies have compared the beneficial effects of probiotic mixtures and single strains against a wide range of disorders, and the results have demonstrated that probiotic mixtures are more effective than single-strain treatments for a variety of end points such as inhibition of pathogen growth and atopic dermatitis[16-21]. Specifically, it has been demonstrated that probiotic mixtures containing multiple strains of more than one genus are even more effective than multistrain probiotics[21]. As a cocktail probiotic capsule, BIFICO contains three strains of probiotic species belonging to the genera Lactobacillus, Enterococcus and Actinobifida. After more than 20 years of clinical use in China, BIFICO has been demonstrated to be more effective than single-strain preparations in treating diarrhea, indigestion and abdominal distention (unpublished observations). The mechanisms underlying these enhanced beneficial effects of the probiotic combination are summarized below. First, Enterococcus faecalis contained in the preparation increases the likelihood of colonization of Bifidobacterium longum. In addition, strains of Enterococcus faecalis are oxygen scavengers and create anaerobic conditions that potentially enhance the growth and survival of the strict anaerobic Bifidobacterium longum. Second, the synergistic health-promoting effects of individual strains exist in BIFICO probiotic mixtures. Growth of probiotic organisms is necessary to maintain sustainable numbers at certain sites in the gastrointestinal tract. In particular, the growth of Bifidobacterium longum strain can be stimulated by Lactobacillus acidophilus, which is known to produce certain growth factors such as bifidogenic growth factors, amino acids and free peptides. Finally, BIFICO capsules containing more than one strain belonging to different genera are able to produce a wider variety of antimicrobial moieties, such as weak organic acids, bacteriocins, hydrogen, coaggregation molecules, biosurfactants, and stimulate sIgA production and mucus secretion by the host. As described above, BIFICO capsules, which have been demonstrated to be more effective than single-strain preparations, may also have synergistic effects for the treatment of H. pylori infection.
H. pylori harbors conserved PAMPs that can be recognized by epithelial cells and innate immune cells via four distinct classes of innate PRRs[3,5,6]. These PRRs differ in their subcellular localization, in addition to their downstream signaling pathways and ligand specificity. The best-defined among the four classes of PRRs are the TLRs. TLRs bind diverse classes of PAMPs, among which are the ligands for TLR2 (lipoteichoic acid and lipoproteins), TLR3 (double-stranded RNA and polyinosinic polycytidylic acid), TLR4 [lipopolysaccharide (LPS)], TLR5 (flagellin) and TLR9 (unmethylated CpG). Studies have suggested that TLR2[22,23] and TLR4[24,25] are the main sensors of H. pylori LPS. Colonization of gastric epithelial cells by H. pylori activates these TLRs, which predominantly activate anti-inflammatory signaling pathways including the NF-κB and MAPK pathways[5,26], leading to increased production of the proinflammatory cytokines TNF-α, IL-1, IL-6 and IL-8[27,28]. In our study, we demonstrated that BIFICO treatment diminished the inflammatory response of gastric mucosal epithelial cells stimulated by H. pylori in vitro via NF-κB and MAPK signaling and reduced the release of subsequent inflammatory cytokines including IL-8, TNF-α, G-CSF and GM-CSF. Furthermore, H. pylori-induced overexpression of TLR2, TLR4 and MyD88 in the mouse stomach was suppressed by BIFICO treatment, suggesting that the TLR/MyD88 pathway is involved in BIFICO-mediated down-regulation of H. pylori-induced immune responses in gastric mucosal epithelial cells.
In conclusion, we investigated the effect of BIFICO treatment on H. pylori-induced gastritis. BIFICO is a clinical drug consisting of three species of probiotic strains. Although unable to reduce the colonization of H. pylori, BIFICO was able to diminish the inflammatory response of gastric mucosal epithelial cells stimulated by H. pylori and the subsequent inflammatory cytokine release. However, because gastric epithelial cells are exposed to a myriad of commensal and pathogenic bacteria, we cannot exclude the possibility that other signaling cascades other than TLR signaling may be stimulated by H. pylori infection. Nevertheless, our results show that NF-κB activation could be reduced by BIFICO treatment. Further studies are necessary to further elucidate these beneficial effects of BIFICO capsules, and a better understanding of how probiotics affect epithelial inflammation could lead to therapeutically relevant strategies for effective H. pylori treatment. Thus, these results could form the basis for future clinical studies for microbiota manipulation to ameliorate H. pylori-induced infection worldwide.
The authors would like to thank Associate Professor Zi-Sheng Ai who is from Tongji University School of Medicine for his contributions to the data analysis of this research.
Helicobacter pylori (H. pylori) is a common human pathogen that colonizes approximately 50% of the world’s population. Chronic gastritis caused by H. pylori infection is considered to be a major risk factor for the development of gastric and duodenal ulcers and gastric malignancies. Although successful eradication of H. pylori using currently available antimicrobial agents is able to cure the majority of patients diagnosed with gastritis and peptic ulcer disease, a dramatic increase in resistant strains has prompted a need for alternative treatments.
H. pylori is usually the dominant species within a community of bacterial species in the stomach and may induce gastritis under conditions of bacterial imbalance or immune dysfunction. Because competition in the bacterial community plays a pivotal role in preventing the invasion of pathogenic bacteria, several probiotics including Lactobacillus spp., Saccharomyces spp., Bifidobacterium spp. and Bifidobacterium clausii have recently been studied in H. pylori infection. However, a combination of multiple probiotics may be more powerful to prevent invasion by pathogenic H. pylori and subsequent inflammation.
This study demonstrates for the first time that a traditional probiotic pharmaceutical (BIFICO) in China composed of Enterococcus faecalis, Bifidobacterium longum and Lactobacillus acidophilus triple viable bacteria, which was approved for treating intestinal disorders, can suppress H. pylori-induced gastritis in experimental mice. Although BIFICO treatment had no effect on the colonization of H. pylori in the stomach, it diminished the inflammatory response of gastric mucosal epithelial cells stimulated by H. pylori via the TLR/MyD88 pathway.
Due to its inhibitory effects on H. pylori-induced gastritis, BIFICO can potentially be used to treat patients with H. pylori infection and the associated inflammation.
BIFICO is a traditional probiotic pharmaceutical in China composed of Enterococcus faecalis, Bifidobacterium longum and Lactobacillus acidophilus triple viable bacteria, which was previously approved for the treatment of intestinal disorders. The TLR/MyD88 pathway is stimulated in the immune response to pathogens; specifically, innate immune cells recognize pathogen-associated molecular patterns via Toll-like receptors, which activates inflammatory signaling pathways and the production of proinflammatory factors.
This is an interesting study where the authors have investigated the effects of a traditional probiotic pharmaceutical, BIFICO, on H. pylori-induced gastritis in vivo. The results suggest that BIFICO, which is commonly used to treat intestinal disorders, could be beneficial for preventing H. pylori-induced gastritis.
P- Reviewer: Amin M, Zhuang Y S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Ma S
| 1. | Patel MK, Ryan GN, Cerny AM, Kurt-Jones EA. Methods for in vivo and in vitro analysis of innate immune responses to Helicobacter pylori infection. Methods Mol Biol. 2012;921:209-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 2. | Wroblewski LE, Peek RM, Wilson KT. Helicobacter pylori and gastric cancer: factors that modulate disease risk. Clin Microbiol Rev. 2010;23:713-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 817] [Cited by in RCA: 1012] [Article Influence: 67.5] [Reference Citation Analysis (1)] |
| 3. | Salama NR, Hartung ML, Müller A. Life in the human stomach: persistence strategies of the bacterial pathogen Helicobacter pylori. Nat Rev Microbiol. 2013;11:385-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 479] [Article Influence: 39.9] [Reference Citation Analysis (2)] |
| 4. | Mitchell HM. The epidemiology of Helicobacter pylori. Curr Top Microbiol Immunol. 1999;241:11-30. [PubMed] |
| 5. | Keates S, Hitti YS, Upton M, Kelly CP. Helicobacter pylori infection activates NF-kappa B in gastric epithelial cells. Gastroenterology. 1997;113:1099-1109. [PubMed] |
| 6. | Maeda S, Yoshida H, Ogura K, Mitsuno Y, Hirata Y, Yamaji Y, Akanuma M, Shiratori Y, Omata M. H. pylori activates NF-kappaB through a signaling pathway involving IkappaB kinases, NF-kappaB-inducing kinase, TRAF2, and TRAF6 in gastric cancer cells. Gastroenterology. 2000;119:97-108. [PubMed] |
| 7. | Bik EM, Eckburg PB, Gill SR, Nelson KE, Purdom EA, Francois F, Perez-Perez G, Blaser MJ, Relman DA. Molecular analysis of the bacterial microbiota in the human stomach. Proc Natl Acad Sci USA. 2006;103:732-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 730] [Cited by in RCA: 776] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 8. | Cremonini F, Di Caro S, Covino M, Armuzzi A, Gabrielli M, Santarelli L, Nista EC, Cammarota G, Gasbarrini G, Gasbarrini A. Effect of different probiotic preparations on anti-helicobacter pylori therapy-related side effects: a parallel group, triple blind, placebo-controlled study. Am J Gastroenterol. 2002;97:2744-2749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 213] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 9. | Cindoruk M, Erkan G, Karakan T, Dursun A, Unal S. Efficacy and safety of Saccharomyces boulardii in the 14-day triple anti-Helicobacter pylori therapy: a prospective randomized placebo-controlled double-blind study. Helicobacter. 2007;12:309-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 89] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 10. | Hurduc V, Plesca D, Dragomir D, Sajin M, Vandenplas Y. A randomized, open trial evaluating the effect of Saccharomyces boulardii on the eradication rate of Helicobacter pylori infection in children. Acta Paediatr. 2009;98:127-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | Vítor JM, Vale FF. Alternative therapies for Helicobacter pylori: probiotics and phytomedicine. FEMS Immunol Med Microbiol. 2011;63:153-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 77] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 12. | Wilhelm SM, Johnson JL, Kale-Pradhan PB. Treating bugs with bugs: the role of probiotics as adjunctive therapy for Helicobacter pylori. Ann Pharmacother. 2011;45:960-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 13. | Armuzzi A, Cremonini F, Bartolozzi F, Canducci F, Candelli M, Ojetti V, Cammarota G, Anti M, De Lorenzo A, Pola P. The effect of oral administration of Lactobacillus GG on antibiotic-associated gastrointestinal side-effects during Helicobacter pylori eradication therapy. Aliment Pharmacol Ther. 2001;15:163-169. [PubMed] |
| 14. | Yu H, Liu L, Chang Z, Wang S, Wen B, Yin P, Liu D, Chen B, Zhang J. Genome Sequence of the Bacterium Bifidobacterium longum Strain CMCC P0001, a Probiotic Strain Used for Treating Gastrointestinal Disease. Genome Announc. 2013;1:pii e00716-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Georgopoulos SD, Papastergiou V, Karatapanis S. Current options for the treatment of Helicobacter pylori. Expert Opin Pharmacother. 2013;14:211-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Dunne C, Murphy L, Flynn S, O’Mahony L, O’Halloran S, Feeney M, Morrissey D, Thornton G, Fitzgerald G, Daly C. Probiotics: from myth to reality. Demonstration of functionality in animal models of disease and in human clinical trials. Antonie Van Leeuwenhoek. 1999;76:279-292. [PubMed] |
| 17. | Rolfe RD. The role of probiotic cultures in the control of gastrointestinal health. J Nutr. 2000;130:396S-402S. [PubMed] |
| 18. | Gionchetti P, Amadini C, Rizzello F, Venturi A, Campieri M. Review article: treatment of mild to moderate ulcerative colitis and pouchitis. Aliment Pharmacol Ther. 2002;16 Suppl 4:13-19. [PubMed] |
| 19. | Shibolet O, Karmeli F, Eliakim R, Swennen E, Brigidi P, Gionchetti P, Campieri M, Morgenstern S, Rachmilewitz D. Variable response to probiotics in two models of experimental colitis in rats. Inflamm Bowel Dis. 2002;8:399-406. [PubMed] |
| 20. | Ulisse S, Gionchetti P, D’Alò S, Russo FP, Pesce I, Ricci G, Rizzello F, Helwig U, Cifone MG, Campieri M. Expression of cytokines, inducible nitric oxide synthase, and matrix metalloproteinases in pouchitis: effects of probiotic treatment. Am J Gastroenterol. 2001;96:2691-2699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 133] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 21. | Timmerman HM, Koning CJ, Mulder L, Rombouts FM, Beynen AC. Monostrain, multistrain and multispecies probiotics--A comparison of functionality and efficacy. Int J Food Microbiol. 2004;96:219-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 355] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 22. | Yokota S, Ohnishi T, Muroi M, Tanamoto K, Fujii N, Amano K. Highly-purified Helicobacter pylori LPS preparations induce weak inflammatory reactions and utilize Toll-like receptor 2 complex but not Toll-like receptor 4 complex. FEMS Immunol Med Microbiol. 2007;51:140-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 23. | Smith SM, Moran AP, Duggan SP, Ahmed SE, Mohamed AS, Windle HJ, O’Neill LA, Kelleher DP. Tribbles 3: a novel regulator of TLR2-mediated signaling in response to Helicobacter pylori lipopolysaccharide. J Immunol. 2011;186:2462-2471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 24. | Ishihara S, Rumi MA, Kadowaki Y, Ortega-Cava CF, Yuki T, Yoshino N, Miyaoka Y, Kazumori H, Ishimura N, Amano Y. Essential role of MD-2 in TLR4-dependent signaling during Helicobacter pylori-associated gastritis. J Immunol. 2004;173:1406-1416. [PubMed] |
| 25. | Kawahara T, Teshima S, Oka A, Sugiyama T, Kishi K, Rokutan K. Type I Helicobacter pylori lipopolysaccharide stimulates toll-like receptor 4 and activates mitogen oxidase 1 in gastric pit cells. Infect Immun. 2001;69:4382-4389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 147] [Article Influence: 6.1] [Reference Citation Analysis (1)] |
| 26. | Münzenmaier A, Lange C, Glocker E, Covacci A, Moran A, Bereswill S, Baeuerle PA, Kist M, Pahl HL. A secreted/shed product of Helicobacter pylori activates transcription factor nuclear factor-kappa B. J Immunol. 1997;159:6140-6147. [PubMed] |
| 27. | Aihara M, Tsuchimoto D, Takizawa H, Azuma A, Wakebe H, Ohmoto Y, Imagawa K, Kikuchi M, Mukaida N, Matsushima K. Mechanisms involved in Helicobacter pylori-induced interleukin-8 production by a gastric cancer cell line, MKN45. Infect Immun. 1997;65:3218-3224. [PubMed] |