CASE REPORT
Case 1
A 58-year-old man was referred to our hospital from a local clinic on December 25, 2013, complaining of abdominal pain with radiation to the right shoulder and back for 2 wk. During that period, he also had intermittent fever with chills. His past medical history included cholangiolithiasis and cholecystolithiasis over 30 years ago, and his surgical history included cholecystectomy and hepatectomy of the left lateral lobe 30 years ago. He reported possible occupational exposure to vinyl chloride because he worked with paint-related chemicals for 10 years. The patient denied any history of hepatitis, diabetes mellitus, or cancer. Physical examination revealed slight tenderness of the right upper abdominal quadrant. Percussion elicited pain over the liver area. Laboratory examinations on admission revealed: white blood cell (WBC) count 9.6 × 109/L, hemoglobin 12.9 g/dL, platelet count 248 × 109/L, alanine aminotransferase (ALT) 64 U/L, aspartate transaminase (AST) 59 U/L, alkaline phosphatase (ALP) 460 U/L, γ-glutamyl transpeptidase (GGT) 329 U/L, albumin 30.5 g/L, total bilirubin (TBIL) 14.6 μmol/L, direct bilirubin (DBIL) 7.1 μmol/L, prothrombin time (PT) 15.4 s, and international normalized ratio (INR) 1.23. Serum tumor markers, including α-fetoprotein (AFP), carcinoembryonic antigen (CEA), and carbohydrate 125 (CA125), were within normal ranges, apart from carbohydrate antigen 19-9 (CA19-9), which was slightly elevated at 58.74 IU/mL. Serology for hepatitis A, B and C tested negative. Abdominal T1-weighted magnetic resonance imaging (MRI) revealed a heterogeneous low-intensity mass about 4.3 cm × 6.2 cm, and high signal intensity was evident on T2-weighted imaging. The focal areas of higher signal intensity on both images indicated hemorrhage (Figure 1). To obtain a definitive diagnosis, computed tomography (CT)-guided fine-needle aspiration (FNA) biopsy was performed, and suggested a diagnosis of spindle cell tumor. The patient underwent hepatectomy of the right posterior lobe (segments VI and VII), had an uneventful postoperative course, and was discharged 16 d after surgery. Specimen cross-sectioning revealed a clearly demarcated yellowish-white lesion measuring 6.3 cm × 4.5 cm (Figure 2). Histology revealed spindle-shaped neoplastic cells exhibiting marked nuclear pleomorphism arranged in a whorled pattern, and present in fascicular clusters (Figure 3A). In addition to these solid areas, necrotic and hemorrhagic regions were also noted (Figure 3B). Immunohistochemical examination revealed staining positive for CD31 (Figure 3C) and Ki-67 (positive rate of 40%-50%), characteristic of tumor cells. Staining for CD34 and S-100 were negative. The histopathological diagnosis was consistent with PHA.
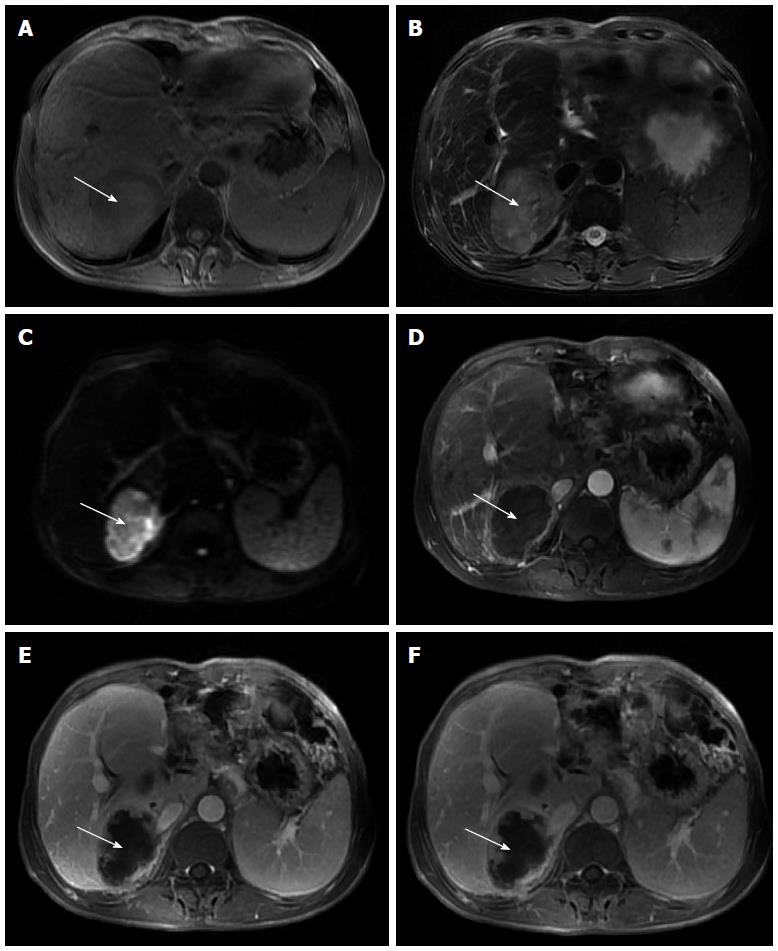
Figure 1 Magnetic resonance imaging.
A: Markedly heterogeneous low signal intensity mass (white arrow) in the right lobe of the liver on T1-weighted imaging, with focal areas of high signal intensity that indicate fresh hemorrhage; B: Heterogeneous high signal intensity mass (white arrow) in the right lobe of the liver on T2-weighted imaging, with focal areas of higher signal intensity that indicate necrosis or hemorrhage; C: Mass (white arrow) shows high signal intensity on diffusion-weighted imaging that reveals restricted diffusion; D: Mass (white arrow) has a ring-like enhancement in the arterial phase; E: Progressive enhancement of the ring (white arrow) in the portal phase, revealing nodular enhanced foci within the ring; F: Progressive enhancement of the ring (white arrow) and nodular enhanced foci within the ring in the delayed phase.
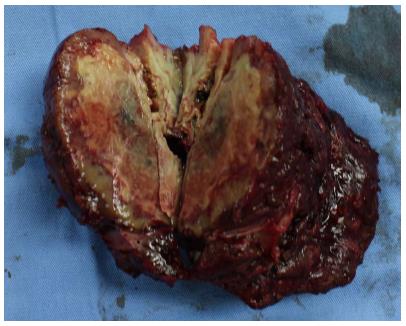
Figure 2 Cross-section of the specimen showing a yellow-white lesion 6.
3 cm × 4.5 cm.
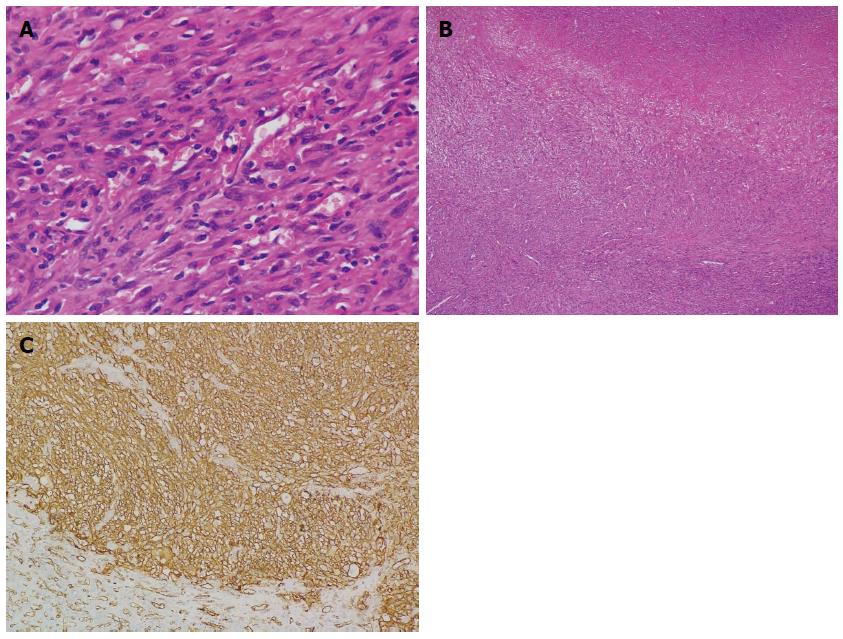
Figure 3 Histological examination.
A: Spindle-shaped neoplastic cells exhibiting marked nuclear pleomorphism with whorled shape and fascicular clusters (HE stain; magnification × 400); B: Necrotic areas seen between the clusters of neoplastic cells (HE stain; magnification × 40); C: Immunohistochemical examination reveals positive staining of CD31 (magnification × 200). HE: Hematoxylin and eosin.
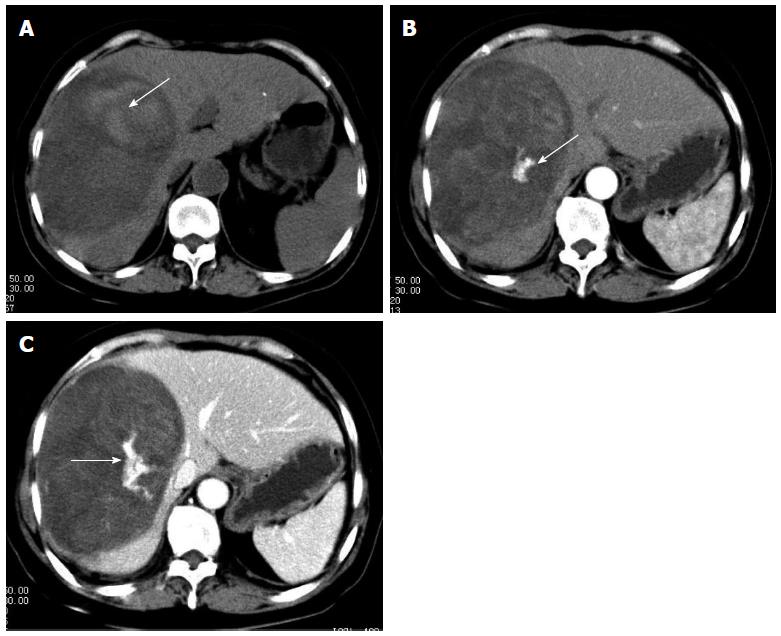
At follow-up 1 mo after surgery, the patient was in good condition. To suppress possible local recurrence or tumor metastasis, transcatheter arterial chemoembolization (TACE) was performed 1 mo after hepatectomy and the patient was initiated on a regimen of cisplatin (50 mg), mitomycin (10 mg), and pirarubicin (50 mg). No significant tumor staining foci were seen at that time. However, the patient was readmitted to our hospital due to fever and right upper abdominal pain 3 mo after surgery. Contrast-enhanced CT revealed emerging lesions in the right liver close to the incisal margin and enlarged retroperitoneal lymph nodes; both of which suggested tumor recurrence (Figure 4). The patient refused further treatment, and was reported to survive for 8 mo after hepatectomy by telephone follow-up.
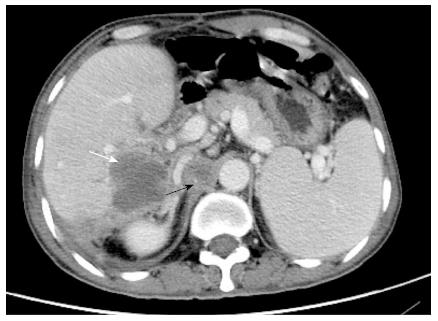
Figure 4 Computed tomography imaging.
Emerging lesions in the right liver (white arrow) and enlarged retroperitoneal lymph nodes (black arrow) suggested postoperative tumor recurrence.
Case 2
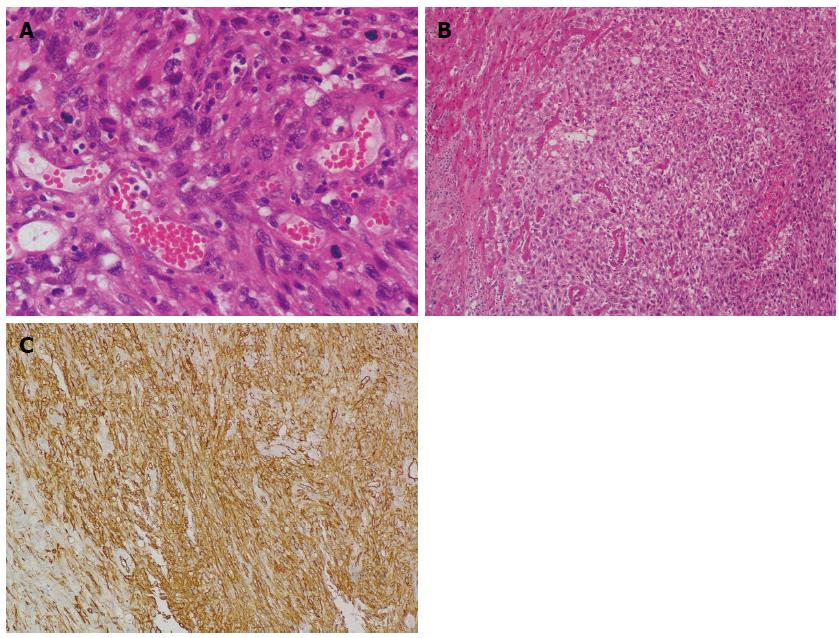
A 58-year-old woman was admitted to our hospital on November 5, 2007 due to recurrent upper abdominal pain for the past 4 years, along with fatigue and weight loss. Her past history was unremarkable except for hypertension. She denied any carcinogen exposure. Physical examination revealed a tender abdominal mass in the right upper abdomen. Complete blood count revealed: WBC count 4.8 × 109/L, hemoglobin 8.7 g/dL, and platelet count 237 × 109/L. Liver function test showed moderate elevation of ALP (129 U/L) and GGT (67 U/L), while ALT, AST, ALB, TBIL and DBIL were within normal ranges. AFP, CEA, CA125 and CA19-9 were also within normal ranges. Serology for hepatitis A, B and C was negative. Contrast-enhanced CT revealed a mass in the right hepatic lobe measuring about 13 cm × 9 cm. The mass was heterogeneously hypoattenuated with non-homogeneous enhancement in both arterial and venous phases. Vascular imaging with contrast suggested a possible diagnosis of PHA (Figure 5). Right hemihepatectomy was performed and the surgical procedure was unremarkable. The patient recovered uneventfully and was discharged 11 d after surgery. Although the margins of the specimen were clear-cut, cross-sectional histology demonstrated a red-gray lesion measuring 13 cm × 9.5 cm, with large necrotic areas. Vascular channels were formed by atypical cells, which exhibited marked nuclear pleomorphism, and were filled with erythrocytes (Figure 6A). Neoplastic cells infiltrated the adjacent liver parenchyma (Figure 6B). Immunohistochemically, the specimen was positive for vascular endothelial markers (CD31 and CD34) (Figure 6C), and negative for cytokeratin, CD68 and S100. A diagnosis of PHA was established based upon the aforementioned pathological findings. The patient was available for follow-up and she survived for 16 mo after the operation.
Figure 5 Computed tomography scan.
A: Plain computed tomography scan reveals an oval shaped heterogeneous hypoattenuating mass with hyperattenuating foci (arrow) which suggests hemorrhage; B: Dominant mass lesion shows heterogenous enhancement with hyperattenuating enhanced foci (arrow), which indicate vasculature, in arterial phase; C: Dominant mass shows progressive enhancement and hyperattenuating enhanced foci enlarged in portal phase (arrows).
Figure 6 Histological examination.
A: Neoplastic cells exhibiting marked nuclear pleomorphism, and vascular channels filled with erythrocytes (HE stain; magnification × 400); B: Clusters of neoplastic cells infiltrating liver parenchyma (HE stain; magnification × 100); C: Immunohistochemical examination reveals positive staining of CD34 (magnification × 200). HE: Hematoxylin and eosin.
DISCUSSION
PHA, arising from endothelial and fibroblastic tissues, accounts for only 0.1%-2% of primary hepatic tumors[1,2], and is rarely seen in practice. It usually develops during the sixth and seventh decades of life and shows an obvious male predominance with a male to female ratio of 3:1[4]. According to an epidemiological study in the United States, vinyl chloride monomer exposure, use of thorotrast in angiography, exposure to inorganic arsenic, and treatment with androgenic anabolic steroids are associated with development of PHA in 25% of all cases. The remaining studied cases were of uncertain etiology[5].
Symptoms of PHA are variable and mimic chronic liver disease. They include abdominal pain, anorexia, fatigue, weight loss, fever, and low back pain, and may even be asymptomatic[2,6,7]. As symptoms tend to be nonspecific, most patients are diagnosed at an advanced stage. Reported physical findings of PHA include jaundice, ascites, hepatomegaly, and splenomegaly[2,4,8]. About 15%-27% of PHA patients present with hemoperitoneum, due to spontaneous tumor rupture; a potentially fatal complication[2,9]. Acute hepatic failure, as a primary symptom in PHA patients, has also been reported[7,8]. In our literature review, PHA was documented to metastasize to the lungs (8 cases), spleen (5 cases), bone (5 cases), peritoneum (3 case) and cerebellum (1 case)[10-14]. Sometimes, patients presented with symptoms due to a metastatic lesion, such as chest pain in a case with lung metastasis.
Laboratory tests of PHA patients often show moderate elevation of liver enzymes, such as ALT, AST, and most commonly, ALP, which suggests a cholestatic manifestation[2,15]. Thrombocytopenia and anemia are relatively common hematological findings. The former, along with the vascular nature of tumor, may contribute to hepatic rupture. Tumor markers such as AFP, CEA, CA19-9, and CA125 are always within normal ranges or only mildly elevated[4,16].
The absence of specific clinical manifestations and laboratory findings in PHA reasonably emphasizes the diagnostic value of imaging studies. From a morphological perspective, PHA appears as multiple nodules, a dominant mass, or a mixed pattern of a dominant mass and multiple nodules, and rarely, manifests as an infiltrative, micronodular subtype[17,18]. Conventional ultrasound examination has been reported as a nonspecific imaging modality and small lesions often appear isoechoic and clearly demarcated, while large lesions appear hypoechoic and poorly demarcated. However, specific characteristics are revealed under contrast-enhanced ultrasound (CEUS), with a central unenhanced area and peripheral irregular enhancement in the arterial and portal phases, and complete late-phase wash-out[19]. PHA presents differently on CT imaging according to its various morphological patterns. Necrosis and hemorrhage, which occur frequently in the solid portion of PHA, both contribute to the complexity of imaging. PHA lesions are generally hypoattenuating, sometimes with hyperattenuating foci on unenhanced CT. On contrast-enhanced CT, most nodular lesions appear hypoattenuating and with enhanced foci, and some nodular lesions show irregularity or ring enhancement. Dominant mass lesions show heterogeneous enhancement, suggesting central necrosis and fibrotic changes. A delayed progressive enhancement pattern in all enhanced lesions has been observed[20,21]. MRI reflects the hemorrhagic, heterogeneous, and hypervascular nature of PHA lesions. On T1-weighted MR images, dominant mass lesions often contain irregular areas of high signal intensity, suggesting hemorrhage. On T2-weighted MR images, mass lesions of PHA reveal a markedly heterogeneous architecture, with focal areas of high intensity along with septum-like or rounded areas of low intensity. Areas of high intensity on T2-weighted images suggest hemorrhage or necrosis, while areas of low signal intensity indicate hemosiderin deposition, fibrous solid portions, or occasionally fresh hemorrhage. On dynamic contrast-enhanced MRI, each lesion reveals heterogeneous enhancement on arterial- and portal-phase images. On delayed imaging, however, there is progressive enhancement of the lesion compared with that of early-phase images[14,20]. Pertinent angiographic findings include multiple nodular or mass lesions present with areas of fluffy staining and early pooling of contrast medium that persists and increases over time. Dominant mass lesions reveal poorly defined, irregular tumor with a nonstaining area. The large dominant masses also show early contrast medium pooling and continuously increasing enhancement[12]. Rademaker et al[22] have suggested that combined use of common hepatic angiography and dual-phase helical CT provides better identification of PHA.
Differentiating PHA from hepatic hemangioma, hepatocellular carcinoma (HCC), cholangiocarcinoma, and hepatic abscess is difficult. Elevation of AFP, past history of hepatitis B virus infection, and liver cirrhosis favor a diagnosis of HCC. Continuing enhancement on the late-phase of MRI may be helpful in differentiating PHA from HCC[14]. Imaging findings of PHA and hepatic hemangioma are sometimes similar. Rapid growth, tumor rupture or increasing pain may, however, suggest PHA rather than hemangioma[21]. Arterioportal shunting, which is often absent in hemangioma, also favors a diagnosis of PHA[23]. According to experienced radiologists, PHA might be distinguished from hepatic hemangioma via biphasic MRI[21,22,24]. It has also been reported that F-18 fluorodeoxyglucose positron emission tomography is helpful in differentiating PHA from giant cavernous hepatic hemangioma[25].
A definitive diagnosis of PHA is always established via pathological analysis. PHA has a vascular nature, therefore, liver biopsy is liable to cause bleeding[15,26]. Additionally, due to the high frequency of necrosis and hemorrhage inside the tumor, samples taken from these areas may produce false-negative results[27]. Therefore, nonsurgical liver biopsy is considered to be a dangerous and unreliable method for PHA diagnosis[26]. Nevertheless, Koyama et al[20] reported promising results for percutaneous biopsies in their series of PHA patients, with a success rate of 78% and no substantial complications. In our first case, preoperative diagnosis was also determined by CT-guided liver biopsy, implying that while PHA is not an absolute contraindication for liver biopsy, scrutinous observation and surgical evaluation are important prior to the procedure.
Histologically, PHA is composed of spindle-shaped and polyhedral cells. This tumor is characterized by a variety of patterns of vascular channels, ranging from dilated sinusoidal or cavernous spaces to slit-like freely anastomosing vessels, formed by spindle-shaped cells[17,18]. Wang et al[28] analyzed 20 cases of PHA immunohistochemically and detected the expression of specific immunohistochemical markers. All cases expressed at least one of the following three markers: CD31, CD34 and FVIIIRag. Ten cases had low expression of PTEN. The Ki-67 proliferative index was > 10% in all cases. Recently, ERG, a transcription factor, was indicated to be a specific and more sensitive diagnostic marker for PHA in comparison to CD31, CD34 and FVIIIRag[29]. Nevertheless, no immunohistochemical marker has been found to be associated with prolonged survival[30].
PHA is an aggressive malignancy that generally carries a poor prognosis. Complete surgical resection is the only definitive treatment that can improve survival. Groeschl et al[31] reported 207 PHA patients with a median overall survival time of only 1 mo. Comparatively, those who had the tumor resected had their survival time prolonged to 6 mo. In our literature review, a total of 32 patients with survival data were analyzed, with a median survival time of 4 mo after initial diagnosis. Nine patients receiving surgical resection with or without adjuvant therapy had longer survival time (median: 10 mo) compared to those who did not have their tumor resected (median: 2 mo). Among them, five patients who underwent tumor excision with negative margins had the best prognoses with a median survival time of 10 mo. Two patients receiving liver transplantation showed rapid recurrence and a subsequent low survival time. Prognosis for four patients presenting with hemoperitoneum due to tumor rupture was dismal (median: 23 d). Curative surgery was difficult to perform in > 80% of PHA patients who were diagnosed at an advanced stage, and presented with a dominant intrahepatic mass and/or extrahepatic metastases[4]. A definite diagnosis of PHA was established only in some cases after surgery, which regretfully did not meet oncological treatment criteria and led to a high risk of tumor recurrence[9]. Spontaneous PHA rupture usually carries a dismal prognosis, even if bleeding is stopped by emergency transcatheter arterial embolization (TAE) or surgery[12]. Due to the high frequency of tumor recurrence (77%) and dismal postoperative survival, PHA is considered to be a contraindication to liver transplantation[32].
PHA is reported to be radioresistant, and radiotherapy is generally not initiated in PHA patients[2,33]. Chemotherapy, including TACE, is considered to be an effective palliative therapy for unresectable PHA[10,15]. However, due to the rarity of PHA and paucity of medical records, articles concerning chemotherapy are few in number. To date, there is no established chemotherapy regimen for advanced PHA. Chemotherapeutic agents such as 5-fluorouracil, carboplatin, doxorubicin and adriamycin have been used in PHA[10,34]. The limited number of studies on this subject have shown that addition of adjuvant chemotherapy may improve survival after surgical resection[35]. Palliative chemotherapy, which is potentially useful, can be an option in advanced hepatic angiosarcoma[10,34]. TACE has shown effectiveness in the treatment of PHA, particularly in patients with dominant masses rather than those with multiple nodules[12]. Although our study reviews the present state of PHA treatment modalities and covers cases viewed in our hospital, a study with a larger sample is required to draw definite conclusions regarding the efficacy of TACE in this setting.
Literature regarding diagnostic methods and treatment regimens for PHA is limited and we hope that our report reminds clinicians of the difficulty in accurately diagnosing this disease, and inspires medical practitioners to study this rare malignancy.