Published online May 28, 2015. doi: 10.3748/wjg.v21.i20.6341
Peer-review started: August 13, 2014
First decision: September 15, 2014
Revised: September 23, 2014
Accepted: November 7, 2014
Article in press: November 11, 2014
Published online: May 28, 2015
Processing time: 290 Days and 18.8 Hours
AIM: To compare the therapeutic effects of proton pump inhibitors vs H2 receptor antagonists for upper gastrointestinal bleeding in patients after successful endoscopy.
METHODS: We searched the Cochrane library, MEDLINE, EMBASE and PubMed for randomized controlled trials until July 2014 for this study. The risk of bias was evaluated by the Cochrane Collaboration’s tool and all of the studies had acceptable quality. The main outcomes included mortality, re-bleeding, received surgery rate, blood transfusion units and hospital stay time. These outcomes were estimated using odds ratios (OR) and mean difference with 95% confidence interval (CI). RevMan 5.3.3 software and Stata 12.0 software were used for data analyses.
RESULTS: Ten randomized controlled trials involving 1283 patients were included in this review; 678 subjects were in the proton pump inhibitors (PPI) group and the remaining 605 subjects were in the H2 receptor antagonists (H2RA) group. The meta-analysis results revealed that after successful endoscopic therapy, compared with H2RA, PPI therapy had statistically significantly decreased the recurrent bleeding rate (OR = 0.36; 95%CI: 0.25-0.51) and receiving surgery rate (OR = 0.29; 95%CI: 0.09-0.96). There were no statistically significant differences in mortality (OR = 0.46; 95%CI: 0.17-1.23). However, significant heterogeneity was present in both the numbers of patients requiring blood transfusion after treatment [weighted mean difference (WMD), -0.70 unit; 95%CI: -1.64 - 0.25] and the time that patients remained hospitalized [WMD, -0.77 d; 95%CI: -1.87 - 0.34]. The Begg’s test (P = 0.283) and Egger’s test (P = 0.339) demonstrated that there was no publication bias in our meta-analysis.
CONCLUSION: In patients with upper gastrointestinal bleeding after successful endoscopic therapy, compared with H2RA, PPI may be a more effective therapy.
Core tip: Recently, the administration of proton pump inhibitors (PPI) or H2 receptor antagonists (H2RA) have been used commonly for upper gastrointestinal bleeding patients after successful endoscopic therapy; however, which drug class is more effective, remains controversial. In this meta-analysis, we concluded that in patients with upper gastrointestinal bleeding after successful endoscopic therapy, compared with H2RA, PPI may be a more effective therapy.
-
Citation: Zhang YS, Li Q, He BS, Liu R, Li ZJ. Proton pump inhibitors therapy
vs H2 receptor antagonists therapy for upper gastrointestinal bleeding after endoscopy: A meta-analysis. World J Gastroenterol 2015; 21(20): 6341-6351 - URL: https://www.wjgnet.com/1007-9327/full/v21/i20/6341.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i20.6341
Gastrointestinal (GI) bleeding is a common cause of hospitalization, resulting in approximately 400000 hospital admissions annually, with a mortality rate of 5%-10%[1]. Re-bleeding has been described as the most important factor that affects patient prognosis; therefore, the re-bleeding rate is associated with mortality[2]. Appropriate endoscopic therapy of patients with non-variceal upper gastrointestinal bleeding improves outcomes, including re-bleeding rates, mortality, surgery, blood transfusions and hospitalization time[3,4]. Moreover, Helicobacter pylori (H. pylori) infection and nonsteroidal anti-inflammatory medication (NSAID) use are believed to be the main causes of non-variceal upper gastrointestinal bleeding (UGIB)[5,6]. Consequently, many factors may affect results of studies that examine UGIB.
Recently, the administration of proton pump inhibitors (PPI) or H2 receptor antagonists (H2RA) has been used commonly for upper gastrointestinal bleeding patients after successful endoscopic therapy[7,8]; however, the two drugs possess different pharmacological acid-suppressing activities. PPIs are substituted benzimidazoles that inhibit the parietal cell hydrogen-potassium adenosine-triphosphatase enzyme system in the gastric mucosa, reducing acid output; whereas H2RAs decrease acid secretion by interfering with the H2 receptor[9]. PPIs decrease hydrogen ion concentration by 95%-99% in humans at doses of 30-40 mg/d; however, H2RAs cause less acid inhibition than PPIs[10].
Several valuable randomized controlled trials (RCTs) have compared the therapeutic effect of PPIs and H2RAs in upper gastrointestinal bleeding patients after successful endoscopic therapy. Additionally, the meta-analysis of Yang et al[11] only evaluated the re-bleeding rate in two groups to perform a one-sided comparison of the curative effect of two drugs. Therefore, in this study, we expanded the sample size to analyze the effect of PPI therapy vs H2RAs therapy from these RCTs, with data pertaining to recurrent bleeding rate, mortality, receive surgery rate, blood transfusion units and hospitalization time.
The following keywords, proton pump inhibitors, PPI, H2 receptor antagonists, H2RA, endoscopic, bleeding, randomized controlled trial and clinical trial, were used as search terms in the Cochrane library, MEDLINE, EMBASE and PubMed until July 2014.
The inclusion criteria for this study were: (1) all patients in the experimental group were diagnosed with any type of upper gastrointestinal bleeding after successful endoscopic therapy; (2) comparison therapies of proton pump inhibitors or H2 receptor antagonists in similar baseline level patients; (3) the end-points included recurrent bleeding rate; (4) randomization, controls, and measurable outcomes were reported; and (5) the articles were written in English.
The articles were extracted independently by two investigators and any disagreements were resolved by discussion or by asking the third investigator. The first author’s name, publication year, sample size, participants’ age, participants’ gender, smoking (%), alcohol abuse (%), positive H. pylori infection (%), NSAID user (%), drug type, intervention measure and outcome assessment time were extracted. The main outcomes included were: (1) mortality (n); (2) re-bleeding (n); (3) received surgery (n); (4) blood transfused (unit/500 mL); and (5) hospitalization stay time (d).
We used the Cochrane Collaboration’s tool[12] to evaluate the quality of the articles. The following seven items of risk of bias in the tool were assessed: random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias) and Other bias, all studies were classified as low risk, high risk and unclear risk. The assessment was performed independently by two investigators; disagreements were be resolved by discussion or by involving the third investigator.
At the end of treatment for the individual trials, the odds ratio (OR) and mean difference (MD) with their 95% confidence interval (CIs) were calculated. The OR and weighted mean difference (WMD) were used as summary estimators. A fixed-effect model weighted by the inverse variance method was used following a homogeneity test. We performed the homogeneity test using a χ2 test on N-1 degrees of freedom. A P value of 0.05 was regarded as statistically significant, rejecting the assumption of homogeneity (P < 0.05), and the random-effect model was then performed using the inverse variance method. Publication biases were evaluated using a Funnel plot, Begg’s test and Egger’s test; P≥ 0.05 indicated there was no publication bias.
All statistical analyses were performed using Review Manager 5.3.3 statistical software (Cochrane Collaboration, Oxford, United Kingdom) and Stata 12.0 statistical software (Stata Co. College Station, TX, United States) for the meta-analysis.
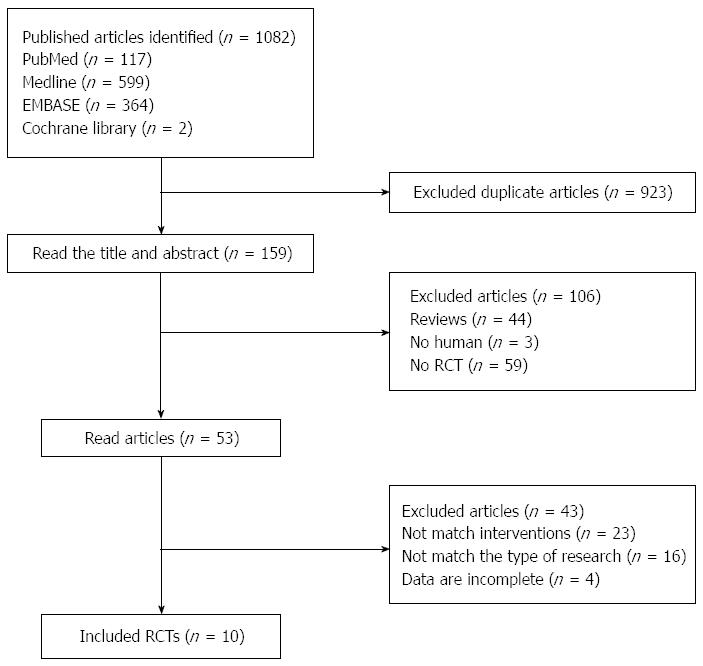
One-thousand and eighty-two publish articles were identified, and we initially excluded 923 duplicate articles. Then, after reading the title and abstract, 53 articles remained. Finally, by reading the full text of each article, 10 RCTs[13-22] were included in this meta-analysis (Figure 1). The characteristics of all included studies are shown in Table 1. This meta-analysis included 1283 patients, with 678 subjects in the PPI group and the remaining 605 subjects in the H2RA group. The maximum sample size of the included studies was 200 cases[16]; the minimum was 77 cases[20]. For the study of Lin et al[16], which used two randomized controlled programs, the programs were divided into a and b programs as two separate studies and included in the meta analysis.
| Ref. | Sample size | Age | Gender (M/F) | Smoking (%) | Alcohol abuse (%) | H. pylori infection positive (%) | NSAID user (%) | Drug type | Intervention | Outcome assessment time | The main outcomes | |
| PPI group | H2RA group | |||||||||||
| Hsu et al[13] | P:52 | P: 63.2 ± 18. | P: 41/11 | P: 32.7 | P: 13.5 | NA | P: 26.9 | P: Pantoprazole | 40 mg intravenous/12 h 3 d, followed by 40 mg/d orally | 50 mg intravenous/8 h, followed by 150 mg/12 h orally | 8 wk | 12345 |
| H:50 | H: 64.7 ± 13.8 | H: 37/13 | H: 32.0 | H: 8.0 | H: 320 | H: Ranitidine | ||||||
| Ye et al[14] | P:41 | P: 61.2 ± 9.0 | P: 28/13 | NA | NA | P: 61.0 | NA | P: Omeprazole | 20 mg/d orally | 20 mg/12 h orally | 28 d | 1 |
| H:41 | H: 58.5 ± 9.4 | H: 24/17 | H: 56.1 | H: Famotidine | ||||||||
| Jensen et al[15] | P:72 | P: 59.6 ± 16.1 | P: 51/21 | NA | NA | NA | P: 69 | P: Pantoprazole | 80 mg bolus and 8 mg/h infusion 3 d | 50 mg bolus and 6.25 mg/h infusion 3 d | 3 d, 7 d, 30 d | 1 |
| H:77 | H: 55.6 ± 16.8 | H: 52/25 | H: 71 | H: Ranitidine | ||||||||
| Lin et al[16] | Pa:67 | Pa: 67 | Pa: 58/9 | NA | NA | NA | Pa: 26.9 | P: Omeprazole | a: 40 mg intravenous/12 h 3 d, followed by 20 mg/d orally | 400 mg intravenous/12 h 3 d, followed by 400 mg/12 h orally | 14 d | 12345 |
| Pb:66 | Pb: 71 | Pb: 57/9 | Pb: 24.2 | H: Cimetidine | b: 40 mg intravenous/6 h 3 d, followed by 20 mg/d orally | |||||||
| H:67 | H: 68 | H: 61/6 | H: 29.9 | |||||||||
| Jeong et al[17] | P:85 | P: 62.9 ± 9.4 | P: 52/33 | NA | NA | P: 61.9 | NA | P: Pantoprazole | 80 mg bolus and 8 mg/h infusion d1, 40 mg intravenous/12 h d2-3, followed by 40 mg/d orally | 20 mg intravenous/12 h d2, followed by 20 mg/d orally | 24 h, 7 d, 14 d | 123 |
| H:79 | H: 63.5 ± 7.8 | H: 53/26 | H: 64.1 | H: Famotidine | ||||||||
| Uedo et al[18] | P:64 | P: 68.1 ± 8.5 | 112/33 | NA | NA | 11.5 | 11.5 | P: Rabeprazole | 20 mg/d orally | 800 mg/d orally | 8 wk | 1 |
| H:66 | H: 65.7± 7.6 | H: Cimetidine | ||||||||||
| Imaeda et al[19] | P:62 | P: 68.4 ± 8.0 | P: 47/15 | P: 58.1 H: 49.2 | NA | P: 61.3 | NA | P: Lansoprazole | 30 mg intravenous/12 h 2 d, followed by 30 mg/d orally | 75 mg intravenous/12 h 2 d, followed by 75 mg/12 h orally | 8 wk | 1 |
| H:61 | H: 67.6 ± 8.5 | H: 52/9 | H: 62.3 | H: Roxatidine | ||||||||
| Sakurada et al[20] | P:40 | P: 65.8 ± 2.5 | P: 35/5 | NA | NA | P: 77.5 | P: 35.0 | P: Omeprazole | 20 mg intravenous/12 h 3 d, followed by 20 mg/d orally | 20 mg intravenous/12 h 3 d, followed by 20 mg/d orally | 6-8 wk | 15 |
| H:37 | H:60.2 ± 1.7 | H: 31/6 | H: 78.4 | H: 29.7 | H: Famotidine | |||||||
| Tomita et al[21] | P:77 | P: 70.4 ± 8.7 | P: 59/18 | NA | NA | NA | NA | P: Omeprazole | 20 mg/12 h intravenous 3 d, followed by 20 mg/d orally | 40 mg bolus/d | 8 wk | 1 |
| H:79 | H: 70.6 ± 9.5 | H: 59/20 | H: Famotidine | |||||||||
| Lin et al[22] | P:50 | P: 65 H: 66.5 | P: 46/4 | P: 34.0 | P:12.0 | NA | NA | P: Omeprazole | 40 mg intravenous and 160 mg infusion 3 d, followed by 20 mg/12 h orally 2 m | 300 mg intravenous and 1200 mg infusion 3 d, followed by 400 mg/12 h orally 2 m | 3 d, 14 d | 12345 |
| H:50 | H:43/7 | H: 28.0 | H: 12.0 | H: Cimetidine | ||||||||
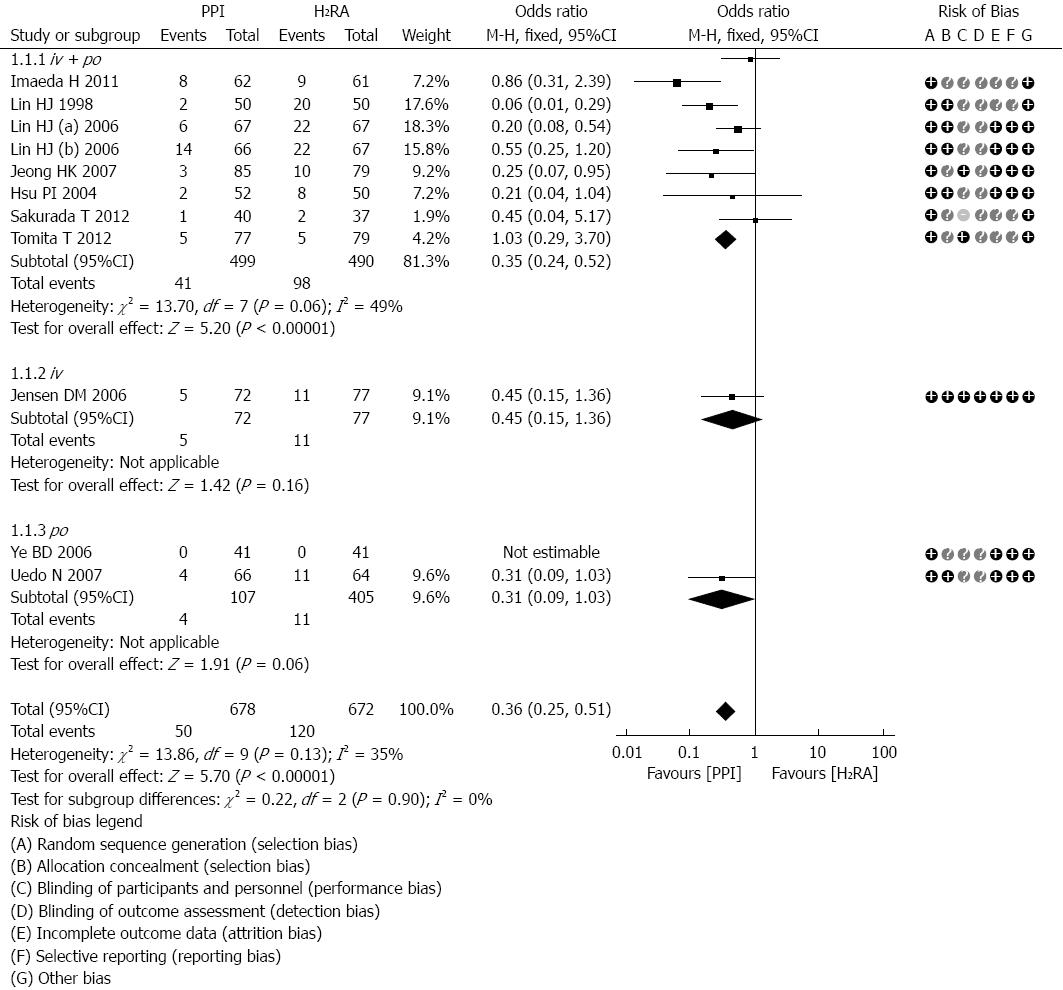
The number of recurrent bleeding subjects reported after treatment was investigated in all included studies[13-22]. According to the different routes of administration, the studies could be divided into three subgroups, intravenous followed by oral, simple intravenous and simple oral. There was not any significant heterogeneity between the included trials (P = 0.13, I2 = 35%) and subgroups (P = 0.90, I2 = 0%); therefore, we used the fixed effects model for the analysis. Seven of the studies[13,16,17,19-22], including 922 subjects, used the first intravenous and then oral administration method; the results of these studies did not statistically significantly reduce the re-bleeding rate (OR = 0.35; 95%CI: 0.24-0.52; Figure 2). However, one trial[15], which included 149 subjects, reported the re-bleeding number after simple intravenous treatment and did observe a statistically significant difference (OR = 0.45; 95%CI: 0.15-1.36; Figure 2). Meanwhile, no statistical difference was observed in two trials[14,18] that reported the re-bleeding number after simple oral treatment (OR = 0.31; 95%CI: 0.09-1.03; Figure 2). The meta-analysis results for re-bleeding rate revealed that after successful endoscopic therapy, compared with the H2RA therapy group, the PPI therapy group had significantly reduced re-bleeding rates (OR = 0.36; 95%CI: 0.25-0.51; Figure 2). Figure 2 shows the quality assessments of these ten articles, as evaluated by the Cochrane Collaboration’s tool.
Inconsistencies within the end-point time, nationality and intervention drugs, may have resulted in significant and substantial heterogeneity in our analysis (Table 2). The first subgroup analysis used the time of recurrent bleeding occurrence after successful endoscopic therapy (d). Only one trial[17] reported the re-bleeding number within 24 h and two trials[15,22] reported the re-bleeding number within 3 d. These trials were significantly heterogeneous in reducing the re-bleeding rate (OR = 0.23; 95%CI: 0.07-0.76; Table 2). An additional two trials[15,17] reported the re-bleeding number within 7 d after successful endoscopic therapy and observed a statistically significant reduction in the re-bleeding rate (OR = 0.32; 95%CI: 0.13-0.79; Table 2). Furthermore, heterogeneity was observed in the three trials[16,17,22] that reported the re-bleeding number within 14 d (OR = 0.26; 95%CI: 0.16-0.43; Table 2). Meanwhile, a statistical difference was observed in two trials[14,15] that reported the re-bleeding number within 28-30 days (OR = 0.45; 95%CI: 0.15-1.36; Table 2). Finally, there were five trials[13,18-21] that reported re-bleeding number within 6 wk or more and no statistical difference was observed (OR = 0.53; 95%CI: 0.30-0.94; Table 2). In summary, this subgroup analysis demonstrated that the end-point time after successful endoscopic therapy was not significantly different between studies (OR = 0.33; 95%CI: 0.20-0.45; Table 2) and subgroups (P = 0.42; Table 2).
| Number of studies | Number of subjects | OR (95%CI) | Heterogeneity withinsubgroups | Difference betweensubgroups | |
| End-point time | |||||
| Overall | 10 | 2076 | 0.33 (0.24-0.45) | No (P = 0.20, I2 = 23%) | No (P = 0.42, I2 = 0%) |
| 24 h | 1 | 164 | 0.08 (0.00-1.46) | NA | |
| 3 d | 2 | 249 | 0.23 (0.07-0.76) | Yes (P = 0.13, I2 = 56%) | |
| 7 d | 2 | 313 | 0.32 (0.13-0.79) | No (P = 0.37, I2 = 0%) | |
| 14 d | 3 | 464 | 0.26 (0.16-0.43) | Yes (P = 0.07, I2 = 58%) | |
| 28-30 d | 2 | 231 | 0.45 (0.15-1.36) | NA | |
| 6 wk or more | 5 | 588 | 0.53 (0.30-0.94) | No (P = 0.42, I2 = 0%) | |
| Nation | |||||
| Overall | 10 | 1283 | 0.36 (0.25-0.51) | No (P = 0.13, I2 = 35%) | No (P = 0.42, I2 = 0%) |
| Japan | 4 | 486 | 0.63 (0.33-1.17) | No (P = 0.51, I2 = 0%) | |
| China | 3 | 402 | 0.25 (0.14-0.42) | Yes (P = 0.06, I2 = 59%) | |
| South Korea | 2 | 246 | 0.25 (0.07-0.95) | NA | |
| United States | 1 | 149 | 0.45 (0.15-1.36) | NA | |
| Intervention drug PPI | |||||
| Overall | 8 | 1030 | 0.32 (0.21-0.48) | No (P = 0.13, I2 = 37%) | No (P = 0.93, I2 = 0%) |
| Omeprazole | 5 | 615 | 0.32 (0.20-0.52) | Yes (P = 0.03, I2 = 61%) | |
| Pantoprazole | 3 | 415 | 0.31 (0.15-0.65) | No (P = 0.69, I2 = 0%) | |
| Intervention drug H2RA | |||||
| Overall | 8 | 996 | 0.33 (0.22-0.48) | No (P = 0.14, I2 = 36%) | No (P = 0.18, I2 = 41.6%) |
| Cimetidine | 3 | 430 | 0.27 (0.16-0.44) | Yes (P = 0.07, I2 = 58%) | |
| Famotidine | 3 | 315 | 0.85 (0.28-2.61) | No (P = 0.56, I2 = 0%) | |
| Ranitidine | 2 | 251 | 0.34 (0.14-0.85) | No (P = 0.45, I2 = 0%) | |
The second subgroup analysis used the nationality of origin for each study. Four Japanese trials[18-21] had an OR = 0.63 (95%CI: 0.33-1.17; Table 2) and did not demonstrate a statistically significantly reduced re-bleeding rate. Three Chinese trials[13,16,22] showed heterogeneity for reducing the re-bleeding rate (OR = 0.25; 95%CI: 0.14-0.42; Table 2). Two South Korea trials[14,17] and one United States trial[15] had an OR of 0.25 (95%CI: 0.07-0.95; Table 2) and 0.45 (95%CI: 0.15-1.36; Table 2), respectively. In summary, in this subgroup analysis for re-bleeding, the nationality of the study did not significant effect drug use after successful endoscopic therapy between the different studies (OR = 0.36; 95%CI: 0.25-0.51; Table 2) and subgroups (P = 0.42; Table 2).
The third subgroup analysis used groupings by the PPI intervention drug type. Five trials[14,16,19,20,22] used omeprazole as the intervention PPI drug and had significant heterogeneity, with an OR of 0.32 (95%CI: 0.20-0.52; Table 2). These five trials were not statistically significantly different compared with the three pantoprazole studies[13,15,17] (OR = 0.31, 95%CI: 0.15-0.65; Table 2). The fourth subgroup used groupings by the H2RA intervention drug type. Three trials used cimetidine[16,18,22] as the H2RA intervention drug and had an OR of 0.27 (95%CI: 0.16-0.44; Table 2) with significant heterogeneity. Three trials used famotidine[14,20,21] as the H2RA intervention drug (OR = 0.85; 95%CI: 0.28-2.61; Table 2) and two trials[13,15] used ranitidine as the H2RA intervention drug (OR = 0.34; 95%CI: 0.14-0.85; Table 2). In summary, the H2RA intervention drug subgroup analysis for the re-bleeding rate revealed that the H2RA drug type after successful endoscopic therapy drug use had no significant effect in reducing the re-bleeding rate (OR = 0.33; 95%CI: 0.22-0.48; Table 2) or between subgroups (P = 0.18; Table 2).
In general, the subgroup analyses results did not reveal statistically significant differences in patients with upper gastrointestinal bleeding after successful endoscopic therapy (Table 2).
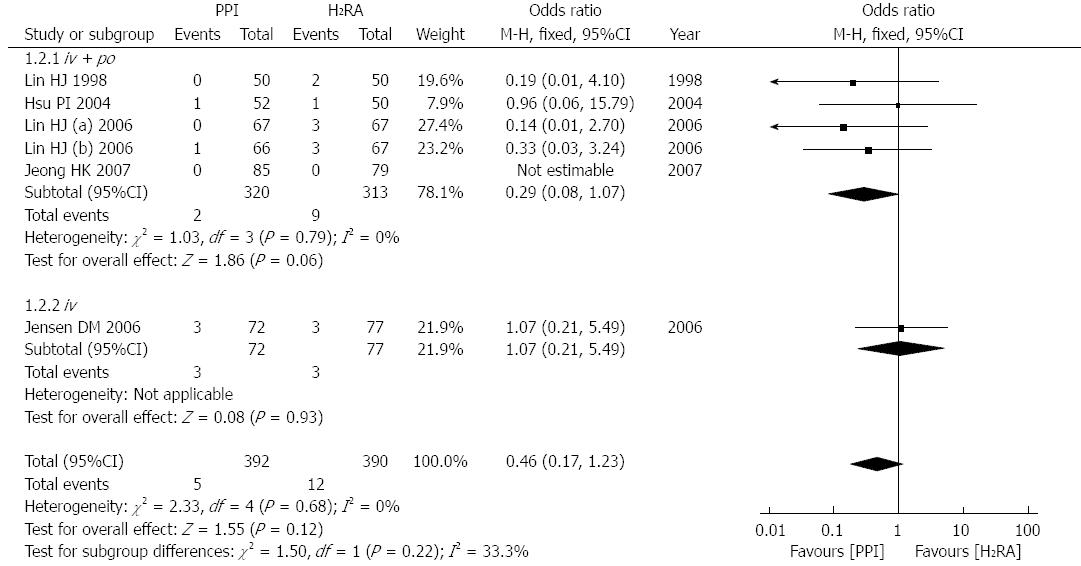
Five studies[13,15-17,22], involving 715 patients, reported mortality after treatment. According to the different routes of administration, the studies could also be divided into three subgroups, intravenous followed by oral, simple intravenous and simple oral. There was no significant heterogeneity between these trials (P = 0.68, I2 = 0%) or subgroups (P = 0.22, I2 = 33.3%); therefore, we used a fixed effects model for the analysis. Four of the studies[13,16,17,22], including 588 subjects, used the first method, intravenous and then oral administration, and the mortality was not statistically significantly reduced (OR = 0.29; 95%CI: 0.08-0.17; Figure 3). Only one trial[15], including 149 subjects, reported the dead number after simple intravenous treatment and no statistically significant difference was observed (OR = 1.07; 95%CI: 0.21-5.49; Figure 3). In summary, this meta-analysis of mortality revealed that after successful endoscopic therapy, there were no significant differences in mortality between the two drugs (OR = 0.46; 95%CI: 0.17-1.23; Figure 3).
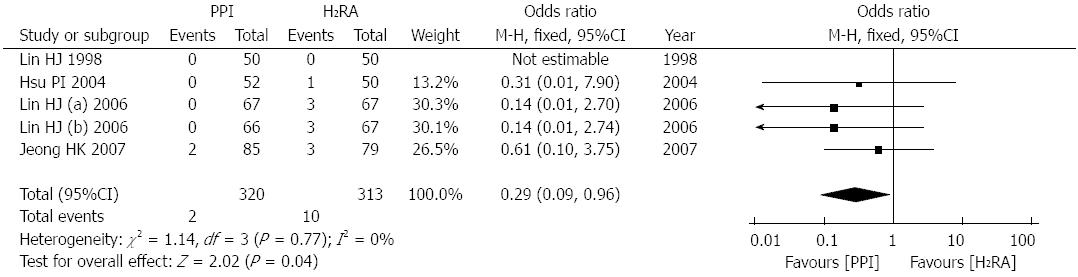
Four studies[13,16,17,22], involving 566 patients, reported the number of patients who received surgery after treatment. There was no significant heterogeneity between these trials (P = 0.77, I2 = 0%); therefore, a fixed effects model was used for the analysis. The meta-analysis revealed that after successful endoscopic therapy, compared with H2RA therapy, PPI therapy significantly decreased the number of patients that received surgery (OR = 0.29; 95%CI: 0.09-0.96; Figure 4).
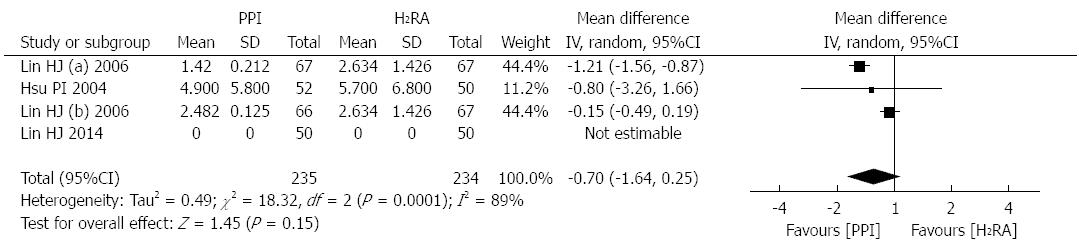
Three studies[13,16,22], involving 402 patients, reported the units (mL; 500 mL per unit) for patients who required blood transfusions after treatment. There was significant heterogeneity between these trials (P = 0.0001, I2 = 89%); therefore, a random effects model was used for the analysis. The meta-analysis revealed that after successful endoscopic therapy, PPI therapy was more effective in decreasing blood transfusion units (WMD: -0.70 unit; 95%CI:-1.64-0.25; Figure 5).
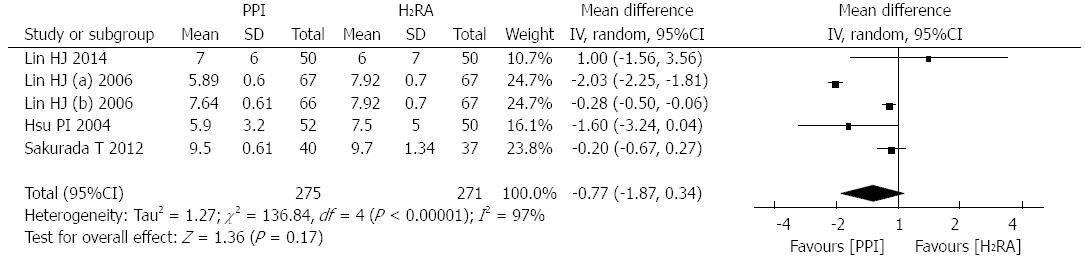
Four studies[13,16,20,22], involving 479 patients, reported the hospitalization time of patients. There was significant heterogeneity between these trials (P < 0.00001, I2 = 97%); therefore, a random effects model was used for the analysis. The meta-analysis revealed that after successful endoscopic therapy, PPI therapy was more effective in decreasing hospitalization time (WMD: -0.77d; 95%CI: -1.87-0.34; Figure 6).
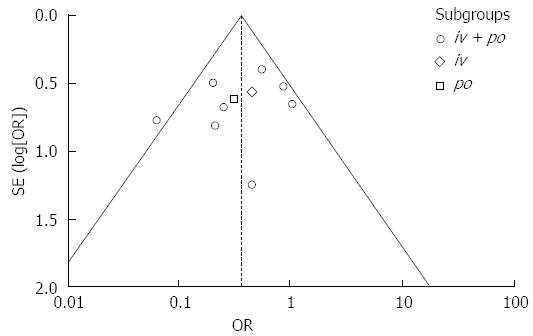
Publication bias was evaluated using a Funnel plot (Figure 7). The asymmetrical scatter plot revealed little publication bias in our meta-analysis. In addition, we also performed the Begg’s test and Egger’s test; both tests demonstrated that there was no publication bias in our meta-analysis (Begg’s test: P = 0.283; Egger’s test: P = 0.339).
In patients with upper gastrointestinal bleeding after successful endoscopic therapy, compared with H2RA therapy, PPI therapy was more effective for reducing the re-bleeding rate and received surgery rate, but there were no significant differences in mortality between the two drugs. There was little heterogeneity between the three different methods of administration (iv + po; iv; po). The results also revealed that intravenous and oral PPI had similar results, which has also been proposed by several published studies[23,24], and the PPI therapeutic results were more effective than H2RA therapy. In this meta-analysis, the intravenous followed by oral administration method had better therapeutic value than either simple intravenous therapy or simple oral therapy, which may prompt a standard drug administration method.
For the subgroup analyses of the re-bleeding time, the risk of recurrent bleeding was highest during the first 3 d after therapy and most re-bleeding events occurred in the first 24 h[6]. In this meta-analysis, we concluded that PPI can significantly reduce the re-bleeding rate during the first 3 d after successful endoscopic therapy compared with H2RA. Additionally, the same result was observed during 7 d and 14 d post endoscopy, which indicated that PPI can decrease the short-term re-bleeding rate. Furthermore, the same result was observed in the group of studies that evaluated 6 wk or more post endoscopy, which illustrated that PPIs may also decrease the long-team re-bleeding rate. However, in the 28-20 d group, the two types of drugs produced similar results, with an OR < 1 (OR = 0.45, Table 2), which indicated that PPI therapy tends to be better than H2RA therapy.
There was no significant difference between the different populations included in the studies in our meta-analysis (P = 0.16, I2 = 41.2%, Table 2); therefore, we concluded that in patients with upper gastrointestinal bleeding after successful endoscopic therapy, PPI has better therapeutic value worldwide. However, a meta-analysis by Leontiadis et al[25] revealed that PPI therapy for ulcer bleeding was more efficacious in Asia than elsewhere, which conflicts with the results in our study. However, in our meta-analysis, nine out of ten studies were conducted in Asian countries, which may result in geographical limitations.
In the subgroup analysis of PPI type, there was no significant difference between the omeprazole group and pantoprazole group (P = 0.93, I2 = 0%, Table 2); i.e., the different PPI types had little influence on outcome. However, in the analysis of the H2RA group, although there was no heterogeneity difference between the groups (P = 0.18, I2 = 41.6%, Table 2), cimetidine and ranitidine had superior curative effects compared with famotidine (Table 2).
The standard dose of PPI used after successful endoscopic therapy is hard to determine, the included studies showed large differences in the dose administered; however, the meta-analysis of Wu et al[26] demonstrated that low-dose intravenous PPI can achieve the same efficacy as high-dose PPI following endoscopic hemostasis. To clarify these conclusions, additional research must be conducted.
In the ten included studies, there were no serious adverse reactions. An evaluation of the acute and chronic adverse reactions of PPIs and H2RAs is necessary because the treatment period ranged from 3 d to a few weeks. The results suggested that these two types of drugs have short-term adverse reactions that are mild or not obvious. PPIs are a well-tolerated pharmaceutical class, with adverse effects occurring at a rate of 1%-3%, and with no significant differences between PPI types[27]. The adverse effects most commonly observed with PPI use are nausea, rash, headaches, constipation, flatulence, diarrhea, abdominal pain and dizziness[28]. However, in patients with a history of ulcer bleeding, long-term oral PPI is necessary and the safety of long-term PPI use is controversial. Insogna et al[29] revealed that long-term PPI use may influence mineral metabolism, specifically calcium absorption, which increased the risk of bone fracture. Ito et al[30] demonstrated that long-term PPI use may influence calcium absorption, as well as influence the absorption of vitamin B12, iron and magnesium, which can have important clinical implications. However, tolerance has been reported to prolonged H2RA therapy, as discussed in several studies. Rackoff et al[31] argued that prolonged hypergastrinemia is induced by long-term or high dose H2RA therapy.
There were several limitations in this study. First, the quality of the included randomized controlled trials was variable, but most of them were of acceptable quality. A number of high-quality, well-designed RCTs are needed for further research. Future studies should describe the grouping method in detail and disclose the number of patients lost to follow-up and exit. Second, heterogeneity between the studies may have skewed the meta-analysis results; the results may be from the baseline and after pharmaceutical therapy. For example, in our meta-analysis, the baseline values of the smoking rate, alcohol abuse rate, positive H. pylori infection rate and NSAID use rate in the included studies were different and these factors may have affected the analysis results. Additionally, the agents used in the included studies were different; most studies used pantoprazole or omeprazole for the PPI group, whereas cimetidine, famotidine and ranitidine were mostly used in the H2RA group. Some factors were influenced by the area, hospital, etc., particularly the mean hospitalization days. Therefore, subject enrollment data should include age, sex, risk and drug types, as well as, follow-up time, endpoint and the number of patients who accepted PPI therapy or H2RA therapy. Third, the potential for publication bias is always a concern. The number of included studies and differences in sample size may have affected the publication bias. Fourth, it is difficult to publish the results when the results do not identify any significant differences, i.e., PPI therapy has similar efficacy to H2RA therapy. This phenomenon may have led to bias.
Further research, specifically large-scale double-blind randomization trials, are required to provide more credible data for PPI or H2RA treatment using different administration methods in upper gastrointestinal bleeding patients.
In patients with upper gastrointestinal bleeding, recurrent bleeding is the most important adverse effect, resulting in morbidity and mortality. Both proton pump inhibitors and H2 receptor antagonists are commonly administered to upper gastrointestinal bleeding patients after successful endoscopic therapy.
A series of randomized controlled trials were conducted to compare proton pump inhibitor (PPI) therapy and H2 receptor antagonists (H2RA) therapy after successful endoscopy to determine the appropriate first-line treatment drug for upper gastrointestinal bleeding.
This is the first meta-analysis to evaluate the efficacy and safety of PPI therapy and H2RA therapy after successful endoscopy in patients with upper gastrointestinal bleeding, using several important endpoints, such as re-bleeding rate, mortality and receiving surgery rate.
The results suggested that PPI therapy is superior to H2RA therapy in upper gastrointestinal bleeding patients after successful endoscopic therapy. This result may provide valuable information to clinicians.
PPI is a type of H+/K+ ATPase inhibitor. Its acid-suppressing activity is potent, highly specific and has a long duration. H2RA can selectively block wall H2 receptors on the cell membrane, reducing gastric acid secretion.
This is a well-performed meta-analysis that aimed to determine whether PPI therapy is more efficacious and safe than H2RA therapy in upper gastrointestinal bleeding patients after successful endoscopic therapy that provides definitive results.
P- Reviewer: Soares RLS, Wasano K, Wagh MS, Yang CH S- Editor: Qi Y L- Editor: Stewart G E- Editor: Wang CH
| 1. | Nadler M, Eliakim R. The role of capsule endoscopy in acute gastrointestinal bleeding. Therap Adv Gastroenterol. 2014;7:87-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | van Rensburg CJ, Hartmann M, Thorpe A, Venter L, Theron I, Lühmann R, Wurst W. Intragastric pH during continuous infusion with pantoprazole in patients with bleeding peptic ulcer. Am J Gastroenterol. 2003;98:2635-2641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Hwang JH, Fisher DA, Ben-Menachem T, Chandrasekhara V, Chathadi K, Decker GA, Early DS, Evans JA, Fanelli RD, Foley K. The role of endoscopy in the management of acute non-variceal upper GI bleeding. Gastrointest Endosc. 2012;75:1132-1138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 218] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 4. | Khamaysi I, Gralnek IM. Acute upper gastrointestinal bleeding (UGIB) - initial evaluation and management. Best Pract Res Clin Gastroenterol. 2013;27:633-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 5. | Yeomans ND. The ulcer sleuths: The search for the cause of peptic ulcers. J Gastroenterol Hepatol. 2011;26 Suppl 1:35-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Crooks CJ, West J, Card TR. Comorbidities affect risk of nonvariceal upper gastrointestinal bleeding. Gastroenterology. 2013;144:1384-193, 1384-193, quiz 1384-1393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 7. | Maggio D, Barkun AN, Martel M, Elouali S, Gralnek IM. Predictors of early rebleeding after endoscopic therapy in patients with nonvariceal upper gastrointestinal bleeding secondary to high-risk lesions. Can J Gastroenterol. 2013;27:454-458. [PubMed] |
| 8. | Bhatia V, Lodha R. Upper gastrointestinal bleeding. Indian J Pediatr. 2011;78:227-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Welage LS, Berardi RR. Evaluation of omeprazole, lansoprazole, pantoprazole, and rabeprazole in the treatment of acid-related diseases. J Am Pharm Assoc (Wash). 2000;40:52-62; quiz 121-3. [PubMed] |
| 10. | Lahner E, Annibale B, Delle Fave G. Systematic review: impaired drug absorption related to the co-administration of antisecretory therapy. Aliment Pharmacol Ther. 2009;29:1219-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 11. | Yang Z, Wu Q, Liu Z, Wu K, Fan D. Proton pump inhibitors versus histamine-2-receptor antagonists for the management of iatrogenic gastric ulcer after endoscopic mucosal resection or endoscopic submucosal dissection: a meta-analysis of randomized trials. Digestion. 2011;84:315-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 77] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 12. | Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18487] [Cited by in RCA: 24863] [Article Influence: 1775.9] [Reference Citation Analysis (3)] |
| 13. | Hsu PI, Lo GH, Lo CC, Lin CK, Chan HH, Wu CJ, Shie CB, Tsai PM, Wu DC, Wang WM. Intravenous pantoprazole versus ranitidine for prevention of rebleeding after endoscopic hemostasis of bleeding peptic ulcers. World J Gastroenterol. 2004;10:3666-3669. [PubMed] |
| 14. | Ye BD, Cheon JH, Choi KD, Kim SG, Kim JS, Jung HC, Song IS. Omeprazole may be superior to famotidine in the management of iatrogenic ulcer after endoscopic mucosal resection: a prospective randomized controlled trial. Aliment Pharmacol Ther. 2006;24:837-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Jensen DM, Pace SC, Soffer E, Comer GM. Continuous infusion of pantoprazole versus ranitidine for prevention of ulcer rebleeding: a U.S. multicenter randomized, double-blind study. Am J Gastroenterol. 2006;101:1991-1999; quiz 2170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 2.6] [Reference Citation Analysis (2)] |
| 16. | Lin HJ, Lo WC, Cheng YC, Perng CL. Role of intravenous omeprazole in patients with high-risk peptic ulcer bleeding after successful endoscopic epinephrine injection: a prospective randomized comparative trial. Am J Gastroenterol. 2006;101:500-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 17. | Jeong HK, Park CH, Jun CH, Lee GH, Kim HI, Kim HS, Choi SK, Rew JS. A prospective randomized trial of either famotidine or pantoprazole for the prevention of bleeding after endoscopic submucosal dissection. J Korean Med Sci. 2007;22:1055-1059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Uedo N, Takeuchi Y, Yamada T, Ishihara R, Ogiyama H, Yamamoto S, Kato M, Tatsumi K, Masuda E, Tamai C. Effect of a proton pump inhibitor or an H2-receptor antagonist on prevention of bleeding from ulcer after endoscopic submucosal dissection of early gastric cancer: a prospective randomized controlled trial. Am J Gastroenterol. 2007;102:1610-1616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 169] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 19. | Imaeda H, Hosoe N, Suzuki H, Saito Y, Ida Y, Nakamura R, Iwao Y, Ogata H, Hibi T. Effect of lansoprazole versus roxatidine on prevention of bleeding and promotion of ulcer healing after endoscopic submucosal dissection for superficial gastric neoplasia. J Gastroenterol. 2011;46:1267-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Sakurada T, Kawashima J, Ariyama S, Kani K, Takabayashi H, Ohno S, Kato S, Yakabi K. Comparison of adjuvant therapies by an H2-receptor antagonist and a proton pump inhibitor after endoscopic treatment in hemostatic management of bleeding gastroduodenal ulcers. Dig Endosc. 2012;24:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Tomita T, Kim Y, Yamasaki T, Okugawa T, Kondo T, Toyoshima F, Sakurai J, Tanaka J, Morita T, Oshima T. Prospective randomized controlled trial to compare the effects of omeprazole and famotidine in preventing delayed bleeding and promoting ulcer healing after endoscopic submucosal dissection. J Gastroenterol Hepatol. 2012;27:1441-1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 22. | Lin HJ, Lo WC, Lee FY, Perng CL, Tseng GY. A prospective randomized comparative trial showing that omeprazole prevents rebleeding in patients with bleeding peptic ulcer after successful endoscopic therapy. Arch Intern Med. 1998;158:54-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 181] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 23. | Yen HH, Yang CW, Su WW, Soon MS, Wu SS, Lin HJ. Oral versus intravenous proton pump inhibitors in preventing re-bleeding for patients with peptic ulcer bleeding after successful endoscopic therapy. BMC Gastroenterol. 2012;12:66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Sung JJ, Suen BY, Wu JC, Lau JY, Ching JY, Lee VW, Chiu PW, Tsoi KK, Chan FK. Effects of intravenous and oral esomeprazole in the prevention of recurrent bleeding from peptic ulcers after endoscopic therapy. Am J Gastroenterol. 2014;109:1005-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 25. | Leontiadis GI, Sharma VK, Howden CW. Systematic review and meta-analysis: enhanced efficacy of proton-pump inhibitor therapy for peptic ulcer bleeding in Asia--a post hoc analysis from the Cochrane Collaboration. Aliment Pharmacol Ther. 2005;21:1055-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 74] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 26. | Wu LC, Cao YF, Huang JH, Liao C, Gao F. High-dose vs low-dose proton pump inhibitors for upper gastrointestinal bleeding: a meta-analysis. World J Gastroenterol. 2010;16:2558-2565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (1)] |
| 27. | Boparai V, Rajagopalan J, Triadafilopoulos G. Guide to the use of proton pump inhibitors in adult patients. Drugs. 2008;68:925-947. [PubMed] |
| 28. | Vakil N, Fennerty MB. Direct comparative trials of the efficacy of proton pump inhibitors in the management of gastro-oesophageal reflux disease and peptic ulcer disease. Aliment Pharmacol Ther. 2003;18:559-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 29. | Insogna KL. The effect of proton pump-inhibiting drugs on mineral metabolism. Am J Gastroenterol. 2009;104 Suppl 2:S2-S4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 30. | Ito T, Jensen RT. Association of long-term proton pump inhibitor therapy with bone fractures and effects on absorption of calcium, vitamin B12, iron, and magnesium. Curr Gastroenterol Rep. 2010;12:448-457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 240] [Cited by in RCA: 199] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 31. | Rackoff A, Agrawal A, Hila A, Mainie I, Tutuian R, Castell DO. Histamine-2 receptor antagonists at night improve gastroesophageal reflux disease symptoms for patients on proton pump inhibitor therapy. Dis Esophagus. 2005;18:370-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 3.3] [Reference Citation Analysis (0)] |