Published online May 28, 2015. doi: 10.3748/wjg.v21.i20.6304
Peer-review started: March 3, 2015
First decision: March 10, 2015
Revised: March 24, 2015
Accepted: April 28, 2015
Article in press: April 28, 2015
Published online: May 28, 2015
Processing time: 88 Days and 5.8 Hours
AIM: To evaluate the efficacy of sequential blood purification therapy in the treatment of critical patients with hyperlipidemic severe acute pancreatitis.
METHODS: Thirty-one intensive care unit (ICU) patients with hyperlipidemic severe acute pancreatitis treated at the Second Affiliated Hospital of Harbin Medical University were divided into either a study group (n = 15; July 1, 2012 to June 30, 2014) or a control group (n = 16; July 1, 2010 to June 30, 2012) based on the implementation of sequential blood purification therapy. The control group received continuous venous-venous hemofiltration (CVVH) on the basis of conventional treatments, and the therapeutic dose of CVVH was 30 mL/kg per hour. The study group received sequential plasma exchange and CVVH on the basis of conventional treatments. The anticoagulation regimen of CVVH is the regional citrate anticoagulation. Mortality rate on day 28, rates of systemic and local complications, duration of ICU, and time to target serum lipid level, as well as physiologic and laboratory indices were compared between the two groups.
RESULTS: The mortality rate on day 28 was significantly lower in the study group than in the control group (13.33% vs 37.50%; P < 0.05). The duration of ICU stay was significantly shorter in the study group than in the control group (7.4 ± 1.35 d vs 9.19 ± 2.99 d, P < 0.05). The time to target serum lipid level was significantly shorter in the study group than in the control group (3.47 ± 0.52 d vs 7.90 ± 1.14 d, P < 0.01). There were no significant differences in the rates of systemic complications and local complications between the two groups (60% vs 50% and 80% vs 81%, respectively). In the comparisons of physiologic and laboratory indices, serum albumin and C-reactive protein were significantly better in the study group than in the control group after treatment (37.8 ± 4.6 g/L vs 38.9 ± 5.7 g/L, and 20.5 ± 6.4 mg/L vs 28.5 ± 7.1 mg/L, respectively, both P < 0.05). With the exception of plateletcrit, no other indices showed significant differences between the two groups.
CONCLUSION: Sequential blood purification therapy is effective in the treatment of ICU patients with hyperlipidemic severe acute pancreatitis and can improve patient prognosis.
Core tip: Plasma exchange and continuous venous-venous hemofiltration have certain clinical effects in the treatment of hyperlipidemia acute severe pancreatitis, but there is currently no standardized combination therapy. Based on the 2012 Atlanta International Pancreatitis Consensus, we designed a sequential mode of combined application of plasma exchange and continuous venous-venous hemofiltration for the treatment of hyperlipidemia severe acute pancreatitis. This sequential blood purification therapy was found to be effective in the treatment of intensive care unit patients with hyperlipidemic severe acute pancreatitis and improved patient prognosis, and should therefore become the standardized treatment process.
- Citation: Wang HL, Yu KJ. Sequential blood purification therapy for critical patients with hyperlipidemic severe acute pancreatitis. World J Gastroenterol 2015; 21(20): 6304-6309
- URL: https://www.wjgnet.com/1007-9327/full/v21/i20/6304.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i20.6304
Acute pancreatitis (AP) is divided into three categories according to the 2012 Atlanta classification: mild, moderate, and severe[1]. In contrast to the low mortality of mild AP, severe AP (SAP) is associated not only with a high mortality rate, but also with a high rate of complications[2]. So far, many treatment strategies for AP have been developed. In addition to common surgical treatment, peritoneal lavage, organ support therapy, and endoscopic retrograde cholangiopancreatography, blood purification therapy is also a promising treatment. Despite these therapies, the treatment of SAP is still a great challenge, and its mortality rate can still reach 15%-25%[3].
SAP is characterized by persistent organ failure, and the risk of death is especially higher in the first several days of organ failure (36%-50%)[4-6]. Therefore, there is an urgent need to further improve the survival rate of patients with SAP. Currently, the utility of a paradigm involving a multidisciplinary team typically including general surgeons, gastroenterologists, radiologists, and intensive care unit (ICU) physicians is advocated in the clinical treatment of SAP[7]. Organ support technology, especially continuous renal replacement therapy(CRRT), has a key role in the critical care of SAP.
The most common causes of AP include gallstone diseases, excessive alcohol consumption, and pregnancy. Hyperlipidemic AP, which occurs when the triglyceride level is > 1000 mg/dL, accounts for only 1%-4% of cases[8,9]. At present, it is believed that hyperlipidemic AP is related to pancreatic tissue (pancreatic ducts and acini cells) injury and microcirculation disturbance caused by free fatty acids that are produced by pancreatic lipase-catalyzed decomposition of triglycerides. With the progression of the disease, the enzymes, fatty acids, and inflammatory mediators in pancreatic tissue enter into the systemic circulation and participate in the development of multiple organ dysfunction, thus leading to the failure of one or multiple organs[2,10]. There is evidence that multiple organ failure subsequent to systemic inflammatory response syndrome caused by pancreatic inflammation is the main cause of death in patients with pancreatitis.
In the past few years, the use of blood purification technology in the treatment of ICU patients with AP has progressively increased. Continuous venous-venous hemofiltration (CVVH) is one of the most commonly used blood purification procedures in ICU patients, and it can selectively remove inflammatory factors in the body and effectively eliminate the inflammatory cytokine storm. For acute kidney injury patients, CVVH can remove toxins in the body and reduce water retention. In patients with hyperlipidemic AP, the application of plasma exchange (PE) allows for rapid and efficient removal of serum lipids, and reduces triglyceride levels and the production of free fatty acids, thus weakening pancreatic self-digestion by trypsin. In addition, PE can improve pancreatic tissue microcirculation and relieve a high blood coagulation state, ultimately achieving the goal of treatment of AP. In theory, PE is very beneficial for the treatment of hyperlipidemic SAP[11-13].
So far, there have been many small sample-sized clinical studies confirming that PE and CVVH are conducive to improving mortality in patients with either SAP or hyperlipidemic SAP. Moreover, a recent study demonstrated that combined use of PE and CVVH has more advantages in patients with SAP[14-16]. However, despite a large number of existing clinical studies, there have been no standardized criteria for the combination of PE and CVVH for SAP, and this has led to conflicting conclusions. In addition, because the patients were selected randomly in many previous studies, they could not effectively evaluate the clinical efficacy of the combination therapy of PE and CVVH. The present study was designed to further evaluate the clinical efficacy of PE combined with CVVH in the treatment of ICU patients with hyperlipidemic SAP.
This study was divided into two stages based on the implementation of sequential blood purification therapy. The patients (n = 16) treated from July 1, 2010 to June 30, 2012 underwent conventional treatments and CVVH and were included in the control group, and those (n = 15) treated from July 1, 2012 to June 30, 2014 received conventional treatments with sequential blood purification therapy and comprised the study group.
Inclusion criteria were: (1) diagnosis of pancreatitis (meeting at least two of the following three criteria): abdominal pain typical of pancreatitis; serum amylase and/or lipase levels ≥ three times the upper limit of normal; evidence of pancreatitis upon abdominal imaging; (2) diagnosis of severe pancreatitis: Marshall score ≥ 2; and (3) diagnosis of hyperlipidemic pancreatitis: serum triglycerides > 1000 mg/dL. Exclusion criteria were: (1) pancreatic cancer; (2) gallstones; (3) > five-year history of heavy drinking (> 50 g/d); (4) younger than 18 years or older than 60 years; and (5) not receiving PE/CVVH within 5 h after admission to ICU. The withdrawal criterion was that the patient himself/herself or the authorized person requested withdrawl from the study. Indications for discontinuation of therapy were: (1) disappearance of specific abdominal symptoms; (2) Marshall score < 2; and (3) serum triglycerides < 500 mg/dL[17].
The study group received sequential blood purification therapy, which involved the initial PE with freshly frozen plasma (≥ 3000 mL/d) until serum triglycerides < 500 mg/dL and subsequent CVVH until the disappearance of specific abdominal symptoms and Marshall score < 2. The control group received conventional treatments and CVVH, i.e., lipid-lowering drugs (simvastatin with fenofibrate, 20 + 200 mg/d[17]) plus CVVH, until the disappearance of specific abdominal symptoms, Marshall score < 2, and serum triglycerides < 500 mg/dL.
PE was performed using a FLEX system with the TPE 2000 set via a polysulfone filter, and the velocity was set at 30 mL/min. CVVH was performed using a FLEX system with the M100 set via an AN69 filter, and the parameters were as follows: therapeutic dose, 30 mL/kg per hour; blood flow velocity, 150-180 mL/min; dilution mode, pre-dilution 100%; frequency of filter replacement, 8-12 h (depending on transfilter pressure); permissible transfilter pressure, 0-300 mmHg; anticoagulation regimen, regional citrate anticoagulation (4% sodium citrate and 100 mmol/L calcium chloride); detection range for free Ca2+ ion before filter, 0.25-0.35 mmol/L; and detection range for free Ca2+ ion after filter, 1.12-1.20 mmol/L[18,19].
Conventional treatments applied to the study and control groups included fasting, fluid resuscitation, oxygen therapy, gastrointestinal decompression, somatostatin, and organ support therapy.
The primary outcome measure was mortality on day 28[14], and secondary outcome measures[15] were rate of systemic complications, rate of local complications, duration of ICU stay, and time to target serum triglyceride level (< 500 mg/dL). Systemic complications included: (1) pulmonary insufficiency (PaO2 < 8 kPa); (2) renal insufficiency (Cr > 2 mg/dL); (3) shock (systolic blood pressure < 12 kPa, systolic blood pressure was decreased > 40 mmHg compared with the baseline); and (4) upper gastrointestinal bleeding > 500 mL/24 h. Local complications included pylorus dysfunction, peripancreatic effusion, pancreatic pseudocyst, spleen vein and portal vein thrombosis, colon necrosis, necrotic collection, and walled-off necrosis. In addition, physiologic and laboratory indices were also compared after treatment between the two groups. The physiologic indices included body temperature (°C), heart rate (beats/min), blood pressure (mm Hg), RR interval (beats/min), mean arterial pressure (mmHg), and PaO2/FiO2. Laboratory indices included WBC (109/L), plateletcrit (109/L), albumin (g/L), alanine transaminase (U/L), total bilirubin (mmol/L), blood urea nitrogen (mmol/L), serum creatinine (mmol/L), Ca2+ (mmol/L), bicarbonate (mmol/L), serum amylase (U/L), urine amylase (U/L), C-reactive protein (mg/L), and procalcitonin (ng/mL).
Measurement data are expressed as mean ± SD, and count data are expressed as number of cases (or percentage). Survival analysis was performed using the Kaplan-Meier method. Mortality on day 28, secondary indices (systemic complications, rate of local complications, duration of ICU stay, and time to target serum triglyceride level), as well as physiologic and laboratory indices after treatment were compared between the two groups using the t test. Statistical analyses were performed using SAS 9.1.3 statistical software (SAS Institute Inc., Cary, NC, United States), and P < 0.05 were considered statistically significant.
Table 1 shows the baseline characteristics of patients in the study and control groups. There were no significant differences in the baseline characteristics between the two groups.
| Characteristic | Study group(n = 15) | Control group(n = 16) | P value |
| Age (yr) | 42.6 ± 9.9 | 40.9 ± 12.6 | 0.6806 |
| BMI (kg/m2) | 27.4 ± 4.1 | 28.5 ± 3.7 | 0.4387 |
| Sex (male/female), n | 10/5 | 11/5 | 1.0000 |
| Bacterial culture positive, n | 3 | 4 | 1.0000 |
| Marshall score | 2.6 ± 1.7 | 2.5 ± 1.4 | 0.8590 |
| APACHE II score | 21.3 ± 2.9 | 22.5 ± 2.1 | 0.1952 |
| Upper gastrointestinal bleeding, n | 7 | 8 | 1.0000 |
| ARDS, n | 12 | 13 | 1.0000 |
| Heart failure/pulmonary edema, n | 3 | 2 | 0.6539 |
| DIC, n | 1 | 2 | 1.0000 |
| Surgical debridement, n | 1 | 1 | 1.0000 |
| Use of vasopressors, n | 15 | 16 | 1.0000 |
| Mechanical ventilation, n | 10 | 10 | 1.0000 |
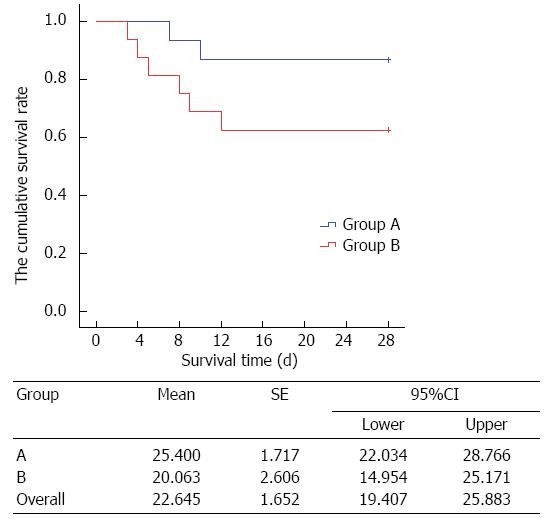
Kaplan-Meier survival analysis showed that the mortality rate on day 28 was 13.33% and 37.50% in the study group and control group, respectively. As time went on, the mortality rate was significantly lower in the study group than in the control group (P < 0.05) (Figure 1).
For secondary outcome measures, the duration of ICU stay and the time to target serum lipid level were significantly shorter in the study group than in the control group (both P < 0.05) (Table 2). The rate of systemic complications was 60% for the study group and 50% for the control group. The rate of local complications was 80% for the study group and 81% for the control group. There were no significant differences in the rates of systemic complications and local complications between the two groups (Table 2).
| Variable | Study group | Control group | P value |
| Duration of ICU stay (d) | 7.40 ± 1.35 | 9.19 ± 2.99 | 0.0420 |
| Time to target TG (d) | 3.47 ± 0.52 | 7.90 ± 1.14 | < 0.0001 |
| Systemic complications, n (%) | 9 (60.00) | 8 (50.00) | 0.7224 |
| Local complications, n (%) | 12 (80.00) | 13 (81.25) | 1.0000 |
The comparisons of physiologic and laboratory indices between the two groups are shown in Tables 3 and 4. After treatment, serum albumin and C-reactive protein were significantly better in the study group than in the control group (both P < 0.05). With exception of procalcitonin, none of the other indices were significantly different between the two groups.
| Variable | Study group(n = 15) | Control group(n = 16) |
| Body temperature (°C) | 37.5 ± 0.6 | 37.3 ± 0.4 |
| Heart rate (beats/min) | 86 ± 14 | 90 ± 19 |
| RR interval (beats/min) | 16 ± 4 | 18 ± 5 |
| Mean arterial pressure (mmHg) | 70.2 ± 9.3 | 67.9 ± 6.0 |
| PaO2/FiO2 | 179.1 ± 41.9 | 167.7 ± 38.9 |
| Variable | Study group (n = 15) | Control group (n = 16) |
| WBC (109/L) | 11.5 ± 2.3 | 13.1 ± 2.9 |
| PLT (109/L) | 196.5 ± 40.5 | 199.6 ± 58.7 |
| ALB (g/L) | 37.8 ± 4.6a | 38.9 ± 5.7 |
| ALT (U/L) | 54.3 ± 20.4 | 59.7 ± 23.1 |
| TBIL (mmol/L) | 20.1 ± 3.9 | 25.3 ± 4.2 |
| BUN (mmol/L) | 7.8 ± 2.6 | 9.7 ± 2.8 |
| Scr (mmol/L) | 149.8 ± 30.2 | 139.3 ± 37.5 |
| Ca2+ (mmol/L) | 2.1 ± 0.5 | 2.0 ± 0.3 |
| Serum amylase (U/L) | 74.4 ± 28.3 | 82.1 ± 20.7 |
| Urine amylase (U/L) | 399.7 ± 59.7 | 387.1 ± 51.4 |
| CRP (mg/L) | 20.5 ± 6.4a | 28.5 ± 7.1 |
| PCT (ng/mL) | 1.33 ± 0.42a | 1.71 ± 0.61 |
Hyperlipidemic SAP is clinically characterized by severe symptoms, many complications, easy recurrence, and poor prognosis. It is common in obese, young, or middle-aged men, most of which have bad living habits such as a high-fat diet. Evidence has shown that control of serum triglyceride < 500 mg/dL can prevent further progression of pancreatitis[17]. It is well known that hyperlipidemia causes AP mainly through the complex interplay among high serum triglyceride level, increased free fatty acids, reduced activity of trypsin, and activated inflammatory factors to eventually lead to pancreatic tissue inflammation. A high level of serum triglyceride destroys the protectional function of the pancreas, causes abnormal pancreatic enzyme activation and pancreatic self-digestion, and results in the release of a large amount of various proinflammatory factors, thereby causing a cascade effect[10]. Therefore, lowering the serum triglyceride level is the primary goal for early treatment of hyperlipidemic SAP.
To lower high serum triglyceride levels in patients with pancreatitis, oral lipid-lowering drugs (typically fibrates) are usually used in clinical settings. Meanwhile, fat-free parenteral nutrition preparations are often administered. When patients with hyperlipidemic SAP become seriously ill and are transferred to the ICU, physicians may utilize the PE technology to rapidly lower serum triglyceride levels so as to prevent the progression of pancreatic inflammation. When hyperlipidemia is effectively relieved, the cause of disease progression and aggravation is effectively eliminated. Previous clinical studies on the use of PE in the treatment of hyperlipidemic SAP have demonstrated that PE has a good curative effect (especially for lowering serum lipid) and is safe[20,21]. The present study compared the time to target serum triglyceride level (< 500 mg/dL) between PE and use of oral lipid-lowering drugs and found that the former has certain advantages.
When serum triglyceride levels in pancreatitis patients are effectively controlled, the clinical treatment goal shifts to regulating the body’s inflammatory response and volume status. CVVH achieves the purpose of treatment by regulating fluid balance and removing inflammatory mediators and toxins[22]. PE combined with CVVH for the treatment of hyperlipidemic SAP can improve the clinical effects of PE alone in terms of rapidly decreasing the body’s inflammatory factors and, at the same time, adjusting and optimizing the patient’s circulatory status[23,24]. For this reason, the present study sequentially utilized PE to lower lipid levels and then CVVH to effectively reduce the systematic inflammatory response syndrome and optimize the circulatory status. In addition, application of CVVH can clear allergic reactions related to antigens and antibodies generated during PE treatment due to the transfusion of allogeneic plasma, and further optimize the therapeutic effect, thus ensuring the safety of the treatment. Fortunately, no allergic transfusion reactions occurred in our patients, thus reducing the impact on the implementation of PE. In view of this, although the rate of systemic complications (60%) in the study group was higher than that in the control group (50%), the duration of ICU stay was significantly shorter in the study group than in the control group.
Although this study is not a randomized controlled trial, its design is different from that of many previous studies with arbitrarily selected patients, in which the duration of blood purification treatment as well as the combination of blood purification therapies are arbitrary. In the present study, a target oriented sequential therapy protocol was used. This allowed us to monitor the relatively complete clinical course of patients with hyperlipidemic SAP, thus ensuring the credibility of subsequent evaluation of disease outcome and prognosis. However, given that hyperlipidemic SAP is not relatively common[25,26], recruitment of many more patients is somewhat difficult. Future larger sample-sized, randomized controlled, multicenter clinical studies are expected. In addition, despite many unpredictable variations in clinical trials and that there were no significant differences in the secondary outcome measures between the two groups, the sequential treatment group was associated with a better survival rate and some significantly improved laboratory indices, such as C-reactive protein, suggesting that the application of sequential blood purification treatment in the management of ICU patients with hyperlipidemic SAP is feasible, effective, and safe.
Hyperlipidemia is either one of the causes or a consequence of AP[27-30], and even may be both in some cases. However, there is still controversy over this point of view. The pathogenesis of hyperlipidemia-induced AP is still under study, and signaling pathways that have an exact association with hyperlipidemic SAP are being explored. Future treatment of pancreatitis may be based on targeted therapies that can block hyperlipidemia-induced pancreatic injury. Currently, there have been no standardized criteria for the selection of blood purification procedures and parameters for ICU patients with different stages of pancreatitis. These will be our future important research topics and directions. It is currently well recognized that the treatment of hyperlipidemic SAP relies greatly on early, rapid lowering of blood lipid levels in combination with routine therapies for AP. For early lipid-lowering effects, plasma exchange has better efficacy than the use of lipid-lowering drugs. The subsequent application of blood filtration technology and conventional treatments for AP is the current practice for the treatment of ICU patients with AP.
Hyperlipidemia is a common cause of acute pancreatitis, and leads to pancreatic tissue inflammation. The pancreatic tissue inflammation can lead to secondary systemic inflammatory response syndrome and multiple organ failure. Multiple organ failure is the main cause of death in patients with severe acute pancreatitis (SAP). Many studies showed that plasma exchange (PE) combined with continuous venous-venous hemofiltration (CVVH) in the treatment of SAP had certain clinical benefits. However, many clinical studies did not have the standardized treatment. Therefore, we cannot assess the validity of their study.
In the past few years, the use of blood purification treatment of pancreatitis in the intensive care unit (ICU) has progressively increased. In the treatment of hyperlipidemic pancreatitis, the current research focus is how to reduce the mortality and improve the prognosis of patients with the combined application of PE and CVVH.
Based on the 2012 Atlanta International Pancreatitis Consensus, the authors designed a sequential application of PE and CVVH for the treatment of hyperlipidemic SAP. Compared with the past research, the authors designed a relatively reasonable treatment process for improving hyperlipidemic SAP patients’ prognosis.
This sequential blood purification therapy is effective for the treatment of ICU patients with hyperlipidemic SAP and can improve their prognosis.
SAP is refers to acute pancreatitis with more than 48 h persistent multiple organ failure (Marshall Score = 2). Hyperlipidemic acute pancreatitis refers to acute pancreatitis with triglyceride levels > 1000 mg/dL.
This observational study is a very interesting manuscript evaluating the efficacy of sequential blood purification therapy for ICU patients with hyperlipidemic SAP and its impact on prognosis.
P- Reviewer: Fischer A, Miyoshi E S- Editor: Yu J L- Editor: AmEditor E- Editor: Zhang DN
| 1. | Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4932] [Cited by in RCA: 4330] [Article Influence: 360.8] [Reference Citation Analysis (45)] |
| 2. | Tenner S, Baillie J, DeWitt J, Vege SS. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. 2013;108:1400-115; 1416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1232] [Cited by in RCA: 1382] [Article Influence: 115.2] [Reference Citation Analysis (3)] |
| 3. | Mao EQ, Tang YQ, Zhang SD. Effects of time interval for hemofiltration on the prognosis of severe acute pancreatitis. World J Gastroenterol. 2003;9:373-376. [PubMed] |
| 4. | Buter A, Imrie CW, Carter CR, Evans S, McKay CJ. Dynamic nature of early organ dysfunction determines outcome in acute pancreatitis. Br J Surg. 2002;89:298-302. [PubMed] |
| 5. | Mofidi R, Duff MD, Wigmore SJ, Madhavan KK, Garden OJ, Parks RW. Association between early systemic inflammatory response, severity of multiorgan dysfunction and death in acute pancreatitis. Br J Surg. 2006;93:738-744. [PubMed] |
| 6. | Johnson CD, Abu-Hilal M. Persistent organ failure during the first week as a marker of fatal outcome in acute pancreatitis. Gut. 2004;53:1340-1344. [PubMed] |
| 7. | Frossard JL, Steer ML, Pastor CM. Acute pancreatitis. Lancet. 2008;371:143-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 524] [Cited by in RCA: 512] [Article Influence: 30.1] [Reference Citation Analysis (1)] |
| 8. | Shimada M. A challenging case of severe acute hypertriglyceridemia-induced pancreatitis. South Med J. 2009;102:999-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 9. | Reper P, Attou R, Gucciardo L, Gottignies P, Devriendt J, Massaut J. Early plasmapheresis as a successful treatment in hypertriglyceridemia-induced acute pancreatitis in first trimester pregnancy following in vitro fertilization. Eur J Obstet Gynecol Reprod Biol. 2014;179:257-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Stefanutti C, Labbadia G, Morozzi C. Severe hypertriglyceridemia-related acute pancreatitis. Ther Apher Dial. 2013;17:130-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 11. | Yu C, Liu ZH, Chen ZH, Gong DH, Ji DX, Li LS. Improvement of monocyte function and immune homeostasis by high volume continuous venovenous hemofiltration in patients with severe acute pancreatitis. Int J Artif Organs. 2008;31:882-890. [PubMed] |
| 12. | Lentini P, Cruz D, Nalesso F, de Cal M, Bobek I, Garzotto F, Zanella M, Brendolan A, Piccinni P, Ronco C. [A pilot study comparing pulse high volume hemofiltration (pHVHF) and coupled plasma filtration adsorption (CPFA) in septic shock patients]. G Ital Nefrol. 2009;26:695-703. [PubMed] |
| 13. | Hirasawa H. Indications for blood purification in critical care. Contrib Nephrol. 2010;166:21-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | He C, Zhang L, Shi W, Liang X, Ye Z, Zhang B, Liu S. Coupled plasma filtration adsorption combined with continuous veno-venous hemofiltration treatment in patients with severe acute pancreatitis. J Clin Gastroenterol. 2013;47:62-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Chen JH, Yeh JH, Lai HW, Liao CS. Therapeutic plasma exchange in patients with hyperlipidemic pancreatitis. World J Gastroenterol. 2004;10:2272-2274. [PubMed] |
| 16. | Kadikoylu G, Yavasoglu I, Bolaman Z. Plasma exchange in severe hypertriglyceridemia a clinical study. Transfus Apher Sci. 2006;34:253-257. [PubMed] |
| 17. | Yadav D, Pitchumoni CS. Issues in hyperlipidemic pancreatitis. J Clin Gastroenterol. 2003;36:54-62. [PubMed] |
| 18. | Tovey L, Dickie H, Gangi S, Terblanche M, McKenzie C, Beale R, Treacher D, Ostermann M. Beyond the randomized clinical trial: citrate for continuous renal replacement therapy in clinical practice. Nephron Clin Pract. 2013;124:119-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Morabito S, Pistolesi V, Tritapepe L, Zeppilli L, Polistena F, Fiaccadori E, Pierucci A. Regional citrate anticoagulation in CVVH: a new protocol combining citrate solution with a phosphate-containing replacement fluid. Hemodial Int. 2013;17:313-320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Stefanutti C, Di Giacomo S, Vivenzio A, Labbadia G, Mazza F, D’Alessandri G, Russi G, De Silvestro G, Marson P. Therapeutic plasma exchange in patients with severe hypertriglyceridemia: a multicenter study. Artif Organs. 2009;33:1096-1102. [PubMed] |
| 21. | Yeh JH, Chen JH, Chiu HC. Plasmapheresis for hyperlipidemic pancreatitis. J Clin Apher. 2003;18:181-185. [PubMed] |
| 22. | Cole L, Bellomo R, Hart G, Journois D, Davenport P, Tipping P, Ronco C. A phase II randomized, controlled trial of continuous hemofiltration in sepsis. Crit Care Med. 2002;30:100-106. [PubMed] |
| 23. | Bellomo R, Tetta C, Ronco C. Coupled plasma filtration adsorption. Intensive Care Med. 2003;29:1222-1228. [PubMed] |
| 24. | Abdul Cader R, Abdul Gafor H, Mohd R, Yen Kong W, Arshad N, Kong N. Coupled Plasma Filtration and Adsorption (CPFA): A Single Center Experience. Nephrourol Mon. 2013;5:891-896. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Qihui C, Xiping Z, Xianfeng D. Clinical study on acute pancreatitis in pregnancy in 26 cases. Gastroenterol Res Pract. 2012;2012:271925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 26. | Mao EQ, Tang YQ, Zhang SD. Formalized therapeutic guideline for hyperlipidemic severe acute pancreatitis. World J Gastroenterol. 2003;9:2622-2626. [PubMed] |
| 27. | Chen TZ, Xie SL, Jin R, Huang ZM. A novel lipoprotein lipase gene missense mutation in Chinese patients with severe hypertriglyceridemia and pancreatitis. Lipids Health Dis. 2014;13:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Brahm A, Hegele RA. Hypertriglyceridemia. Nutrients. 2013;5:981-1001. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 29. | Bălănescu NR, Topor L, Ulici A, Djendov FB. Acute pancreatitis secondary to hyperlipidemia in an 11-year-old girl: a case report and review of literature. J Med Life. 2013;6:2-6. [PubMed] |
| 30. | Sandhu S, Al-Sarraf A, Taraboanta C, Frohlich J, Francis GA. Incidence of pancreatitis, secondary causes, and treatment of patients referred to a specialty lipid clinic with severe hypertriglyceridemia: a retrospective cohort study. Lipids Health Dis. 2011;10:157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 115] [Article Influence: 8.2] [Reference Citation Analysis (0)] |