Published online May 21, 2015. doi: 10.3748/wjg.v21.i19.6032
Peer-review started: September ??, 2014
First decision: September 30, 2014
Revised: October 1, 2014
Accepted: November 19, 2014
Article in press: November 19, 2014
Published online: May 21, 2015
Processing time: 258 Days and 22.2 Hours
AIM: To evaluate the efficacy and safety of endoscopic submucosal dissection (ESD) for early gastric cancer (EGC) with undifferentiated-type histology.
METHODS: A systematic literature review was conducted using the core databases. Complete resection, curative resection, en bloc resection, recurrence and adverse event rate were extracted and analyzed. A random effect model was applied. The methodological quality of the enrolled studies was assessed using the Newcastle-Ottawa Scale. Publication bias was evaluated using a funnel plot, the trim and fill method, Egger’s test, and a rank correlation test.
RESULTS: Fourteen retrospective studies between 2009 and 2014 were identified (972 EGC lesions with undifferentiated-type histology). The total en bloc and complete resection rates were estimated as 92.1% (95%CI: 87.4%-95.2%) and 77.5% (95%CI: 69.3%-84%), respectively. The total curative resection rate was 61.4% (95%CI: 44.5%-75.9%). The overall recurrence rate was 7.6% (95%CI: 3.4%-16%). Limited to histologically diagnosed expanded-criteria lesions, the en bloc and complete resection rates were 91.2% and 85.6%, respectively. The curative resection rate was 79.8%.
CONCLUSION: In this analysis, ESD is a technically feasible treatment modality for EGC with undifferentiated-type histology. Long-term studies are needed to confirm these therapeutic outcomes.
Core tip: Controversies regarding proposed expansions of the indication for endoscopic submucosal dissection (ESD) for early gastric cancer (EGC) with undifferentiated-type histology still remain. In this meta-analysis, ESD is a technically feasible treatment modality for EGC with undifferentiated-type histology. However, cautious interpretation is needed because of heterogeneity among studies. Inconsistent implementation of indication, insufficient follow-up duration, and different outcome criteria are causes of heterogeneity. Further studies using common primary outcomes or large-scale, long-term studies will elucidate the feasibility of ESD for EGC with undifferentiated-type histology.
- Citation: Bang CS, Baik GH, Shin IS, Kim JB, Suk KT, Yoon JH, Kim YS, Kim DJ, Shin WG, Kim KH, Kim HY, Lim H, Kang HS, Kim JH, Kim JB, Jung SW, Kae SH, Jang HJ, Choi MH. Endoscopic submucosal dissection for early gastric cancer with undifferentiated-type histology: A meta-analysis. World J Gastroenterol 2015; 21(19): 6032-6043
- URL: https://www.wjgnet.com/1007-9327/full/v21/i19/6032.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i19.6032
Gastric cancer is a prevalent malignancy in East Asian countries[1]. With the widespread implementation of endoscopic screening programs in these countries, the proportion of patients with early gastric cancer (EGC) at the time of diagnosis has been increasing. Currently, endoscopic submucosal dissection (ESD) is the widely accepted treatment modality for a specific subset of EGC patients in South Korea and Japan[2,3]. However, the absolute indications for ESD for EGC have been criticized, because very strict criteria result in unnecessary operations[4].
Based on previous research, which stratified the risk of lymph node metastasis in patients with EGC, an expanded set of indications was proposed[5-7]. The proposed expanded criteria include the following: (1) differentiated-type mucosal adenocarcinoma without ulceration and lymphovascular invasion, irrespective of size; (2) differentiated-type mucosal adenocarcinoma 30 mm or smaller with ulceration and without lymphovascular invasion; (3) undifferentiated-type mucosal adenocarcinoma 20 mm or smaller without ulceration and lymphovascular invasion; and (4) differentiated-type adenocarcinoma 30 mm or smaller with minute submucosal invasion (SM1), but without lymphovascular invasion[3]. However, the results of clinical observations based on these expanded criteria have been conflicting, and endoscopic resection based on these indications is regarded as an investigational treatment[3].
EGC with undifferentiated-type histology generally refers to a poorly differentiated adenocarcinoma or signet ring cell carcinoma, although there are no such criteria in the WHO classification[8]. This group of cancers is included in the expanded indications in the Japanese guidelines based on clinical observations[3,5,9,10]. However, the results of clinical studies, including studies on EGC with undifferentiated-type histology, are conflicting. Thus, a meta-analysis was conducted to assess the feasibility of ESD for EGC patients with undifferentiated-type histology based on the expanded criteria.
MEDLINE (through PubMed), EMBASE, and the Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library were searched using common keywords related to ESD for EGC with undifferentiated-type histology (from inception to April 2014). Medical Subject Headings (MeSH) terminology was used because all 3 databases permit searching using MeSH terminology. The keywords used included “gastric cancer”, “endoscopic submucosal dissection”, “ESD”, “poorly differentiated”, “signet ring cell carcinoma” or “undifferentiated” using Boolean operators. Only publications on human subjects were searched, and the bibliographies of relevant articles were also reviewed to identify additional studies. The language of publication was not restricted.
Due to a lack of randomized-controlled studies relevant to this topic, we included non-randomized studies meeting both of the following criteria: (1) designed to evaluate ESD for EGC with undifferentiated-type histology in the target or control group; and (2) included at least one outcome (complete resection rate, curative resection rate, en bloc resection rate, recurrence rate or procedure-related adverse event rate) that enabled an evaluation of feasibility of ESD for EGC with undifferentiated-type histology. The exclusion criteria were as follows: (1) incomplete data; (2) review article; or (3) abstract only (study not published as full-text article).
Two of the authors (Bang CS and Baik GH) independently evaluated the eligibility of all studies retrieved from the databases based on the predetermined selection criteria. The abstracts of all identified studies were reviewed to exclude irrelevant articles. Full-text reviews were performed to determine whether the inclusion criteria were satisfied by the remaining studies. Disagreements between the two evaluators were resolved by discussion or consultation with a third author (Kim JH).
The methodological quality of the enrolled studies was assessed using the Newcastle-Ottawa Scale. This tool comprises three parameters: the selection of the study population, the comparability of the groups, and the ascertainment of the exposure or outcome. Each parameter consists of subcategorized questions: selection (n = 4), comparability (n = 1), and exposure or outcome (n = 3)[11,12]. The stars awarded for each item allow for a rapid visual assessment of the methodological quality of the studies. A study can be awarded a maximum of nine stars, indicating the highest quality. Two of the authors (Bang CS and Baik GH) independently evaluated the methodological quality of all the studies, and disagreements between the two evaluators were resolved by discussion or consultation with a third author (Kim DJ).
Two of the authors (Bang CS and Baik GH) independently extracted the outcomes of all the studies, and disagreements between the two evaluators were resolved by discussion or consultation with a third author (Kim JH). The primary outcomes were as follows: (1) en bloc resection rate: the proportion of cancers removed as a single piece without fragmentation: (2) complete resection rate: the proportion of cancer with no neoplastic components at the lateral or vertical margins on microscopic analysis, and without lymphovascular invasion; (3) curative resection rate: the proportion of cancers with 20 mm or less of intramucosal cancer without ulceration, without neoplastic components at the lateral or vertical margins, and without lymphovascular invasion; (4) recurrence rate: the proportion of cancers that reappeared at the site of the lesion (local recurrence) or synchronous, metachronous, or distant metastatic lesions, and (5) ESD adverse event rate: the proportion of cancers whose treatment resulted in procedure-related gastric hemorrhage or perforation. We also performed sensitivity analyses based on the indications for ESD (expanded vs beyond-expanded indication) and follow-up duration (long-term vs short-term follow-up). Both a cumulative analysis and a one-study-removed analysis were also performed.
Comprehensive Meta-Analysis (CMA) software (version 2.2.064, Borenstein M, Hedges L, Higgins J and Rothstein H. Englewood, NJ: Biostat; United States) was used for this meta-analysis. We calculated the pooled en bloc resection, complete resection, curative resection, recurrence and adverse event rates divided by gastric hemorrhage and perforation. To compare the efficacy of ESD according to treatment criteria (expanded vs beyond-expanded criteria), we calculated odds ratios (ORs) with 95% confidence intervals (CIs) using 2 × 2 tables from the original articles. Heterogeneity was tested using the I2 test, which measures the percentage of total variation across studies[13]. I2 was calculated as follows: I2 (%) = 100 × (Q-df)/Q, where Q is Cochrane’s heterogeneity statistic and df is the degrees of freedom. Negative values for I2 were set to zero, and an I2 value over 50% was considered to indicate substantial heterogeneity (range: 0%-100%)[14]. Pooled effect sizes with 95%CIs were calculated using a random effects model and the DerSimonian and Laird method due to methodological heterogeneity[15]. These results were confirmed again by the I2 test. A fixed effects model using the inverse variance-weighted (Woolf’s) method was used in the sensitivity analyses, including the cumulative and one-study-removed analyses, based on the assumption of a common effect size shared by the subgrouped studies[16,17]. Significance was set at P = 0.05 in both models. Publication bias was evaluated using Begg’s funnel plot, Egger’s test of the intercept, Duval and Tweedie’s trim and fill, and Begg and Mazumdar’s rank correlation test[18-22].
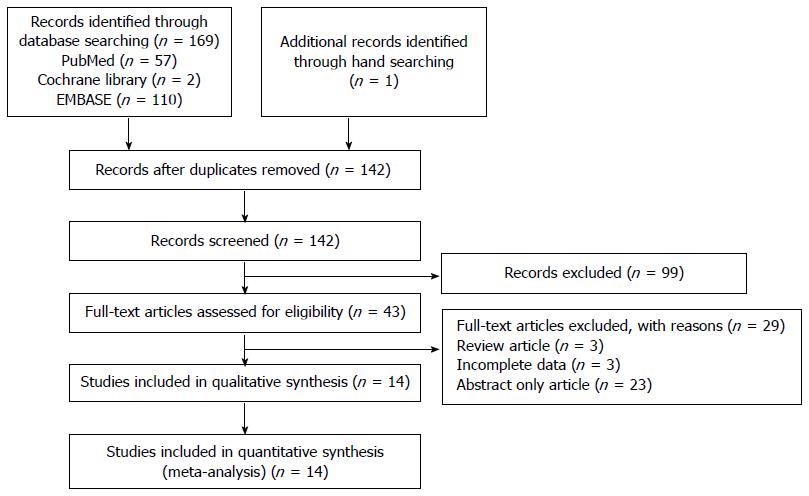
Figure 1 shows a flow diagram of how relevant studies were identified. A total of 170 articles were identified by a search of 3 core databases and a manual search of relevant bibliographies. In all, 28 duplicate studies and an additional 99 studies were excluded during the initial screening through a review of the titles and abstracts. The full texts of the remaining 43 studies were thoroughly reviewed. Among these studies, 29 were excluded from the final analysis. The reasons for study exclusion during the final review were as follows: review articles (n = 3), incomplete data (n = 3), or abstract only articles (n = 23). The remaining 14 non-randomized studies were included in the final analysis.
Among the 14 studies[23-36], we identified a total of 972 patients with EGC with undifferentiated-type histology. The clinical characteristics of the enrolled studies are shown in Tables 1-2 and Table 3. The enrolled studies were published between 2009 and 2014. All of the studies were conducted in Asia (10 studies in South Korea and 4 studies in Japan). Two studies were conducted in a multicenter setting[28,33], whereas the remaining studies were conducted in a single center setting. Twelve English and 2 Korean studies were selected. The duration of follow-up ranged from a median of 13.5 mo in one study to a mean of 101.9 mo in another. Ten studies reported en bloc resection rates, and 9 studies reported complete resection rates. Curative resection and recurrence rates were reported in 6 studies. The procedure-related adverse events included hemorrhage in 10 studies and perforation in 9 studies (Table 1).
| Ref. | Duration of follow up | Location, language | Complete resection | Curative resection | En bloc resection | Total recurrence | Adverse events | Total patients |
| Kim et al[23], 2009 | Mean 17.1 ± 9.1 mo | South Korea (English) | 1/32 | Bleeding 6/32 | 32 | |||
| Kang et al[24], 2010 | Mean 16 mo | South Korea (English) | 33/60 | 60/60 | 0/60 | Bleeding 1/60 perforation 1/60 | 60 | |
| Lee et al[25], 2010 | Median 13.5 mo | South Korea (English) | 22/581 | 48/58 | 0/16 (3 yr) 0/2 (5 yr) | 58 | ||
| Yamamoto et al[26], 2010 | Japan (English) | 46/58 | 57/58 | Bleeding 5/58 perforation 2/58 | 58 | |||
| Goh et al[27], 2011 | Mean 19.39 ± 11.2 mo | South Korea (English) | 10/18 | 3/14 | 18 | |||
| Park et al[28], 2012 | Mean 41 mo | South Korea, multicenter (2) (English) | 23/55 | 55 | ||||
| Kamada et al[29], 2012 | Mean 3.8 yr | Japan (English) | 37/46 | 42/46 | 1/462 | Bleeding 2/46 perforation 2/46 | 46 | |
| Okada et al[30], 2012 | Median 36 mo | Japan (English) | 85/103 | 102/103 | Bleeding 9/101 perforation 1/101 | 103 | ||
| Park et al[31], 2013 | Median 24.1 mo (absolute indication group), 30 mo (expanded indication group) | South Korea (English) | 91/116 | 106/116 | Bleeding 7/116 perforation 6/116 | 116 | ||
| Choi et al[32], 2013 | Mean 37.4 mo | South Korea (Korean) | 66/82 | 72/82 | 3/823 | 82 | ||
| Kim et al[33], 2013 | Median 34 mo | South Korea, multicenter (6) (English) | 54/74 | 23/74 | 67/74 | 4/74 | Bleeding 1/74 perforation 3/74 | 74 |
| Abe et al[34], 2013 | Median 76.4 mo | Japan (English) | 88/97 | 62/97 | 96/97 | 2/794 | Bleeding 4/97 perforation 3/97 delayed perforation 1/97 | 97 |
| Chung et al[35], 2014 | Mean 41.7 ± 22.6 mo | South Korea (Korean) | 58/76 | 64/76 | 9/64 | Bleeding 4/76 perforation 0/76 | 76 | |
| Oka et al[36], 2014 | Mean 101.9 ± 38.9 mo | Japan (English) | 86/97 | 60/97 | Bleeding 6/97 perforation 1/97 | 97 |
| Ref. | Complete resection | Curative resection | En bloc resection | Total recurrence | Total patients | Adverse events |
| Kang et al[24], 2010 | 17/18 | 18 | ||||
| Lee et al[25], 2010 | 11/17 | 17 | ||||
| Yamamoto et al[26], 2010 | 46/471 | 46/47 | 47/47 | 47 | Bleeding 5/47 perforation 2/47 | |
| Park et al[28], 2012 | 23/55 | 55 | ||||
| Kamada et al[29], 2012 | 32/34 | 34 | ||||
| Okada et al[30], 2012 | 85/103 | 102/103 | 103 | |||
| Park et al[31], 2013 | 91/116 | 106/116 | 116 | Bleeding 7/116 perforation 6/116 | ||
| Choi et al[32], 2013 | 66/82 | 72/82 | 82 | |||
| Kim et al[33], 2013 | 23/29 | 23/29 | 25/29 | 29 | Bleeding 0/29 perforation 2/29 | |
| Chung et al[35], 2014 | 50/58 | 51/58 | 58 | Bleeding 4/76 perforation 0/76 | ||
| Oka et al[36], 2014 | 57/60 | 60 |
| Ref. | Location, language | Complete resection | En bloc resection | Recurrence | |||||
| EI effective | EI total | Beyond EI effective | Beyond EI total | EI | Beyond EI | EI | Beyond EI | ||
| Kang et al[24], 2010 | South Korea (English) | 17 | 18 | 16 | 42 | 18/18 | 42/42 | 0/18 | 0/42 |
| Lee et al[25], 2010 | South Korea (English) | 11 | 17 | 11 | 30 | ||||
| Yamamoto et al[26] 2010 | Japan (English) | 46 | 47 | 6 | 11 | 47/47 | 10/11 | ||
| Kamada et al[29], 2012 | Japan (English) | 32 | 34 | 7 | 12 | 0/34 | 1/12 | ||
| Kim et al[33], 2013 | South Korea, multicenter (6) (English) | 223 | 29 | 31 | 45 | 0/29 | 4/45 | ||
| Chung et al[35], 2014 | South Korea (Korean) | 50 | 58 | 8 | 18 | 51/58 | 13/18 | ||
| Oka et al[36], 2014 | Japan (English) | 57 | 60 | 29 | 37 | ||||
In the evaluation of ESD based on the expanded criteria, we identified a total of 619 EGC patients with undifferentiated-type histology (11 studies). The clinical characteristics of the included studies are shown in Table 2.
For a comparison of ESD based on the expanded vs beyond expanded criteria, we identified a total of 458 EGC patients (263 patients satisfying the expanded criteria vs 195 patients satisfying the beyond expanded criteria) with undifferentiated-type histology (7 studies). The clinical characteristics of the included studies are shown in Table 3.
In terms of the methodological quality, the mean value of the awarded star was 7.6 [6 stars (1 study), 7 stars (5 studies), 8 stars (7 studies), and 9 stars (1 study) (Table 4)]. The majority of studies were classified as high quality, thus sensitivity analysis based on the methodological quality was not performed.
| Ref. | Selection | Comparability | Exposure or outcome | Total |
| Kim et al[23], 2009 | 4 | 1 | 2 | 7 |
| Kang et al[24], 2010 | 4 | 1 | 2 | 7 |
| Lee et al[25], 2010 | 4 | 2 | 2 | 8 |
| Yamamoto et al[26], 2010 | 4 | 1 | 3 | 8 |
| Goh et al[27], 2011 | 3 | 1 | 2 | 6 |
| Park et al[28], 2012 | 3 | 1 | 3 | 7 |
| Kamada et al[29], 2012 | 4 | 1 | 3 | 8 |
| Okada et al[30], 2012 | 4 | 3 | 7 | |
| Park et al[31], 2013 | 4 | 2 | 2 | 8 |
| Choi et al[32], 2013 | 4 | 2 | 3 | 9 |
| Kim et al[33], 2013 | 4 | 1 | 3 | 8 |
| Abe et al[34], 2013 | 4 | 1 | 3 | 8 |
| Chung et al[35], 2014 | 3 | 2 | 3 | 8 |
| Oka et al[36], 2014 | 3 | 1 | 3 | 7 |
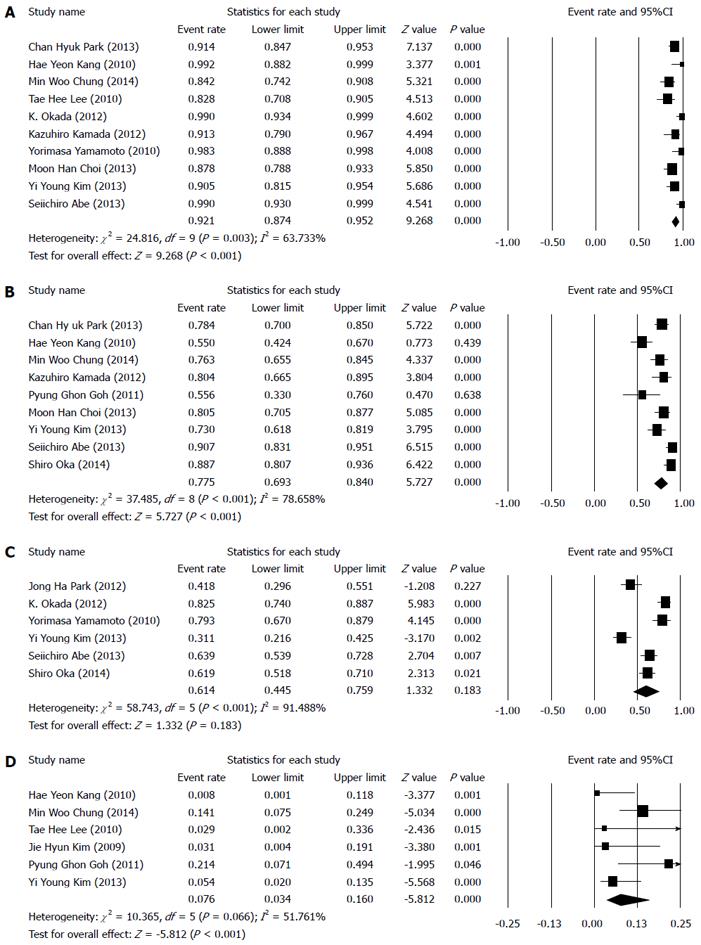
The overall efficacy of ESD for EGC with undifferentiated-type histology was evaluated using the en bloc resection, complete resection, curative resection, and recurrence rates. The total en bloc resection and complete resection rates were estimated as 92.1% (95%CI: 87.4%-95.2%, P < 0.001) and 77.5% (95%CI: 69.3%-84%, P < 0.001), respectively (Figure 2A, B).
The total curative resection rate was 61.4% (95%CI: 44.5%-75.9%, P = 0.183) (Figure 2C), and the overall recurrence rate was 7.6% (95%CI: 3.4%-16%, P < 0.001) (Figure 2D).
The overall safety of ESD for EGC with undifferentiated-type histology was evaluated according to procedure-related adverse events divided by gastric hemorrhage and perforation. The total procedure-related gastric hemorrhage and perforation rates were estimated as 6.5% (95%CI: 4.5%-9.4%, P < 0.001) and 3.3% (95%CI: 2.1%-5.0%, P < 0.001), respectively.
In the histologically diagnosed expanded-criteria lesions, the overall efficacy of ESD for EGC with undifferentiated-type histology was evaluated using the en bloc resection, complete resection, and curative resection rates. The total en bloc resection and complete resection rates were estimated as 91.2% (95%CI: 85.3%-94.8%, P < 0.001) and 85.6% (95%CI: 78.5%-90.7%, P < 0.001), respectively. The total curative resection rate was 79.8% (95%CI: 51.4%-93.6%, P = 0.041).
The overall safety of ESD for EGC with undifferentiated-type histology based on expanded criteria was evaluated according to procedure-related adverse events divided by gastric hemorrhage and perforation. The total procedure-related gastric hemorrhage and perforation rates were estimated as 6.7% (95%CI: 4.1%-10.8%, P < 0.001) and 4.8% (95%CI: 2.6%-8.6%, P < 0.001), respectively.
To compare the efficacy of ESD between the expanded and beyond expanded criteria, ORs with 95%CIs for en bloc resection, complete resection, and recurrence were calculated. ESD based on the expanded criteria showed an OR of 3.475 (95%CI: 1.039-11.622, P = 0.043) for en bloc resection compared to ESD based on the beyond expanded criteria. ESD based on the expanded criteria showed an OR of 7.461 (95%CI: 3.027-18.394, P < 0.001) for complete resection compared to ESD based on the beyond expanded criteria. ESD based on the expanded criteria showed an OR of 0.134 (95%CI: 0.015-1.203, P = 0.073) for recurrence compared to ESD based on the beyond expanded criteria.
The cumulative meta-analysis of the enrolled studies in the order of year published showed a constant but slightly increasing trend in en bloc resection rate. With regard to complete resection, the cumulative meta-analysis of the enrolled studies showed an increasing trend in complete resection rate. However, the curative resection rate showed a decreasing trend in the cumulative meta-analysis. In the evaluation of recurrence, the cumulative meta-analysis showed an increasing trend in the recurrence rate after ESD.
In the histologically diagnosed expanded-criteria lesions, the cumulative meta-analysis of the enrolled studies in the order of year published showed a constant but slightly decreasing trend in the en bloc resection rate. As for complete resection, the cumulative meta-analysis showed 2 outlier effect sizes[24,25]. Kang et al[24] showed the biggest effect size (complete resection rate: 17/18), and Lee et al[25] showed the smallest effect size (complete resection rate: 11/17). These 2 studies included the smallest numbers of patients of histologically diagnosed expanded-criteria lesions in the analysis (Table 2), whereas the other remaining studies showed relatively consistent effect sizes. The curative resection rate showed a decreasing trend in the cumulative meta-analysis of the enrolled studies.
The one-study-removed meta-analysis of the enrolled studies in the order of year published showed consistent results and no specific outlier for the en bloc resection rate. As for complete resection, the one-study-removed meta-analysis also showed consistent results. For the analysis of curative resection, the one-study-removed meta-analysis highlighted 2 influential studies[28,33]. These studies reported relatively lower curative resection rates; however, the methodological quality of these studies was not low (Table 4). Moreover, these 2 studies were performed in a multicenter setting. In the evaluation of recurrence, the one-study-removed analysis identified 3 influential studies[27,33,35]. Two studies[27,35] reported relatively higher recurrence rates, and 1 study[33] reported a relatively lower recurrence rate (Table 1). The follow-up duration was relatively short in the Goh et al[27]’s study (mean 19.39 ± 11.2 mo), and this study had the lowest methodological quality among the included studies (Tables 1 and 4). However, the follow-up durations in the studies by Chung et al[35] and Kim et al[33] were not in the short-term category (mean: 41.7 ± 22.6 mo), and the methodological quality was not low (Tables 1 and 4).
In the histologically diagnosed expanded-criteria lesions, the one-study-removed meta-analysis of the enrolled studies in the order of year published showed consistent results and no specific outlier for the en bloc resection rate. As for complete resection, the cumulative meta-analysis showed consistent results. For the analysis of curative resection, the one-study-removed meta-analysis identified 2 influential studies[28,30]. As in the total population analysis, Park et al[28] reported a relatively lower curative resection rate, whereas Okada et al[30] reported a relatively higher curative resection rate. The methodological quality of these studies was not low (Table 4).
To determine the total recurrence rate, a sensitivity analysis was performed by dividing the studies into a shorter follow-up duration group and a longer follow-up duration group. The distribution of the follow-up duration was as follows: mean 13.5, 16, 17.1, 19.39 mo, median 34 mo, and mean 41.7 mo. The studies with the former 4 follow-up duration times[23-25,27] were categorized into the shorter duration group, and the studies with the latter 2 follow-up duration times[33,35] were sorted into the longer duration group. An analysis of the studies in the shorter follow-up duration group showed a recurrence rate of 8.1% (95%CI: 0.033-0.185, P < 0.001). However, an analysis of the studies in the longer follow-up duration group showed a recurrence rate of 10.4% (95%CI: 0.061-0.171, P < 0.001).
A funnel plot for the enrolled studies is presented. For the studies of en bloc resection rate, the funnel plot is asymmetrical. Egger’s regression test showed that the intercept was 3.334 [95%CI: 2.066-4.602, t-value: 6.064, df = 8, P < 0.001 (1-tailed) and P < 0.001 (2-tailed)]. A trim and fill analysis showed that 3 studies were missed or trimmed. The rank correlation test showed a Kendall’s tau of 0.444 with a continuity correction [P = 0.037 (1-tailed) and P = 0.074 (2-tailed)].
For the studies of complete resection rate, the funnel plot is symmetrical. Egger’s regression test showed that the intercept was 1.452 [95%CI: -7.706-10.611, t-value: 0.375, df = 7, P = 0.359 (1-tailed) and P = 0.719 (2-tailed)]. A trim and fill analysis showed that no study was missed or trimmed. The rank correlation test showed a Kendall’s tau of 0.250 with a continuity correction [P = 0.174 (1-tailed) and P = 0.348 (2-tailed)].
For the studies of curative resection rate, the funnel plot is symmetrical. Egger’s regression test showed that the intercept was 2.878 [95%CI: -26.503-32.259, t-value: 0.272, df = 4, P = 0.400 (1-tailed) and P = 0.799 (2-tailed)]. A trim and fill analysis showed that 1 study was missed or trimmed. The rank correlation test showed a Kendall’s tau of 0.133 with a continuity correction (P = 0.354 (1-tailed) and P = 0.707 (2-tailed)).
For the studies of recurrence rate, the funnel plot is symmetrical. Egger’s regression test showed that the intercept was -1.964 [95%CI: -4.832-0.905, t-value: 1.901, df = 4, P = 0.065 (1-tailed) and P = 0.130 (2-tailed)]. A trim and fill analysis showed that no study was missed or trimmed. The rank correlation test showed a Kendall’s tau of -0.267 with a continuity correction [P = 0.226 (1-tailed) and P = 0.452 (2-tailed)].
For the studies of en bloc resection rate for EGC with expanded criteria, the funnel plot is asymmetrical. Egger’s regression test showed that the intercept was 2.437 [95%CI: -0.299-5.173, t-value: 2.473, df = 4, P = 0.034 (1-tailed) and P = 0.069 (2-tailed)]. A trim and fill analysis showed that 1 study was missed or trimmed. The rank correlation test showed a Kendall’s tau of 0.267 with a continuity correction [P = 0.226 (1-tailed) and P = 0.452 (2-tailed)].
For the studies of complete resection rate for EGC with expanded criteria, the funnel plot is asymmetrical. Egger’s regression test showed that the intercept was 2.340 [95%CI: 0.164-4.515, t-value: 2.543, df = 7, P = 0.019 (1-tailed) and P = 0.039 (2-tailed)]. A trim and fill analysis showed that 3 studies were missed or trimmed. The rank correlation test showed a Kendall’s tau of 0.417 with a continuity correction [P = 0.059 (1-tailed) and P = 0.118 (2-tailed)].
For the studies of curative resection rate for EGC with expanded criteria, the funnel plot is asymmetrical. Egger’s regression test showed that the intercept was 3.814 [95%CI: -15.749-23.376, t-value: 0.839, df = 4, P = 0.245 (1-tailed) and P = 0.490 (2-tailed)]. A trim and fill analysis showed that 1 study was missed or trimmed. The rank correlation test showed a Kendall’s tau of 0.000 with a continuity correction [P = 0.500 (1-tailed) and P > 0.999 (2-tailed)].
For the studies of complete resection rate by expanded criteria (vs beyond-expanded criteria), the funnel plot is asymmetrical. Egger’s regression test showed that the intercept was 4.188 [95%CI: 0.779-7.598, t-value: 3.411, df = 4, P = 0.014 (1-tailed) and P = 0.027 (2-tailed)]. A trim and fill analysis showed that 3 studies were missed or trimmed. The rank correlation test showed a Kendall’s tau of 0.800 with a continuity correction [P = 0.012 (1-tailed) and P = 0.024 (2-tailed)].
Overall, publication bias was detected in the analysis of en bloc resection rate for total EGC lesions. However, there was no evidence of publication bias in the analysis of total lesions, except for en bloc resection rate. In the histologically diagnosed expanded-criteria lesions, publication bias was detected in all of the analyses (en bloc, complete, and curative resection rates). The comparison of complete resection rate divided by expanded and beyond expanded criteria showed publication bias.
In this meta-analysis, ESD is a technically feasible treatment modality for the treatment of EGC with undifferentiated-type histology. The overall en bloc resection rate was 92.1%, and the overall complete resection rate was 77.5%. If limited to histologically diagnosed expanded criteria lesions, the overall complete resection rate increased (85.6%). In terms of the procedure-related adverse events, the reported gastric hemorrhage or perforation rate for the treatment of EGC with undifferentiated-type histology was not different from the rates reported in previous studies including intestinal type EGC[37]. This finding was confirmed again in the sensitivity analyses. The cumulative meta-analysis of the total en bloc and complete resection rates showed a recent increasing trend. The advancement of ESD instruments and technique seem to be the cause of technical feasibility for EGC with undifferentiated-type histology.
However, the therapeutic outcomes are not totally satisfactory. The overall curative resection rate was 61.4%, although it increased to 79.8% if limited to histologically diagnosed expanded-criteria lesions. This finding was confirmed again in the sensitivity analyses. The one-study-removed meta-analysis showed that multicenter studies[28,33] reported relatively lower curative resection rates. Additionally, the overall recurrence rate was 7.6%, which is slightly higher than that of previous studies[35,38-40]. Some studies concluded that ESD for EGC with undifferentiated-type histology is a feasible treatment modality despite relatively lower complete or curative resection rates and a higher recurrence rate compared to other studies. However, there is no acceptable complete or curative resection rate standard for determining the feasibility of ESD. Moreover, all of the enrolled studies were performed retrospectively. Thus, selection bias could influence the therapeutic outcomes of ESD.
To obtain higher complete and curative resection rates based on this meta-analysis, performing ESD according to the expanded criteria rather than the beyond expanded criteria seems to be the appropriate approach for the treatment of undifferentiated-type EGC. However, the expanded criteria were developed based on retrospective studies of surgically treated EGC patients[3,5,7]. Discrepancies between pre- and post-ESD indication or pre- and post-ESD histology have been also reported[24,25,41]. ESD performed based on the expanded criteria could be found to have been based on the beyond expanded criteria after the procedure. A more serious problem is the difficulty in determining tumor extent and depth of invasion of EGC with undifferentiated-type histology. EGC with undifferentiated-type histology is known to extend laterally along the proliferative zone in the intermediate layer of mucosa and the development pattern from the intermediate layer type to the superficial type makes non-exposure to the surface mucosa[42]. The accuracy of EUS in the assessment of depth of invasion for EGC with undifferentiated-type histology is known to be declining compared to intestinal type EGC[43]. Accurately defining tumor extent and depth of invasion could be difficult for EGC with undifferentiated-type histology.
Another issue is the histologic heterogeneity (mixture of undifferentiated components with differentiated EGC). Neither the characteristics nor feasibility of ESD for this type of EGC have been settled[44-46]. As previously mentioned, the discrepancy between pre- and post-ESD histology could be a problem in the assessment of surgery indications or the risk of lymph node metastasis.
The majority of studies on EGC with undifferentiated-type histology do not report outcomes according to whether the tumors are signet ring cell carcinoma or poorly differentiated adenocarcinoma. Only 2 studies among the included articles performed separate analyses[23,32]. These studies commonly reported slightly better therapeutic outcomes (complete resection or en bloc resection rates) in signet ring cell carcinoma than in poorly differentiated adenocarcinoma or poorly differentiated adenocarcinoma with signet ring cell features, although the differences were statistically insignificant. However, more studies are needed to confirm these findings.
In addition to the results of this study, there is a fundamental criticism about the expanded criteria for ESD. As previously mentioned, there is a discrepancy in the term “EGC with undifferentiated-type histology” between the WHO classifications and the Japanese literature[3,8]. There is no such term in the WHO classification system. However, the term “undifferentiated-type EGC” is frequently used, and studies using this terminology are being published. Moreover, in terms of the therapeutic outcomes, curative resection was generally accepted as being complete resection satisfying the expanded criteria proposed by Japanese groups in the literature. However, analyzing results based on this definition could lead to misinterpretations. Curative resection implies neither a cure nor a low risk of recurrence. Furthermore, the expanded indication was developed based on a retrospective analysis of surgically resected EGCs. Regarding EGC tumor size in the expanded indication, some data on lymph node metastasis that are not completely consistent with Japanese studies have been reported[47-49]. Despite the criticism of the expanded indication for ESD to treat EGC, nearly all of the studies are being performed based on the definition proposed by Japanese groups. To solve these fundamental problems related to the indication for ESD, randomized or well-organized large-scale studies separately focused on size, depth of invasion, histologic type, and lymph node metastasis are needed.
This study is the first meta-analysis of the therapeutic outcomes of ESD for EGC with undifferentiated-type histology. A strength of this study is the rigorous search of the literature, which was not limited by language, although data from Western studies were lacking. Potential modifiers were detected when possible, and sensitivity analyses were performed to confirm the robustness of the results.
Despite these strengths, there are several limitations of the present study. First, there are no data on lymph node metastasis. Lymph node metastasis is one of the most important prognostic factors for EGC patients. The rate of lymph node metastasis is known to be approximately 2% in mucosal cancers and approximately 20% in submucosal invasive cancers[5,50]. The risk of lymph node metastasis is known to be higher in EGC with undifferentiated-type histology compared to intestinal type EGC due to lymphovascular invasion, which was estimated to be 14% in a study of post-gastrectomy patients[51]. Moreover, micrometastasis which is associated with worse disease-free survival was reported as 13.3% in the EGC with undifferentiated-type histology[52]. However, there is no definitive method to detect lymph node metastasis accurately before surgery. Second, there was substantial methodological heterogeneity between the included studies, which potentially affected the effect size estimates. The most noticeable modifier was the heterogeneity in the reported outcomes and the inconsistent implementation of the indication. The reported outcomes for the en bloc resection, complete resection (R0 resection), curative resection, en bloc complete resection, and recurrence, rates were various and not consistent between the enrolled studies. Moreover, the outcomes were not defined in detail. For example, the recurrence rate was not divided according to local recurrence, synchronous or metachronous recurrence. The indication was also inconsistently implemented; thus, the beyond-expanded criteria were used for a substantial portion of the patients, despite the discrepancies between the pre- and post-ESD indications. The follow-up duration was another significant modifier. The sensitivity analysis of longer follow-up duration studies showed that the recurrence rate was higher than in shorter follow-up duration studies. These limitations are sources of heterogeneity and contributed to publication bias. Due to the lack of prospective or randomized studies on this topic, large-scale, well-organized, long-term follow-up studies are needed to elucidate the feasibility of ESD on EGC with undifferentiated-type histology. Prospective clinical trial by Japan Clinical Oncology Group completed recruiting patients with EGC with undifferentiated-type histology and outcomes are anticipated[53].
Based on this analysis, ESD is a technically feasible treatment modality for EGC with undifferentiated-type histology. However, cautious interpretation is needed because of heterogeneity among studies. Inconsistent implementation of the indication, insufficient follow-up duration, and differences in outcome measures are causes of heterogeneity. Further studies using common primary outcomes or large-scale, long-term studies will determine the feasibility of ESD for EGC with undifferentiated-type histology.
Endoscopic submucosal dissection (ESD) is the widely accepted treatment modality for a specific subset of early gastric cancer (EGC) patients in gastric cancer prevalent Asian countries. EGC with undifferentiated-type histology generally refers to a poorly differentiated adenocarcinoma or signet ring cell carcinoma, although there are no such criteria in the WHO classification. This group of cancers is included in the expanded indications in the Japanese guidelines based on clinical observations. However, the results of clinical studies, including studies on EGC with undifferentiated-type histology, are conflicting.
Some studies concluded that ESD for EGC with undifferentiated-type histology is a feasible treatment modality despite relatively lower complete or curative resection rates and a higher recurrence rate compared to other studies. However, there is no acceptable complete or curative resection rate standard for determining the feasibility of ESD. Moreover, all of the enrolled studies were performed retrospectively. Thus, selection bias could influence the therapeutic outcomes of ESD.
From the fourteen retrospective studies, therapeutic outcomes were calculated. The total en bloc and complete resection rates were estimated as 92.1% (95%CI: 87.4%-95.2%) and 77.5% (95%CI: 69.3%-84%), respectively. The total curative resection rate was 61.4% (95%CI: 44.5%-75.9%). The overall recurrence rate was 7.6% (95%CI: 3.4%-16%). Limited to histologically diagnosed expanded-criteria lesions, the en bloc and complete resection rates were 91.2% and 85.6%, respectively. The curative resection rate was 79.8%.
In this analysis, ESD is a technically feasible treatment modality for EGC with undifferentiated-type histology. However, cautious interpretation is needed because of heterogeneity among studies. Inconsistent implementation of indication, insufficient follow-up duration, and different outcome criteria are causes of heterogeneity. Further studies using common primary outcomes or large-scale, long-term studies will elucidate the feasibility of ESD for EGC with undifferentiated-type histology.
EGC: EGC is defined as gastric cancer that invades no more deeply than the submucosa, irrespective of lymph node metastasis. ESD: ESD has been developed for en bloc removal of large (usually more than 2 cm), flat GI tract lesions using specialized endoscopic knife to dissect lesions from the submucosa. It offers the potential to remove mucosal and submucosal tumors en bloc.
Authors used appropriate methods of analysis and elaborated this interesting meta-analysis on endoscopic submucosal dissection in the treatment of EGC with undifferentiated-type histology.
P- Reviewer: Fassan M, Fujiwara T, Nishida T S- Editor: Ma YJ L- Editor: A E- Editor: Zhang DN
| 1. | Leung WK, Wu MS, Kakugawa Y, Kim JJ, Yeoh KG, Goh KL, Wu KC, Wu DC, Sollano J, Kachintorn U. Screening for gastric cancer in Asia: current evidence and practice. Lancet Oncol. 2008;9:279-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 578] [Cited by in RCA: 648] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 2. | Lee JH, Kim JG, Jung HK, Kim JH, Jeong WK, Jeon TJ, Kim JM, Kim YI, Ryu KW, Kong SH. [Synopsis on clinical practice guideline of gastric cancer in Korea: an evidence-based approach]. Korean J Gastroenterol. 2014;63:66-81. [PubMed] |
| 3. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011;14:113-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1723] [Cited by in RCA: 1897] [Article Influence: 135.5] [Reference Citation Analysis (0)] |
| 4. | Gotoda T. Endoscopic resection of early gastric cancer. Gastric Cancer. 2007;10:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 495] [Article Influence: 27.5] [Reference Citation Analysis (1)] |
| 5. | Gotoda T, Yanagisawa A, Sasako M, Ono H, Nakanishi Y, Shimoda T, Kato Y. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer. 2000;3:219-225. [PubMed] |
| 6. | Soetikno R, Kaltenbach T, Yeh R, Gotoda T. Endoscopic mucosal resection for early cancers of the upper gastrointestinal tract. J Clin Oncol. 2005;23:4490-4498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 395] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 7. | Hirasawa T, Gotoda T, Miyata S, Kato Y, Shimoda T, Taniguchi H, Fujisaki J, Sano T, Yamaguchi T. Incidence of lymph node metastasis and the feasibility of endoscopic resection for undifferentiated-type early gastric cancer. Gastric Cancer. 2009;12:148-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 368] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 8. | The International Agency for Research on Cancer; Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO Classification of Tumours of the Digestive System (IARC WHO Classification of Tumours). 4th ed. Publisher: World Health Organization 2010; 418. |
| 9. | Abe N, Watanabe T, Sugiyama M, Yanagida O, Masaki T, Mori T, Atomi Y. Endoscopic treatment or surgery for undifferentiated early gastric cancer? Am J Surg. 2004;188:181-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 67] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 10. | Abe N, Watanabe T, Suzuki K, Machida H, Toda H, Nakaya Y, Masaki T, Mori T, Sugiyama M, Atomi Y. Risk factors predictive of lymph node metastasis in depressed early gastric cancer. Am J Surg. 2002;183:168-172. [PubMed] |
| 11. | Deeks JJ, Dinnes J, D’Amico R, Sowden AJ, Sakarovitch C, Song F, Petticrew M, Altman DG. Evaluating non-randomised intervention studies. Health Technol Assess. 2003;7:iii-ix, iii-ix. [PubMed] |
| 12. | Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8858] [Cited by in RCA: 12667] [Article Influence: 844.5] [Reference Citation Analysis (0)] |
| 13. | Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21630] [Cited by in RCA: 25813] [Article Influence: 1122.3] [Reference Citation Analysis (0)] |
| 14. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39087] [Cited by in RCA: 46547] [Article Influence: 2115.8] [Reference Citation Analysis (3)] |
| 15. | DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177-188. [PubMed] |
| 16. | Borenstein M, Hedges LV, Higgins J, Rothstein HR. Fixed-Effect Versus Random-Effects Models. Introduction to Meta-analysis. Chichester: Wiley 2009; 77-86. |
| 17. | Olkin I. Statistical methods for meta-analysis. San Diego, CA: Academic 1985; . |
| 18. | Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455-463. [PubMed] |
| 19. | Sutton AJ, Abrams KR, Jones DR, Sheldon TA, Song F. Methods for meta-analysis in medical research. Chichester: Wiley 2000; . |
| 20. | Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54:1046-1055. [PubMed] |
| 21. | Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088-1101. [PubMed] |
| 22. | Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629-634. [PubMed] |
| 23. | Kim JH, Lee YC, Kim H, Song KH, Lee SK, Cheon JH, Kim H, Hyung WJ, Noh SH, Kim CB. Endoscopic resection for undifferentiated early gastric cancer. Gastrointest Endosc. 2009;69:e1-e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 24. | Kang HY, Kim SG, Kim JS, Jung HC, Song IS. Clinical outcomes of endoscopic submucosal dissection for undifferentiated early gastric cancer. Surg Endosc. 2010;24:509-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 83] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 25. | Lee TH, Cho JY, Chang YW, Kim JO, Lee JS, Cho WY, Kim HG, Kim WJ, Park YS, Jin SY. Appropriate indications for endoscopic submucosal dissection of early gastric cancer according to tumor size and histologic type. Gastrointest Endosc. 2010;71:920-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Yamamoto Y, Fujisaki J, Hirasawa T, Ishiyama A, Yoshimoto K, Ueki N, Chino A, Tsuchida T, Hoshino E, Hiki N. Therapeutic outcomes of endoscopic submucosal dissection of undifferentiated-type intramucosal gastric cancer without ulceration and preoperatively diagnosed as 20 millimetres or less in diameter. Dig Endosc. 2010;22:112-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 27. | Goh PG, Jeong HY, Kim MJ, Eun HS, Kim HJ, Kim ES, Kim YJ, Lee SY, Moon HS, Lee ES. Clinical outcomes of endoscopic submucosal dissection for undifferentiated or submucosal invasive early gastric cancer. Clin Endosc. 2011;44:116-122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Park J, Choi KD, Kim MY, Lee JH, Song HJ, Lee GH, Jung HY, Kim JH. Is endoscopic resection an acceptable treatment for undifferentiated EGC? Hepatogastroenterology. 2012;59:607-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | Kamada K, Tomatsuri N, Yoshida N. Endoscopic submucosal dissection for undifferentiated early gastric cancer as the expanded indication lesion. Digestion. 2012;85:111-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 30. | Okada K, Fujisaki J, Yoshida T, Ishikawa H, Suganuma T, Kasuga A, Omae M, Kubota M, Ishiyama A, Hirasawa T. Long-term outcomes of endoscopic submucosal dissection for undifferentiated-type early gastric cancer. Endoscopy. 2012;44:122-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 107] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 31. | Park CH, Shin S, Park JC, Shin SK, Lee SK, Lee YC, Lee H. Long-term outcome of early gastric cancer after endoscopic submucosal dissection: expanded indication is comparable to absolute indication. Dig Liver Dis. 2013;45:651-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 32. | Choi MH, Hong SJ, Han JP, Song JY, Kim DY, Seo SW, Ha JS, Lee YN, Ko BM, Lee MS. [Therapeutic outcomes of endoscopic submucosal dissection in undifferentiated-type early gastric cancer]. Korean J Gastroenterol. 2013;61:196-202. [PubMed] |
| 33. | Kim YY, Jeon SW, Kim J, Park JC, Cho KB, Park KS, Kim E, Chung YJ, Kwon JG, Jung JT. Endoscopic submucosal dissection for early gastric cancer with undifferentiated histology: could we extend the criteria beyond? Surg Endosc. 2013;27:4656-4662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 34. | Abe S, Oda I, Suzuki H, Nonaka S, Yoshinaga S, Odagaki T, Taniguchi H, Kushima R, Saito Y. Short- and long-term outcomes of endoscopic submucosal dissection for undifferentiated early gastric cancer. Endoscopy. 2013;45:703-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 120] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 35. | Chung MW, Jeong O, Park YK, Lee KH, Lee JH, Lee WS, Joo YE, Choi SK, Cho SB. [Comparison on the long term outcome between endoscopic submucosal dissection and surgical treatment for undifferentiated early gastric cancer]. Korean J Gastroenterol. 2014;63:90-98. [PubMed] |
| 36. | Oka S, Tanaka S, Higashiyama M, Numata N, Sanomura Y, Yoshida S, Arihiro K, Chayama K. Clinical validity of the expanded criteria for endoscopic resection of undifferentiated-type early gastric cancer based on long-term outcomes. Surg Endosc. 2014;28:639-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 37. | Oda I, Suzuki H, Nonaka S, Yoshinaga S. Complications of gastric endoscopic submucosal dissection. Dig Endosc. 2013;25 Suppl 1:71-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 163] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 38. | Isomoto H, Shikuwa S, Yamaguchi N, Fukuda E, Ikeda K, Nishiyama H, Ohnita K, Mizuta Y, Shiozawa J, Kohno S. Endoscopic submucosal dissection for early gastric cancer: a large-scale feasibility study. Gut. 2009;58:331-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 470] [Cited by in RCA: 520] [Article Influence: 32.5] [Reference Citation Analysis (1)] |
| 39. | Choi MK, Kim GH, Park do Y, Song GA, Kim DU, Ryu DY, Lee BE, Cheong JH, Cho M. Long-term outcomes of endoscopic submucosal dissection for early gastric cancer: a single-center experience. Surg Endosc. 2013;27:4250-4258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 40. | Kosaka T, Endo M, Toya Y, Abiko Y, Kudara N, Inomata M, Chiba T, Takikawa Y, Suzuki K, Sugai T. Long-term outcomes of endoscopic submucosal dissection for early gastric cancer: a single-center retrospective study. Dig Endosc. 2014;26:183-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 101] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 41. | Min YW, Lee JH. Endoscopic Resection for Early Gastric Cancer beyond Absolute Indication with Emphasis on Controversial Issues. J Gastric Cancer. 2014;14:7-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 42. | Sawada S, Fujisaki J, Yamamoto N, Kato Y, Ishiyama A, Ueki N, Hirasawa T, Yamamoto Y, Tsuchida T, Tatewaki M. Expansion of indications for endoscopic treatment of undifferentiated mucosal gastric cancer: analysis of intramucosal spread in resected specimens. Dig Dis Sci. 2010;55:1376-1380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 43. | Choi J, Kim SG, Im JP, Kim JS, Jung HC, Song IS. Comparison of endoscopic ultrasonography and conventional endoscopy for prediction of depth of tumor invasion in early gastric cancer. Endoscopy. 2010;42:705-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 169] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 44. | Hanaoka N, Tanabe S, Mikami T, Okayasu I, Saigenji K. Mixed-histologic-type submucosal invasive gastric cancer as a risk factor for lymph node metastasis: feasibility of endoscopic submucosal dissection. Endoscopy. 2009;41:427-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 45. | Mita T, Shimoda T. Risk factors for lymph node metastasis of submucosal invasive differentiated type gastric carcinoma: clinical significance of histological heterogeneity. J Gastroenterol. 2001;36:661-668. [PubMed] |
| 46. | Min BH, Kim KM, Park CK, Lee JH, Rhee PL, Rhee JC, Kim JJ. Outcomes of endoscopic submucosal dissection for differentiated-type early gastric cancer with histological heterogeneity. Gastric Cancer. 2014;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 47. | An JY, Baik YH, Choi MG, Noh JH, Sohn TS, Kim S. Predictive factors for lymph node metastasis in early gastric cancer with submucosal invasion: analysis of a single institutional experience. Ann Surg. 2007;246:749-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 139] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 48. | Jee YS, Hwang SH, Rao J, Park DJ, Kim HH, Lee HJ, Yang HK, Lee KU. Safety of extended endoscopic mucosal resection and endoscopic submucosal dissection following the Japanese Gastric Cancer Association treatment guidelines. Br J Surg. 2009;96:1157-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 49. | Kang HJ, Kim DH, Jeon TY, Lee SH, Shin N, Chae SH, Kim GH, Song GA, Kim DH, Srivastava A. Lymph node metastasis from intestinal-type early gastric cancer: experience in a single institution and reassessment of the extended criteria for endoscopic submucosal dissection. Gastrointest Endosc. 2010;72:508-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 106] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 50. | Lee JH, Kim HH. The Extended Indications of Endoscopic Submucosal Dissection (ESD) for Early Gastric Cancer Are Thus Not Entirely Safe. J Gastric Cancer. 2010;10:87-90. |
| 51. | Nasu J, Nishina T, Hirasaki S, Moriwaki T, Hyodo I, Kurita A, Nishimura R. Predictive factors of lymph node metastasis in patients with undifferentiated early gastric cancers. J Clin Gastroenterol. 2006;40:412-415. [PubMed] |
| 52. | Lee T, Tanaka H, Ohira M, Okita Y, Yoshii M, Sakurai K, Toyokawa T, Kubo N, Muguruma K, Tanaka S. Clinical impact of the extent of lymph node micrometastasis in undifferentiated-type early gastric cancer. Oncology. 2014;86:244-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 53. | UMIN-CTR Clinical Trial. A phase II trial of endoscopic submucosal dissection for expand indication to early gastric cancer of undifferentiated type (JCOG1009/1010, Undiff GC ESD P2). Available from: https://upload.umin.ac.jp/cgi-open-bin/ctr/ctr.cgi?function=brows&action=brows&recptno=R000005789&type=summary&language=E. |