Published online May 21, 2015. doi: 10.3748/wjg.v21.i19.6044
Peer-review started: September 25, 2014
First decision: October 29, 2014
Revised: November 25, 2014
Accepted: December 16, 2014
Article in press: December 16, 2014
Published online: May 21, 2015
Processing time: 238 Days and 1.2 Hours
AIM: To compare the efficacy and safety of biological agents for the treatment of active ulcerative colitis (UC).
METHODS: PubMed, MEDLINE, EMBASE and the Cochrane library were searched to screen relevant articles from January 1996 to August 2014. The mixed treatment comparison meta-analysis within a Bayesian framework was performed using WinBUGS14 software. The proportions of patients reaching clinical response, clinical remission and mucosal healing in induction and maintenance phases were analyzed as efficacy indicators. Serious adverse events in maintenance phase were analyzed as safety indicators.
RESULTS: The meta-analysis results showed that biological agents achieved better clinical response, clinical remission and mucosal healing than placebo. Indirect comparison indicated that in induction phase, infliximab was more effective than adalimumab in inducing clinical response (OR = 0.41, 95%CI: 0.29-0.57), clinical remission (OR = 0.33, 95%CI: 0.19-0.56) and mucosal healing (OR = 0.33, 95%CI: 0.19-0.56), and golimumab in inducing clinical response (OR = 0.66, 95%CI: 0.39-2.33) and mucosal healing (OR = 2.15, 95%CI: 1.18-4.22). No significant difference was found between placebo and biological agents regarding their safety.
CONCLUSION: All biological agents were superior to placebo for UC treatment in both induction and maintenance phases with a similar safety profile, and infliximab had a better clinical effect than the other biological agents.
Core tip: Currently the selection of biological agents in ulcerative colitis (UC) therapy is still controversial. We performed this meta-analysis to compare the efficacy and safety of biological agents for the treatment of active UC, and finally found that all biological agents were superior to placebo for UC treatment in both induction and maintenance phases with a similar safety profile, and infliximab had a better clinical effect than the other biological agents.
- Citation: Mei WQ, Hu HZ, Liu Y, Li ZC, Wang WG. Infliximab is superior to other biological agents for treatment of active ulcerative colitis: A meta-analysis. World J Gastroenterol 2015; 21(19): 6044-6051
- URL: https://www.wjgnet.com/1007-9327/full/v21/i19/6044.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i19.6044
Ulcerative colitis (UC) is a form of chronic inflammatory bowel disease (IBD) characterized by recurrent rectal bleeding, increased stool frequency and urgency, abdominal cramps and pain, and systemic symptoms (such as fever, anemia and weight loss)[1,2]. It is reported that the incidence of UC is 1.2-20.3 per 100000 person-years and its prevalence is 7.6-246.0 per 100000 persons[3]. Current options of treatment include aminosalicylates, corticosteroids, immunosuppressive medications such as azathioprine and 6-mercaptopurine, and biological agents including tumor necrosis factor-α (TNF-α) antibodies and integrin antagonists.
5-aminosalicylic acid (5-ASA) is the first-line medication used to induce and maintain remission in patients with mild-to-moderate active UC[4]. Patients who do not have an adequate response to 5-ASA are recommended to receive corticosteroid treatments[5]. Moreover, traditional immunosuppressive azathioprine (AZA) and 6-mercaptopurine are suggested to treat patients with moderate active UC who are not responsive to oral corticosteroids[6]. However, conventional treatments often lead to a series of adverse events and have a limited effect in long-term disease control.
Anti-TNF-α agents including infliximab, adalimumab and golimumab have been approved by United States Food and Drug Administration for the treatment of moderate-to-severe UC. All the 3 anti-TNF-α agents are demonstrated to be effective for the induction and maintenance of remission in moderate or severe UC. In addition, these agents can also induce mucosal healing and reduce glucocorticoid dependence[7]. Vedolizumab is a humanized immunoglobulin G1 monoclonal antibody to α4β7 integrin[8]. A phase 3 study investigating the efficacy and safety of vedolizumab in patients with moderate to severe active UC indicated that vedolizumab was significantly more effective in terms of clinical response and remission compared to placebo in both induction and maintenance phases[9].
Currently, the selection of biological agents in UC therapy is still controversial. Traditional methods cannot be applied for the comparison for lack of head-to-head studies comparing different biological agents. Therefore, we used a mixed treatment comparison (MTC) to compare the efficacy of biological agents, as MTC was available for indirect comparisons between drugs with different comparators[10,11].
Four databases (PubMed, EMBASE, MEDLINE and the Cochrane library) were screened to obtain articles from January 1996 to August 2014 for inclusion in this study. The key words “ulcerative colitis”and (“infliximab” or “adalimumab” or “golimumab” or “vedolizumab”) were used to search relevant articles. We included those studies meeting the following two criteria: (1) the study evaluated the efficacy of biological treatments using a random case-control design; and (2) trials had to be placebo controlled.
The following information was extracted from each study: the first author’s name; the year of publication; the number of patients; the number of patients achieving clinical response; the number of patients achieving clinical remission; the number of patients achieving mucosal healing; the outcome of serious adverse events; endpoints; and study duration. The Jadad score was used to assess the quality of the included studies. Different doses of the same biological agent were regarded as separate interventions. Odds ratios (ORs) were used to measure the outcome of clinical response, clinical remission, mucosal healing and serious adverse events in induction and maintenance phases. Sandborn et al[12,13] and Feagan et al[14] presented induction phase results at week 6, and their studies were analyzed with trials presenting results at week 8[9]. Sandborn et al[12,13] presented maintenance phase results at week 54, and this study was analyzed with trials presenting results at week 52.
To evaluate the relative effectiveness of each biological agent, an MTC meta-analysis within a Bayesian framework was performed. For all Bayesian analyses, Markov-chain-Monte-Carlo methods were used[15]. A random effect model was used to estimate the ORs as the measurement of relative treatment effect. We carried out 60000 iterations. The first 10000 iterations were discarded after the burn-in period and estimates were based on the subsequent 50000 ones. Heterogeneity between studies was assessed by Cochrane Q statistics and I2 test. A significant level of no less than 50% for I2 test was considered as evidence of heterogeneity. A fixed effects model was used when there was no evidence of heterogeneity, otherwise a random effects model was chosen. Data analysis was performed using WinBUGS version 1.4.3 (MRC Biostatistics Unit, Cambridge, United Kingdom) and STATA12 (Stata Corp, College Station, Texas, United States). The statistical methods of this study were reviewed by Shanghai 2med Biotechnology Co., Ltd (Shanghai, China).
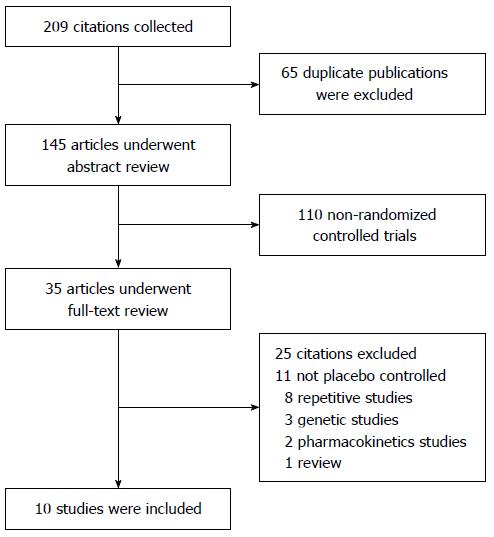
A total of 209 articles were obtained via database searches; ten met the inclusion criteria for this study (Figure 1). A total of 4237 patients with moderate-to-severe active UC were involved. Among the UC patients, 484 were treated with infliximab; 685 with adalimumab; 970 with golimumab; 746 with vedolizumab; and 1352 with placebo. The information of these articles is summarized in Table 1.
| Study | Age (yr) | Drug and dose | Case | Severity | Treatment | Duration (wk) |
| ACT 1 | 41.4 ± 13.7 | Placebo | 121 | Moderate to-severe active UC | Intravenous infusions at weeks 0, 2 and 6 and then every eight weeks or matching placebo | 54 |
| (Rutgeerts et al[16], Feagan et al[17], Sandborn et al[18]) | 42.4 ± 14.3 | Infliximab 5 mg/kg | 121 | |||
| 41.8 ± 14.9 | Infliximab 10 mg/kg | 122 | ||||
| ACT 2 | 39.3 ± 13.5 | Placebo | 123 | Moderate-to-severe active UC | Intravenous infusions at weeks 0, 2 and 6 and then every eight weeks or matching placebo | 30 |
| (Rutgeerts et al[16], Feagan et al[17], Sandborn et al[18]) | 40.5 ± 13.1 | Infliximab 5 mg/kg | 121 | |||
| 40.3 ± 13.3 | Infliximab 10 mg/kg | 120 | ||||
| Suzuki et al[19] | 41.3 ± 13.6 | Placebo | 96 | Moderate-to-severe active UC | Subcutaneous injections 160/80 mg at week 0, 80/40 mg at week 2 and then 40 mg beginning at week 4 every other week or matching placebo | 52 |
| 44.4 ± 15.0 | Adalimumab 80/40 mg | 87 | ||||
| 42.5 ± 14.6 | Adalimumab 160/80 mg | 90 | ||||
| ULTRA 2 | 41.3 ± 13.2 | Placebo | 246 | Moderate-to-severe active UC | Subcutaneous injections 160 mg at week 0, 80 mg at week 2 and then 40 mg beginning at week 4 every other week or matching placebo | 52 |
| (Sandborn et al[20]) | 39.6 ± 12.5 | Adalimumab | 248 | 8 | ||
| Reinisch et al[21] | 37.0 ± 9.0 | Placebo | 130 | Moderate-to-severe active UC | Subcutaneous injections 160/80 mg at week 0, 80/40 mg at week 2 and then 40 mg beginning at week 4 every other week or matching placebo | |
| 40.0 ± 9.5 | Adalimumab 80/40 mg | 130 | ||||
| 36.5 ± 9.5 | Adalimumab 160/80 mg | 130 | 6 | |||
| PURSUIT-SC | 39.0 ± 13.0 | Placebo | 331 | Moderate-to-severe active UC | Subcutaneous injections 400/200 mg at week 0 and 200/100 at week 2 or matching placebo | |
| (Sandborn et al[12]) | 40.0 ± 13.5 | Golimumab 200/100 mg | 331 | |||
| 40.7 ± 13.7 | Golimumab 400/200 mg | 331 | 54 | |||
| PURSUIT-M | 40.2 ± 14.1 | Placebo | 156 | Moderate-to-severe active UC | Subcutaneous injections 100/50 mg every 4 wk or matching placebo | |
| (Sandborn et al[13]) | 41.4 ± 13.8 | Golimumab 50 mg | 154 | |||
| 39.1 ± 13.1 | Golimumab 100 mg | 154 | 52 | |||
| GEMINI 1 | 41.2 ± 12.5 | Placebo | 149 | Moderate-to-severe active UC | Intravenous infusions every 4 wk or every 8 wk or matching placebo | |
| (Feagan et al[9,14]) | 40.1 ± 13.2 | Vedolizumab 300 mg | 746 |
Before performing MTC meta-analysis, we analyzed the effect of single biological agent on response, remission, mucosal healing and serious adverse events compared to placebo. No heterogeneity was found between studies (Table 2).
| Response | Remission | Mucosal healing | Serious adverse events | ||||
| Induction | Maintenance | Induction | Maintenance | Induction | Maintenance | Maintenance | |
| Infliximab | 0 | 0 | 47.4 | 0 | 0 | 0 | 0 |
| Adalimumab | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Golimumab | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Vedolizumab | - | 0 | - | 0 | - | 0 | - |
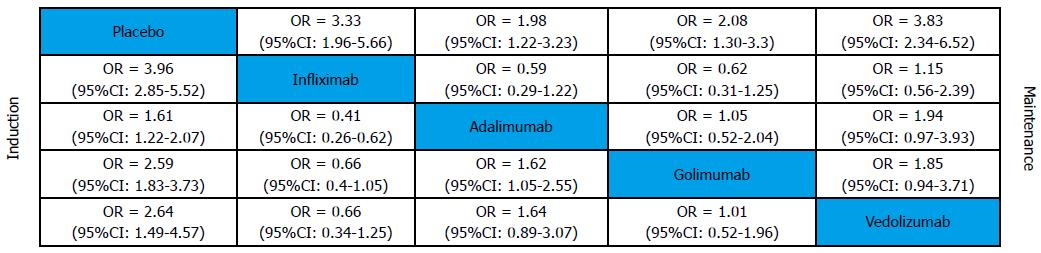
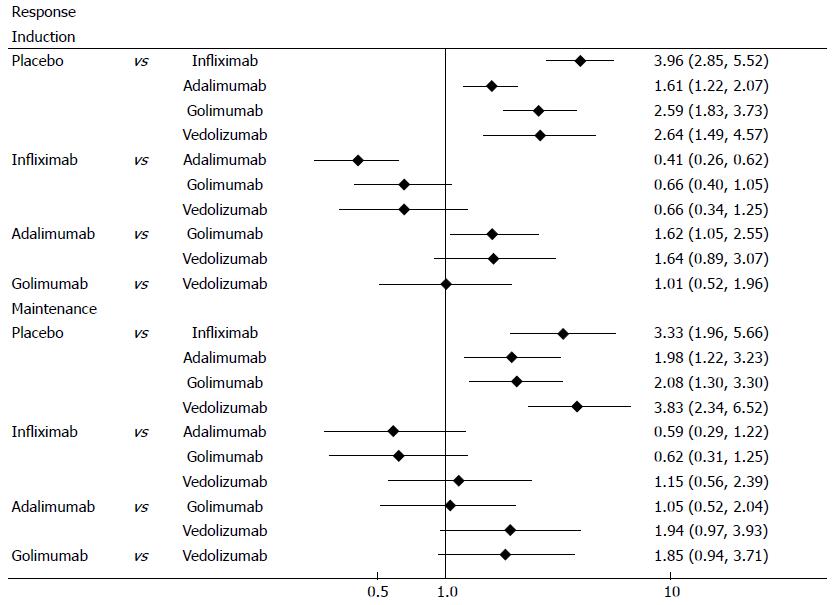
Clinical response was defined as a decrease from baseline in the total Mayo score of at least 3 points and by at least 30%, with an accompanying decrease in the subscore for rectal bleeding of at least 1 point or an absolute subscore for rectal bleeding of 0 or 1. All biological agents were superior to placebo in both induction and maintenance phases (Figure 2). The results of MTC meta-analysis showed that in induction phase infliximab was more effective than adalimumab (OR = 0.41, 95%CI: 0.29-0.57) and golimumab (OR = 0.66, 95%CI: 0.44-0.97), while golimumab had a better effect than adalimumab (OR = 1.62, 95%CI: 1.13-2.33). In maintenance phase, vedolizumab was more effective than adalimumab (OR = 1.94, 95%CI: 1.11-3.44) and golimumab (OR = 1.85, 95%CI: 1.08-3.2). Forest plots are summarized in Figure 3.
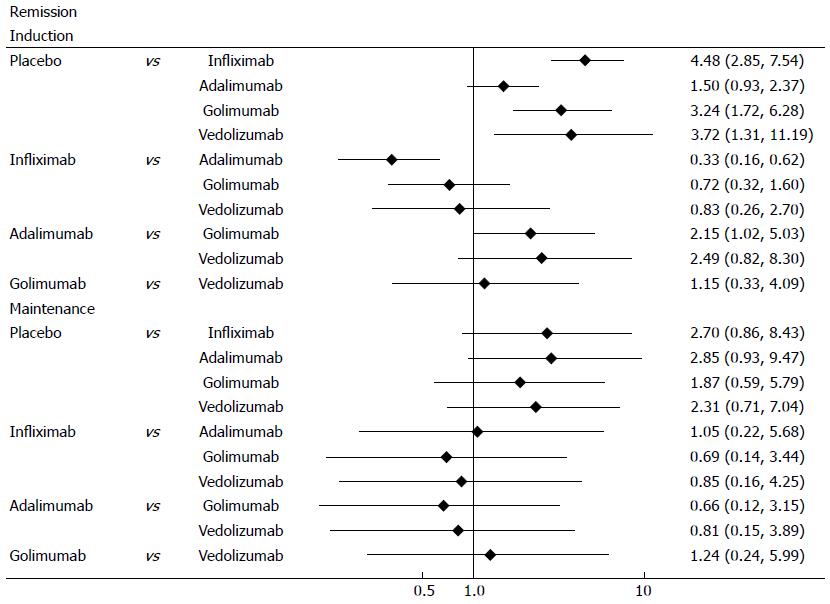
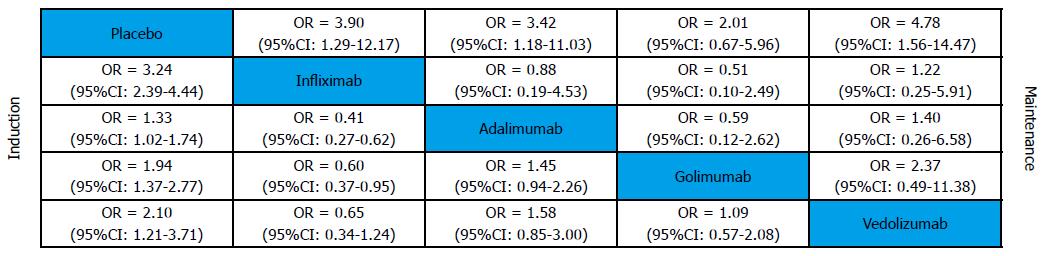
Clinical remission was defined as a total Mayo score of 2 points or lower, with no individual subscore exceeding 1 point. All biological agents were better than placebo for clinical remission in induction and maintenance phases (Figure 4). In induction phase, adalimumab was less effective than infliximab (OR = 0.33, 95%CI: 0.19-0.56), golimumab (OR = 2.15, 95%CI: 1.18-4.22) and vedolizumab (OR = 2.49, 95%CI: 0.99-6.64). However, there was no significant difference between the biological agents in maintenance phase. Forest plots are summarized in Figure 5.
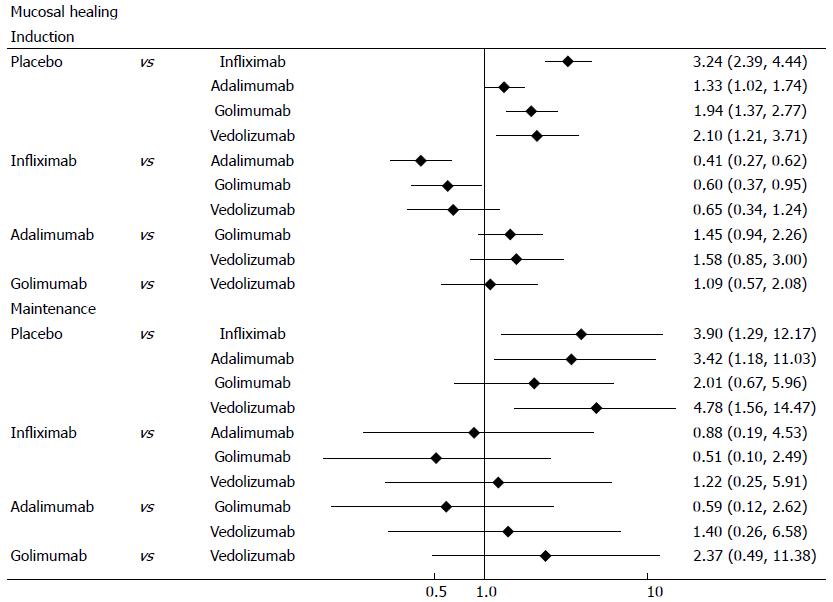
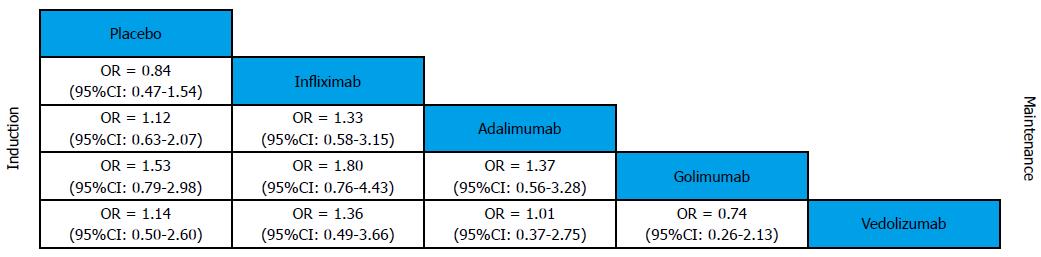
Mucosal healing was defined as an absolute subscore for endoscopy of 0 or 1. Biological agents were better than placebo for mucosal healing in induction and maintenance phases (Figure 6). In induction phase, infliximab was more effective than adalimumab (OR = 0.41, 95%CI: 0.29-0.57) and golimumab (OR = 0.6, 95%CI: 0.41-0.87), while golimumab had a better effect than adalimumab (OR = 1.45, 95%CI: 1.02-2.09). However, no significant difference was found between the biological agents in maintenance phase. Forest plots are summarized in Figure 7.
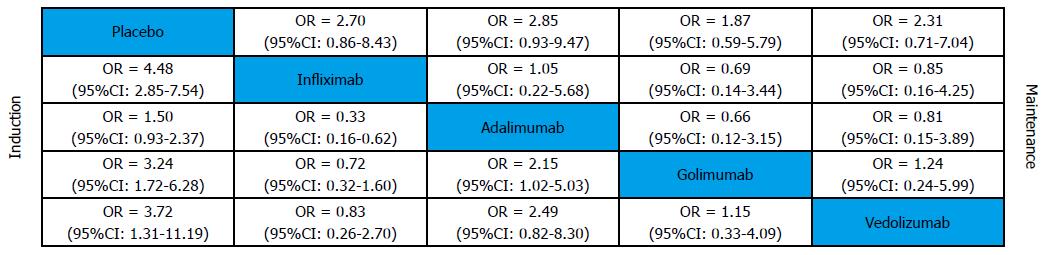
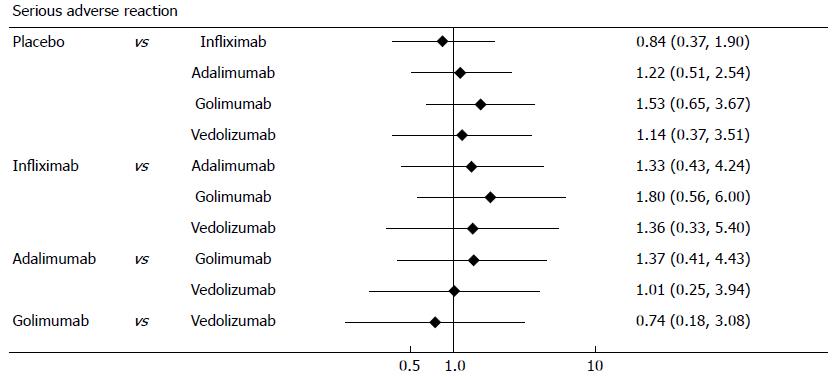
This analysis used random trial data on serious adverse events from maintenance phase. The MTC meta-analysis results showed that biological agents had a similar safety profile to placebo (Figure 8). Forest plots are summarized in Figure 9.
The appearance of biological agents dramatically changed the treatment landscape for UC. Biological agents have been used for the treatment of moderate to severe UC patients failing conventional treatment. Previous randomized controlled trials (RCTs) proved that biological agents were effective and safe for the treatment of UC in both induction and maintenance phases. Danese et al[3] compared the biological agents by performing a multiple-treatment meta-analysis. They illustrated that infliximab is more effective to induce clinical response and mucosal healing than adalimumab in induction phase[3]. However, there was still lack of head-to-head RCTs to compare the different treatment options for long-term efficacy and safety.
This meta-analysis assessing biological agents for the treatment of moderate to severe active UC included 9 RCTs, all of which were placebo controlled trials. No heterogeneity was found when assessing the effect of single biological agent. Meta-analysis results showed that all biological agents were effective for UC treatment in induction and maintenance phases. Indirect comparisons of induction studies indicated that infliximab had a more favorable clinical outcome than golimumab, vedolizumab and adalimumab, while adalimumab was less effective than the others. However, in maintenance phase, all biological agents had a similar effect without statistical difference. The incidence of serious adverse events was not different between the biological agents and placebo.
However, it should be noted that there were some limitations in our study. First, a potential weakness of this meta-analysis was caused by the fact that the included trials were likely different in study design. For example, the studies by Sandborn et al[12,13] and Feagan et al[14] reported efficacy and safety results at week 6 while the others at week 8[9]. Patient characteristics such as previous treatments also varied slightly across studies. Second, the small sample size and lack of head-to-head trials may increase the uncertainty of the results. Finally, we could not assess the publication bias. Despite these limitations, we believe that our analysis could contribute to the evaluation of biological agents that might support clinical decision making.
In conclusion, the results of our meta-analysis suggested that all biological agents were superior to placebo in terms of clinical effects in both induction and maintenance phases. It was also showed that infliximab had a better clinical effect than the other biological agents. By analyzing the incidence of serious adverse events, it was found that biological agents had a similar safety profile to placebo. However, head-to-head comparisons, continuous data collection and benefit-risk assessment are needed to confirm our findings.
Ulcerative colitis (UC) is a form of chronic inflammatory bowel disease (IBD) characterized by recurrent rectal bleeding, increased stool frequency and urgency, abdominal cramps and pain, and systemic symptoms (such as fever, anemia and weight loss). Current options of treatment include aminosalicylates, corticosteroids, immunosuppressive medications and biological agents. However, conventional treatments often lead to a series of adverse events and have a limited effect in long-term disease control.
Biological agents include tumor necrosis factor-α (TNF-α) antibodies and integrin antagonists. Anti-TNF-α agents including infliximab, adalimumab and golimumab have been approved by United States Food and Drug Administration for the treatment of moderate-to-severe UC. All the 3 anti-TNF-α agents are demonstrated to be effective for the induction and maintenance of remission in moderate or severe UC. In addition, these agents can also induce mucosal healing and reduce glucocorticoid dependence. Vedolizumab is a humanized immunoglobulin G1 monoclonal antibody to α4β7 integrin. A phase 3 study investigating the efficacy and safety of vedolizumab in patients with moderate to severe active UC indicated that vedolizumab was significantly more effective in terms of clinical response and remission compared to placebo in both induction and maintenance phases.
Previous studies have shown that biological agents were effective in treatment of UC. However, the selection of biological agents in UC therapy was still controversial. Traditional methods cannot be applied for the comparison for lack of head-to-head studies comparing different biological agents. Therefore the authors used a mixed treatment comparison (MTC) to compare the efficacy of biological agents, as MTC was available for indirect comparisons between drugs with different comparators.
The study results suggest that all biological agents were superior to placebo for UC treatment in both induction and maintenance phases with a similar safety profile, and infliximab had a better clinical effect than the other biological agents.
UC is a form of chronic IBD characterized by recurrent rectal bleeding, increased stool frequency and urgency, abdominal cramps and pain, and systemic symptoms. Anti-TNF-α agents including infliximab, adalimumab and golimumab are monoclonal antibodies that bind to TNF-α with high affinity and specificity. Vedolizumab, a representative for integrin antagonists, is a humanized immunoglobulin G1 monoclonal antibody to α4β7 integrin.
This manuscript is a very interesting article.
P- Reviewer: Kawalec P S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Ma S
| 1. | Ochsenkühn T, D’Haens G. Current misunderstandings in the management of ulcerative colitis. Gut. 2011;60:1294-1299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 2. | Stange EF, Travis SP, Vermeire S, Reinisch W, Geboes K, Barakauskiene A, Feakins R, Fléjou JF, Herfarth H, Hommes DW. European evidence-based Consensus on the diagnosis and management of ulcerative colitis: Definitions and diagnosis. J Crohns Colitis. 2008;2:1-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 372] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 3. | Danese S, Fiorino G, Peyrin-Biroulet L, Lucenteforte E, Virgili G, Moja L, Bonovas S. Biological agents for moderately to severely active ulcerative colitis: a systematic review and network meta-analysis. Ann Intern Med. 2014;160:704-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 167] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 4. | Sonu I, Lin MV, Blonski W, Lichtenstein GR. Clinical pharmacology of 5-ASA compounds in inflammatory bowel disease. Gastroenterol Clin North Am. 2010;39:559-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Eugène C. Ulcerative colitis practice guidelines in adults. Clin Res Hepatol Gastroenterol. 2012;36:107-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Lichtenstein GR, Abreu MT, Cohen R, Tremaine W. American Gastroenterological Association Institute medical position statement on corticosteroids, immunomodulators, and infliximab in inflammatory bowel disease. Gastroenterology. 2006;130:935-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 179] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 7. | Samaan MA, Bagi P, Vande Casteele N, D’Haens GR, Levesque BG. An update on anti-TNF agents in ulcerative colitis. Gastroenterol Clin North Am. 2014;43:479-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Gilroy L, Allen PB. Is there a role for vedolizumab in the treatment of ulcerative colitis and Crohn’s disease? Clin Exp Gastroenterol. 2014;7:163-172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Induction and maintenance therapy with vedolizumab, a novel biologic therapy for ulcerative colitis. Gastroenterol Hepatol (N Y). 2014;10:64-66. [PubMed] |
| 10. | Mills EJ, Thorlund K, Ioannidis JP. Demystifying trial networks and network meta-analysis. BMJ. 2013;346:f2914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 456] [Cited by in RCA: 583] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 11. | Salanti G, Higgins JP, Ades AE, Ioannidis JP. Evaluation of networks of randomized trials. Stat Methods Med Res. 2008;17:279-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 872] [Cited by in RCA: 845] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 12. | Sandborn WJ, Feagan BG, Marano C, Zhang H, Strauss R, Johanns J, Adedokun OJ, Guzzo C, Colombel JF, Reinisch W. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2014;146:85-95; quiz e14-e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 563] [Cited by in RCA: 683] [Article Influence: 62.1] [Reference Citation Analysis (0)] |
| 13. | Sandborn WJ, Feagan BG, Marano C, Zhang H, Strauss R, Johanns J, Adedokun OJ, Guzzo C, Colombel JF, Reinisch W. Subcutaneous golimumab maintains clinical response in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2014;146:96-109.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 516] [Article Influence: 46.9] [Reference Citation Analysis (0)] |
| 14. | Feagan BG, Rutgeerts P, Sands BE, Hanauer S, Colombel JF, Sandborn WJ, Van Assche G, Axler J, Kim HJ, Danese S. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369:699-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1576] [Cited by in RCA: 1865] [Article Influence: 155.4] [Reference Citation Analysis (1)] |
| 15. | Callhoff J, Sieper J, Weiß A, Zink A, Listing J. Efficacy of TNFα blockers in patients with ankylosing spondylitis and non-radiographic axial spondyloarthritis: a meta-analysis. Ann Rheum Dis. 2015;74:1241-1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 156] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 16. | Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, Travers S, Rachmilewitz D, Hanauer SB, Lichtenstein GR. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462-2476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2744] [Cited by in RCA: 2884] [Article Influence: 144.2] [Reference Citation Analysis (2)] |
| 17. | Feagan BG, Reinisch W, Rutgeerts P, Sandborn WJ, Yan S, Eisenberg D, Bala M, Johanns J, Olson A, Hanauer SB. The effects of infliximab therapy on health-related quality of life in ulcerative colitis patients. Am J Gastroenterol. 2007;102:794-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 128] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 18. | Sandborn WJ, Rutgeerts P, Feagan BG, Reinisch W, Olson A, Johanns J, Lu J, Horgan K, Rachmilewitz D, Hanauer SB. Colectomy rate comparison after treatment of ulcerative colitis with placebo or infliximab. Gastroenterology. 2009;137:1250-1260; quiz 1520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 338] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 19. | Suzuki Y, Motoya S, Hanai H, Matsumoto T, Hibi T, Robinson AM, Mostafa NM, Chao J, Arora V, Camez A. Efficacy and safety of adalimumab in Japanese patients with moderately to severely active ulcerative colitis. J Gastroenterol. 2014;49:283-294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 137] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 20. | Sandborn WJ, van Assche G, Reinisch W, Colombel JF, D’Haens G, Wolf DC, Kron M, Tighe MB, Lazar A, Thakkar RB. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2012;142:257-265.e1-e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 817] [Cited by in RCA: 942] [Article Influence: 72.5] [Reference Citation Analysis (0)] |
| 21. | Reinisch W, Sandborn WJ, Hommes DW, D’Haens G, Hanauer S, Schreiber S, Panaccione R, Fedorak RN, Tighe MB, Huang B. Adalimumab for induction of clinical remission in moderately to severely active ulcerative colitis: results of a randomised controlled trial. Gut. 2011;60:780-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 588] [Cited by in RCA: 671] [Article Influence: 47.9] [Reference Citation Analysis (0)] |