Published online May 21, 2015. doi: 10.3748/wjg.v21.i19.6018
Peer-review started: September 29, 2014
First decision: December 2, 2014
Revised: December 20, 2014
Accepted: March 19, 2015
Article in press: March 19, 2015
Published online: May 21, 2015
Processing time: 236 Days and 16 Hours
AIM: To evaluate vascular endothelial growth factor (VEGF) and tryptase in hepatocellular cancer (HCC) before and after trans-arterial chemoembolization (TACE).
METHODS: VEGF and tryptase serum concentrations were assessed from 71 unresectable HCC patients before and after hepatic TACE performed by binding DC-Beads® to doxorubicin. VEGF levels were examined for each serum sample using the Quantikine Human VEGF-enzyme-linked immuno-absorbent assay (ELISA), whereas tryptase serum concentrations were assessed for each serum sample by means of fluoro-enzyme immunoassay (FEIA) using the Uni-CAP100 tool. Differences between serum VEGF and tryptase values before and after TACE were evaluated using Student t test. Person's correlation was used to assess the degree of association between the two variables.
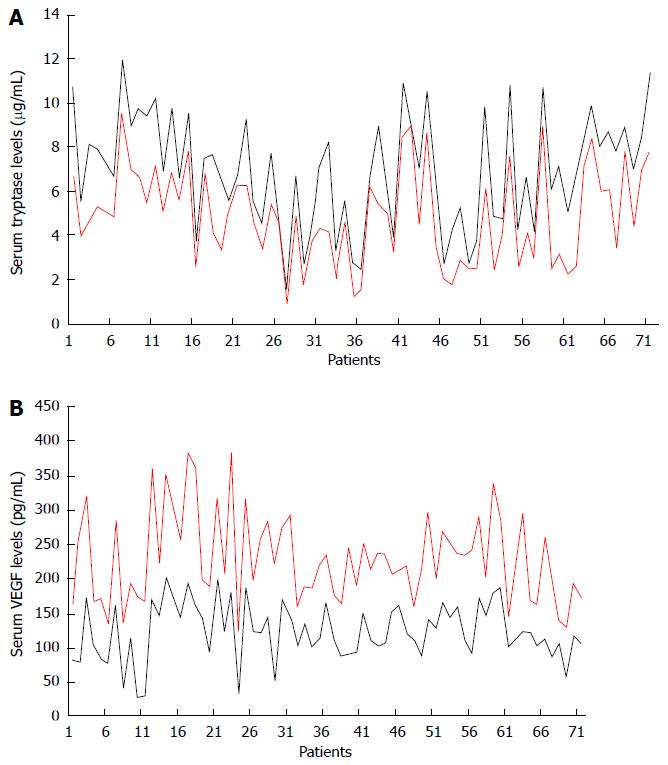
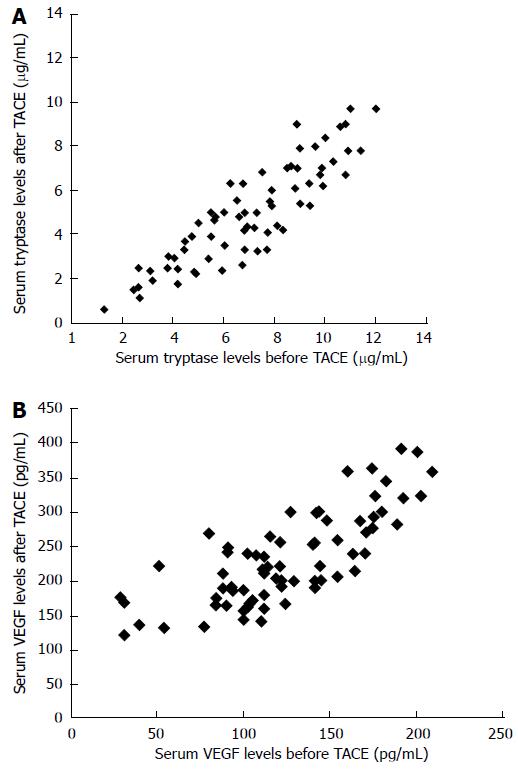
RESULTS: VEGF levels and serum tryptase in HCC patients before TACE had a mean value and standard deviation (SD) of 114.31 ± 79.58 pg/mL and 8.13 ± 3.61 μg/L, respectively. The mean levels and SD of VEGF levels and serum tryptase in HCC patients after TACE were 238.14 ± 109.41 pg/mL and 4.02 ± 3.03 μg/L. The changes between the mean values of concentration of VEGF and tryptase before treatment and after treatment was statistically significant (P < 0.000231 and P < 0.00124, by Wilcoxon-Mann-Whitney respectively). A significant correlation between VEGF levels before and after TACE and between tryptase levels before and after TACE was demonstrated (r = 0.68, P = 0.003; r = 0.84, P = 0.000 respectively).
CONCLUSION: Our pilot results suggest that the higher serum VEGF levels and the lower tryptase levels following TACE may be potential biomarkers changing in response to therapy.
Core tip: Experimental data suggest that vascular endothelial growth factor (VEGF) and tryptase play a role in tumour angiogenesis. This study aims to assess VEGF and tryptase serum concentrations from 71 hepatocellular cancer patients before and after hepatic trans-arterial chemoembolization (TACE) by mean of enzyme-linked immuno-absorbent assay and fluoro-enzyme immunoassay methods respectively. Here, we demonstrated higher serum VEGF levels and lower tryptase levels following TACE as compared to pre-TACE levels. We suggest that changes of VEGF and tryptase levels may be biomarkers of response to therapy. In this context tryptase and VEGF receptor axis inhibitors may be evaluated as adjuvant therapies.
- Citation: Ranieri G, Ammendola M, Marech I, Laterza A, Abbate I, Oakley C, Vacca A, Sacco R, Gadaleta CD. Vascular endothelial growth factor and tryptase changes after chemoembolization in hepatocarcinoma patients. World J Gastroenterol 2015; 21(19): 6018-6025
- URL: https://www.wjgnet.com/1007-9327/full/v21/i19/6018.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i19.6018
Hepatocellular carcinoma (HCC) is a well-established hypervascular tumour with a high rate of angiogenesis[1]. Interestingly, published data suggest that the expression levels of classical surrogate angiogenic factors such as vascular endothelial growth factor (VEGF) or non-classical pro-angiogenic factors, such as tryptase, in primary tumour tissue or in circulating blood fractions could express the biological aggressiveness of malignancies and provide prognostic information[2-9].
For what concern VEGF it is a powerful angiogenic cytokine that induces proliferation of both endothelial cells and tumoural cells[10]. The soluble isoform of VEGF is a dimeric glycoprotein of 36-46 kDa, mainly induced by hypoxia in tumoural and stromal cells that in turn release VEGF. VEGF binds to two specific tyrosine-kinase receptors so called: VEGF-1 (flt-1) and VEGF-2 (KDR/flk1) respectively[11]. From a therapeutic point of view, the axis VEGF/VEGF-receptors is an important anti-angiogenic target for the treatment of HCC patients[12]. Several published studies suggest that the assessment of VEGF in the serum of HCC patients may predict the biological aggressiveness of tumours and indicate response to therapy. However, no conclusive data are available[13-17].
With special regard to tryptase, it is a neutral serine protease stored in the cytoplasmic granules of mast cells (MCs). To date, four different forms of tryptase have been identified: -γ, -β, -α and -δ[18]. Of these, α- and β-tryptase are the two best circulating isoforms described and they are released from MCs[19]. Interesting experimental studies show tryptase is as a potent angiogenic factor able to stimulate the neovascularization in both in vitro and in vivo laboratory experiments[20-28]. In particular, it induces vascular tube formation by either directly acting through mitogen action on endothelial cells[29-38]. Tryptase acts as an agonist of a receptor activated by an endogenous protease, PAR-2, which is expressed on endothelial cells and involved in their proliferation[39,40]. Interestingly, in several pet and human malignancies a correlation has also been demonstrated between angiogenesis and MCs positive to tryptase[41-46]. With particular reference to HCC cells, recently a possible role of tryptase positive MCs in the development of the disease has been suggested[47].
This prospective study aimed to assess levels of both VEGF and tryptase in HCC serum before and after hepatic trans-arterial chemoembolization (TACE) treatment to see if the above pro-angiogenic factors levels change in response to TACE. In addition the correlation between VEGF and tryptase each to other and important clinico-pathological features has been also analysed.
Between April 2008 and March 2012, 71 patients 22 females, 49 males, median age 74 years (range: 47-86 years) with intermediate grade [stage B according to the Barcelona Clinic Liver Cancer (BCLC) staging classification] unresectable HCC underwent TACE of the liver at the Interventional Radiology Unit with Integrated Section of Translational Medical Oncology of National Cancer Research Centre "Giovanni Paolo II", Bari, Italy. All patients were enrolled in this prospective study and underwent measurement of serum VEGF and tryptase before and after TACE. All participants signed a written informed consent. The pre-treatment evaluation included: biochemical liver function, complete blood count, coagulation profile, dose serum alpha-fetoprotein (AFP), chest X-ray, liver ultrasound with contrast medium (CEUS), and computed tomography (CT) scan of the abdomen. The diagnosis of HCC was histologically confirmed by echo-guided needle aspiration or, alternatively, on classic imaging findings for HCC associated with pathological increase of AFP levels higher than the cut-off 200 ng/mL.
The selection criteria for TACE at our institute includes: (1) absence of extrahepatic metastases; (2) patency of the portal vein; and (3) adequate functional reserve of the liver (stage A or B according to Child-Pugh classification, serum bilirubin ≤ 2.4 mg/dL, absence of ascites and hepatic encephalopathy) as shown in Table 1.
| HCC |
| Cytohistologically confirmed |
| Unresectable (technical reasons, comorbidities, refusal of treatment) |
| Adequate liver function level |
| Child-Pugh class (A) or (B) |
| Bilirubin ≤ 2.4 mg/dL |
| Absence of ascites |
| BCLC intermediate stage (B) |
| N1 tumor nodule → diameter > 3.0 cm |
| Max N3 tumor nodules → diameter ≤ 3.0 cm |
| ECOG performance status of 0 to 2 |
The baseline clinical data of 71 patients studied are listed in Table 2. Fifty-two (73%) patients were positive for the hepatitis C antibody, eight (11%) patients were positive for the hepatitis B surface antigen (HBsAg), seven (10%) patients were affected by alcoholic liver disease and four (6%) were affected by nonalcoholic steatohepatitis (NASH). The serum AFP median level of patients was 79 ng/mL. Forty-eight (67%) patients had normal levels (< 20 ng/mL) of AFP, while the other twenty-three (33%) had higher levels.
TACE was performed under general anesthesia by binding DC-Beads® (Biocompatibles, Farnham, GB) to a total dose of doxorubicin of 100 mg/50 mL and injecting through the percutaneous insertion of a microcatheter into the femoral artery of the patient under fluoroscopic guidance (X-ray), corresponding to the artery of the liver. When possible, the artery that feeds the tumor was cannulated in a superselective approach.
All subjects avoided aspirin or non-steroidal anti-inflammatory drug ingestion for 4 wk before blood collection. The peripheral blood samples were taken between 7:00 am and 9:00 am after overnight fasting the day before and one day after TACE treatment. They were immediately dispensed in test tubes with serum separator tubes without additives (Becton Dickinson Vacutainer Systems Hemogard, Plymouth, United Kingdom) and left for at least 30 min at room temperature to allow for a complete clotting process. The samples were then centrifuged at 1500 ×g for 15 min at room temperature and the supernatant recovered. Patient sera thus obtained were collected, aliquoted and frozen at -80 °C until the analysis phase.
VEGF levels were examined for each serum sample using the Quantikine Human VEGF-enzyme-linked immuno-absorbent assay (ELISA) (R&D Systems Inc., Minneapolis, MN, United States), which recognises VEGF-165[3,4] According to the manufacturer, the minimum detectable dose of VEGF is typically less than 9.0 pg/mL. Values below 9.0 pg/mL were considered as zero. Tryptase concentrations were assessed for each serum sample by means of fluoro-enzyme immunoassay (FEIA) using the Uni-CAP100 tool (Pharmacia Diagnostics AB, Uppsala, Sweden).
Statistical analysis was performed using SPSS software (version 17.0). Descriptive statistics of serum VEGF and tryptase levels were used to calculate means and ranges of distribution (range and standard deviation). Differences between serum VEGF and tryptase values before and after TACE were evaluated using Wilcoxon-Mann-Whitney test. Person’s correlation was used to assess the degree of association between the two variables. A P value of < 0.05 was considered significant.
The levels of VEGF and serum tryptase in patients studied at the time of pre-treatment (24 h before TACE) had a mean value and standard deviation (SD) of 114.31 ± 79.58 pg/mL and 8.13 ± 3.61 μg/L respectively. No significant correlation between serum levels of VEGF and tryptase each to other was shown. Again no significant correlation between serum levels of VEGF and tryptase and the main clinico-pathological features was shown. The mean levels and SD of VEGF and tryptase in serum determined at the time of the post-treatment (+ 24 h after TACE) were 238.14 ± 109.41 pg/mL and 4.02 ± 3.03 μg/L. The changes between the mean values of concentration of VEGF and tryptase before treatment and after locoregional treatment was statistically significant significant (P < 0.000231 and P < 0.00124, by Wilcoxon-Mann-Whitney test respectively (Figure 1A and B; Table 3). A significant correlation between VEGF levels before and after TACE and between tryptase levels before and after TACE was demonstrated (r = 0.68, P = 0.003; r = 0.84, P = 0.000 respectively) (Figure 2A and B).
| Sample collectiontime | n | Mean concentrations of serum VEGF ± SD(pg/mL) | Mean concentrations of serum tryptase ± SD (μg/L) |
| 24 h before TACE | 71 | 114.31 ± 79.58 | 8.13 ± 3.61 |
| 24 h after TACE | 71 | 238.14 ± 109.41 | 4.02 ± 3.03 |
| P value | < 0.000231 | < 0.00124 |
HCC is the fifth leading cause of cancer mortality in the world. The identification of serum biomarkers of easy execution and surrogate of the presence or persistence of disease may improve clinical outcome. To this end we analyzed VEGF and tryptase serum levels in HCC before and after TACE. Our data indicated the lack of correlation between serum tryptase and VEGF levels before TACE and between serum tryptase and VEGF levels after TACE suggesting an independent role of tryptase and VEGF in the angiogenic process. Interestingly, this data may be important from a therapeutic point of view suggesting that angiogenesis involved in HCC may be inhibited at two different molecular levels. Here, we also demonstrated that VEGF significantly increased after TACE (Figure 1A). Our data agree with results from Shim et al[15] and Sergio et al[17]. The first Author’s group found that VEGF levels were significantly higher 1-2 d after TACE performed with Doxorubicin plus lipiodol than at baseline. In this study, over-expression of VEGF was associated with extrahepatic metastases. The second Author’s group also found that when TACE is not totally effective, it may induce a significant neoangiogenic reaction, as suggested by an increase in VEGF following treatment; this affected patient survival[17]. Differently to the above studies we detected VEGF after TACE performed with doxorubicin loaded with microspheres called DC-Bead. Although TACE represents the a main treatment for stage B HCC patients (BCLC classification), TACE also induces hypoxia and stimulates angiogenesis via VEGF expression that in turns may help residual cancer cells to survival[12,14,48,49]. Due to this VEGF serum rebound patients with higher serum VEGF levels post treatment may be select to receive an adjuvant therapy with sorafenib that inhibits the VEGF/VEGF-receptor axis.
With particular reference to tryptase, experimental data indicate that it plays an important role in tumour angiogenesis[37-43,50-54]. Our results show high basal levels of serum tryptase as compared to serum tryptase levels after TACE. It is therefore likely that the levels of serum tryptase may be a surrogate indicator of the magnitude of the angiogenic process and of the presence of HCC tumor tissue. Substantiating this assumption, our results showed that following TACE and subsequent tumor tissue necrosis serum tryptase levels decrease (Figure 1B), as if due to the destruction of MC content in the tumor nodule the source production of tryptase itself ceases. Hence, these serin-proteases may play a role as a predictive factor of response to locoregional treatment in HCC patients. In the present study, we measured tryptase level 24 h before treatment as a circulating biomarker surrogate for the presence of neoplastic disease, and again 24 h after treatment to evaluate the reduction of the concentration of the same. The rationale for assessing levels of tryptase after 24 h lies in the short life of tryptase, which is about 4 hours. Therefore, if the primary source of tryptase production no longer exists, after 24 h you would expect a significant reduction in serum concentration. This assumption was reflected in the data we obtained, as the difference between men pre-and post-treatment concentrations, expressed in g/L that was statistically significant. Should tryptase levels not fall during post-treatment in some patients, this could be indicative of residual disease and therefore patients should undergo further diagnostic studies and therapies. For these patients tryptase inhibitors, such as Nafamostat mesilate or Gabexate, may be evaluated in future awaited clinical trials. Although preliminary, and therefore worthy of further investigation, the results of this pilot study suggest a role of both VEGF and tryptase as possible biomarkers in HCC patients underwent to TACE able to select patients in which an adjuvant anti-angiogenic therapy may be recommended.
We would like to thank Caroline Oakley for assistance with the English language.
Hepatocellular carcinoma (HCC) is a hypervascular tumour in which angiogenesis has a crucial role in progression, as already demonstrated in other tumours. Pro-angiogenic factors, such as vascular endothelial growth factor (VEGF) and tryptase, inducing proliferation of both endothelial and tumoural cells, may predict the biological aggressiveness of tumours and represent important anti-angiogenic targets in HCC. However, there are no conclusive data available about the assessment of VEGF serum levels of HCC patients.
To establish the role of tryptase and VEGF in HCC progression angiogenesis-mediated, by mean the assessment of their serum levels in HCC patients. To identificate serum biomarkers of easy execution and surrogate of the presence or persistence of disease that may improve clinical outcome. To determine if these pro-angiogenic factors may be considerable as possible biomarkers able to select patients in which an adjuvant anti-angiogenic therapy may be recommended after TACE in HCC patients.
Several published studies suggest that the assessment of VEGF in the serum of HCC patients may predict the biological aggressiveness of tumour, however, no conclusive data are available. This is the unique study, in which both serum levels of VEGF and tryptase were assessment after hepatic chemoembolization with DC-Beads® (DEB-TACE) in HCC patients and that they could be indicative of residual disease.
In this patients setting tryptase inhibitors (Nafamostat mesilate or Gabexate) or anti-VEGF/VEGFR therapy could slow HCC progression, even if these therapeutic approaches may be evaluated in future awaited clinical trials in HCC patients underwent to DEB-TACE.
VEGF is an angiogenic cytokine (induced by hypoxia in tumoral microenvironment) that binding VEGF receptors-1/2 promotes proliferation of both endothelial and tumoural cells; Tryptase is an angiogenic serine protease (stored in mast cells) that binding a receptor activated by an endogenous protease stimulates proliferation of endothelial cells; TACE means trans-arterial chemoembolization (hepatic locoregional treatment used in stage B of HCC patients according to Barcelona Clinic Liver Cancer staging classification in which the blood supply to the tumor is blocked from chemotherapeutic agent (doxorubicin, mitomycin) plus lipiodol; DEB-TACE is TACE with drug-eluting beads (DC-Beads®) that combines the drug with the embolization device by using microsphere.
This pilot study could be interesting for the reader because provides good explanation of the potential benefits of pro-angiogenic factors with possible clinical impact in HCC patients undergone to DEB-TACE.
P- Reviewer: Murata S, Meister T S- Editor: Yu J L- Editor: A E- Editor: Zhang DN
| 1. | Gadaleta CD, Ranieri G. Trans-arterial chemoembolization as a therapy for liver tumours: New clinical developments and suggestions for combination with angiogenesis inhibitors. Crit Rev Oncol Hematol. 2011;80:40-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 2. | Schoenleber SJ, Kurtz DM, Talwalkar JA, Roberts LR, Gores GJ. Prognostic role of vascular endothelial growth factor in hepatocellular carcinoma: systematic review and meta-analysis. Br J Cancer. 2009;100:1385-1392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 165] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 3. | Ranieri G, Labriola A, Achille G, Florio G, Zito AF, Grammatica L, Paradiso A. Microvessel density, mast cell density and thymidine phosphorylase expression in oral squamous carcinoma. Int J Oncol. 2002;21:1317-1323. [PubMed] |
| 4. | Ranieri G, Ammendola M, Patruno R, Celano G, Zito FA, Montemurro S, Rella A, Di Lecce V, Gadaleta CD, Battista De Sarro G. Tryptase-positive mast cells correlate with angiogenesis in early breast cancer patients. Int J Oncol. 2009;35:115-120. [PubMed] |
| 5. | Ammendola M, Sacco R, Sammarco G, Donato G, Zuccalà V, Romano R, Luposella M, Patruno R, Vallicelli C, Verdecchia GM. Mast Cells Positive to Tryptase and c-Kit Receptor Expressing Cells Correlates with Angiogenesis in Gastric Cancer Patients Surgically Treated. Gastroenterol Res Pract. 2013;2013:703163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Ammendola M, Sacco R, Donato G, Zuccalà V, Russo E, Luposella M, Vescio G, Rizzuto A, Patruno R, De Sarro G. Mast cell positivity to tryptase correlates with metastatic lymph nodes in gastrointestinal cancer patients treated surgically. Oncology. 2013;85:111-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 7. | Ammendola M, Zuccalà V, Patruno R, Russo E, Luposella M, Amorosi A, Vescio G, Sammarco G, Montemurro S, De Sarro G. Tryptase-positive mast cells and angiogenesis in keloids: a new possible post-surgical target for prevention. Updates Surg. 2013;65:53-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 8. | Ammendola M, Sacco R, Sammarco G, Donato G, Zuccalà V, Luposella M, Patruno R, Marech I, Montemurro S, Zizzo N. Mast cells density positive to tryptase correlates with angiogenesis in pancreatic ductal adenocarcinoma patients having undergone surgery. Gastroenterol Res Pract. 2014;2014:951957. [PubMed] |
| 9. | Ammendola M, Sacco R, Sammarco G, Donato G, Montemurro S, Ruggieri E, Patruno R, Marech I, Cariello M, Vacca A. Correlation between serum tryptase, mast cells positive to tryptase and microvascular density in colo-rectal cancer patients: possible biological-clinical significance. PLoS One. 2014;9:e99512. [PubMed] |
| 10. | Ranieri G, Patruno R, Ruggieri E, Montemurro S, Valerio P, Ribatti D. Vascular endothelial growth factor (VEGF) as a target of bevacizumab in cancer: from the biology to the clinic. Curr Med Chem. 2006;13:1845-1857. [PubMed] |
| 11. | Ranieri G, Coviello M, Chiriatti A, Stea B, Montemurro S, Quaranta M, Dittadi R, Paradiso A. Vascular endothelial growth factor assessment in different blood fractions of gastrointestinal cancer patients and healthy controls. Oncol Rep. 2004;11:435-439. [PubMed] |
| 12. | Ranieri G, Gadaleta-Caldarola G, Goffredo V, Patruno R, Mangia A, Rizzo A, Sciorsci RL, Gadaleta CD. Sorafenib (BAY 43-9006) in hepatocellular carcinoma patients: from discovery to clinical development. Curr Med Chem. 2012;19:938-944. [PubMed] |
| 13. | Gadaleta C, Coviello M, Catino A, Venneri MT, Stea B, Quaranta M, Mattioli V, Ranieri G. Serum vascular endothelial growth factor concentrations in hepatocellular cancer patients undergoing percutaneously radiofrequency thermal ablation. J Chemother. 2004;16 Suppl 5:7-10. [PubMed] |
| 14. | Niizeki T, Sumie S, Torimura T, Kurogi J, Kuromatsu R, Iwamoto H, Aino H, Nakano M, Kawaguchi A, Kakuma T. Serum vascular endothelial growth factor as a predictor of response and survival in patients with advanced hepatocellular carcinoma undergoing hepatic arterial infusion chemotherapy. J Gastroenterol. 2012;47:686-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Shim JH, Park JW, Kim JH, An M, Kong SY, Nam BH, Choi JI, Kim HB, Lee WJ, Kim CM. Association between increment of serum VEGF level and prognosis after transcatheter arterial chemoembolization in hepatocellular carcinoma patients. Cancer Sci. 2008;99:2037-2044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 113] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 16. | Zhong C, Wei W, Su XK, Li HD, Xu FB, Guo RP. Serum and tissue vascular endothelial growth factor predicts prognosis in hepatocellular carcinoma patients after partial liver resection. Hepatogastroenterology. 2012;59:93-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 17. | Sergio A, Cristofori C, Cardin R, Pivetta G, Ragazzi R, Baldan A, Girardi L, Cillo U, Burra P, Giacomin A. Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): the role of angiogenesis and invasiveness. Am J Gastroenterol. 2008;103:914-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 397] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 18. | Hallgren J, Pejler G. Biology of mast cell tryptase. An inflammatory mediator. FEBS J. 2006;273:1871-1895. [PubMed] |
| 19. | Schwartz LB, Yunginger JW, Miller J, Bokhari R, Dull D. Time course of appearance and disappearance of human mast cell tryptase in the circulation after anaphylaxis. J Clin Invest. 1989;83:1551-1555. [PubMed] |
| 20. | Rodriguez-Caballero A, Garcia-Montero AC, Almeida J, Balanzategui A, Munoz-Criado S, Orfao A. Association between the HLA haplotype and the TCR-Vbeta repertoire of anti-hCMV specific memory T-cells in immunocompetent healthy adults. Cytometry B Clin Cytom. 2007;72:371-379. [PubMed] |
| 21. | Metz M, Grimbaldeston MA, Nakae S, Piliponsky AM, Tsai M, Galli SJ. Mast cells in the promotion and limitation of chronic inflammation. Immunol Rev. 2007;217:304-328. [PubMed] |
| 22. | Ackermann MR. Acute inflammation. Philadelphia: Mosby Elsevier 2007; 117-118. |
| 23. | Ackermann MR. Chronic inflammation and wound healing. Pathologic Basis of Veterinary Disease, 4th ed. St Louis, Missouri: Mosby Elsevier 2007; 153-191. |
| 24. | Kovanen PT. Mast cells: multipotent local effector cells in atherothrombosis. Immunol Rev. 2007;217:105-122. [PubMed] |
| 25. | Theoharides TC, Conti P. Mast cells: the Jekyll and Hyde of tumor growth. Trends Immunol. 2004;25:235-241. [PubMed] |
| 26. | Ribatti D, Ranieri G. Tryptase, a novel angiogenic factor stored in mast cell granules. Exp Cell Res. 2015;332:157-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 27. | Blair RJ, Meng H, Marchese MJ, Ren S, Schwartz LB, Tonnesen MG, Gruber BL. Human mast cells stimulate vascular tube formation. Tryptase is a novel, potent angiogenic factor. J Clin Invest. 1997;99:2691-2700. [PubMed] |
| 28. | Ribatti D, Ranieri G, Nico B, Benagiano V, Crivellato E. Tryptase and chymase are angiogenic in vivo in the chorioallantoic membrane assay. Int J Dev Biol. 2011;55:99-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 29. | Ruoss SJ, Hartmann T, Caughey GH. Mast cell tryptase is a mitogen for cultured fibroblasts. J Clin Invest. 1991;88:493-499. [PubMed] |
| 30. | Hartmann T, Ruoss SJ, Raymond WW, Seuwen K, Caughey GH. Human tryptase as a potent, cell-specific mitogen: role of signaling pathways in synergistic responses. Am J Physiol. 1992;262:L528-L534. [PubMed] |
| 31. | Abe M, Kurosawa M, Ishikawa O, Miyachi Y, Kido H. Mast cell tryptase stimulates both human dermal fibroblast proliferation and type I collagen production. Clin Exp Allergy. 1998;28:1509-1517. [PubMed] |
| 32. | Frungieri MB, Albrecht M, Raemsch R, Mayerhofer A. The action of the mast cell product tryptase on cyclooxygenase-2 (COX2) and subsequent fibroblast proliferation involves activation of the extracellular signal-regulated kinase isoforms 1 and 2 (erk1/2). Cell Signal. 2005;17:525-533. [PubMed] |
| 33. | Briggaman RA, Schechter NM, Fraki J, Lazarus GS. Degradation of the epidermal-dermal junction by proteolytic enzymes from human skin and human polymorphonuclear leukocytes. J Exp Med. 1984;160:1027-1042. [PubMed] |
| 34. | Kaminska R, Helisalmi P, Harvima RJ, Naukkarinen A, Horsmanheimo M, Harvima IT. Focal dermal-epidermal separation and fibronectin cleavage in basement membrane by human mast cell tryptase. J Invest Dermatol. 1999;113:567-573. [PubMed] |
| 35. | Huttunen M, Harvima IT. Mast cell tryptase and chymase in chronic leg ulcers: chymase is potentially destructive to epithelium and is controlled by proteinase inhibitors. Br J Dermatol. 2005;152:1149-1160. [PubMed] |
| 36. | Zizzo N, Patruno R, Zito FA, Di Summa A, Tinelli A, Troilo S, Misino A, Ruggieri E, Goffredo V, Gadaleta CD. Vascular endothelial growth factor concentrations from platelets correlate with tumor angiogenesis and grading in a spontaneous canine non-Hodgkin lymphoma model. Leuk Lymphoma. 2010;51:291-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 37. | Kemik O, Sumer A, Kemik SA, Purisa S, Tuzun S. Circulating levels of VEGF family and their receptors in hepatocellular carcinoma. Bratisl Lek Listy. 2010;111:485-488. [PubMed] |
| 38. | Shimanuki Y, Takahashi K, Cui R, Hori S, Takahashi F, Miyamoto H, Fukurchi Y. Role of serum vascular endothelial growth factor in the prediction of angiogenesis and prognosis for non-small cell lung cancer. Lung. 2005;183:29-42. [PubMed] |
| 39. | Carvalho RF, Nilsson G, Harvima IT. Increased mast cell expression of PAR-2 in skin inflammatory diseases and release of IL-8 upon PAR-2 activation. Exp Dermatol. 2010;19:117-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 40. | Ribatti D, Vacca A. Overview of angiogenesis during tumor growth. Angiogenesis: An Integrative Approach from Science to Medicine. Berlin: Springer-Verlag 2008; 161-168. |
| 41. | Ribatti D, Nico B, Vacca A, Marzullo A, Calvi N, Roncali L, Dammacco F. Do mast cells help to induce angiogenesis in B-cell non-Hodgkin’s lymphomas? Br J Cancer. 1998;77:1900-1906. [PubMed] |
| 42. | Ribatti D, Molica S, Vacca A, Nico B, Crivellato E, Roccaro AM, Dammacco F. Tryptase-positive mast cells correlate positively with bone marrow angiogenesis in B-cell chronic lymphocytic leukemia. Leukemia. 2003;17:1428-1430. [PubMed] |
| 43. | Molica S, Vacca A, Crivellato E, Cuneo A, Ribatti D. Tryptase-positive mast cells predict clinical outcome of patients with early B-cell chronic lymphocytic leukemia. Eur J Haematol. 2003;71:137-139. [PubMed] |
| 44. | Ribatti D, Vacca A, Nico B, Quondamatteo F, Ria R, Minischetti M, Marzullo A, Herken R, Roncali L, Dammacco F. Bone marrow angiogenesis and mast cell density increase simultaneously with progression of human multiple myeloma. Br J Cancer. 1999;79:451-455. [PubMed] |
| 45. | Ribatti D, Finato N, Crivellato E, Marzullo A, Mangieri D, Nico B, Vacca A, Beltrami CA. Neovascularization and mast cells with tryptase activity increase simultaneously with pathologic progression in human endometrial cancer. Am J Obstet Gynecol. 2005;193:1961-1965. [PubMed] |
| 46. | Benítez-Bribiesca L, Wong A, Utrera D, Castellanos E. The role of mast cell tryptase in neoangiogenesis of premalignant and malignant lesions of the uterine cervix. J Histochem Cytochem. 2001;49:1061-1062. [PubMed] |
| 47. | Cervello M, Foderàa D, Florena AM, Soresi M, Tripodo C, D’Alessandro N, Montalto G. Correlation between expression of cyclooxygenase-2 and the presence of inflammatory cells in human primary hepatocellular carcinoma: possible role in tumor promotion and angiogenesis. World J Gastroenterol. 2005;11:4638-4643. [PubMed] |
| 48. | Wang B, Xu H, Gao ZQ, Ning HF, Sun YQ, Cao GW. Increased expression of vascular endothelial growth factor in hepatocellular carcinoma after transcatheter arterial chemoembolization. Acta Radiol. 2008;49:523-529. [PubMed] |
| 49. | Li X, Feng GS, Zheng CS, Zhuo CK, Liu X. Expression of plasma vascular endothelial growth factor in patients with hepatocellular carcinoma and effect of transcatheter arterial chemoembolization therapy on plasma vascular endothelial growth factor level. World J Gastroenterol. 2004;10:2878-2882. [PubMed] |
| 50. | Marech I, Ammendola M, Gadaleta C, Zizzo N, Oakley C, Gadaleta CD, Ranieri G. Possible biological and translational significance of mast cells density in colorectal cancer. World J Gastroenterol. 2014;20:8910-8920. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 37] [Reference Citation Analysis (0)] |
| 51. | Marech I, Ammendola M, Sacco R, Capriuolo GS, Patruno R, Rubini R, Luposella M, Zuccalà V, Savino E, Gadaleta CD. Serum tryptase, mast cells positive to tryptase and microvascular density evaluation in early breast cancer patients: possible translational significance. BMC Cancer. 2014;14:534. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 52. | Marech I, Gadaleta CD, Ranieri G. Possible prognostic and therapeutic significance of c-Kit expression, mast cell count and microvessel density in renal cell carcinoma. Int J Mol Sci. 2014;15:13060-13076. [PubMed] |
| 53. | Patruno R, Marech I, Zizzo N, Ammendola M, Nardulli P, Gadaleta C, Introna M, Capriuolo G, Rubini RA, Ribatti D. c-Kit expression, angiogenesis, and grading in canine mast cell tumour: a unique model to study c-Kit driven human malignancies. Biomed Res Int. 2014;2014:730246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 54. | Ranieri G, Marech I, Lorusso V, Goffredo V, Paradiso A, Ribatti D, Gadaleta CD. Molecular targeting agents associated with transarterial chemoembolization or radiofrequency ablation in hepatocarcinoma treatment. World J Gastroenterol. 2014;20:486-497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |