Published online May 21, 2015. doi: 10.3748/wjg.v21.i19.5934
Peer-review started: November 2, 2014
First decision: November 27, 2014
Revised: January 7, 2015
Accepted: February 5, 2015
Article in press: February 5, 2015
Published online: May 21, 2015
Processing time: 198 Days and 19.6 Hours
AIM: To characterize patterns of gastric cancer recurrence and patient survival and to identify predictors of early recurrence after surgery.
METHODS: Clinicopathological data for 417 consecutive patients who underwent curative resection for gastric cancer were retrospectively analyzed. Tumor and node status was reclassified according to the 7th edition of the American Joint Committee on Cancer tumor-node-metastasis classification for carcinoma of the stomach. Survival data came from both the patients’ follow-up records and telephone follow-ups. Recurrent gastric cancer was diagnosed based on clinical imaging, gastroscopy with biopsy, and/or cytological examination of ascites, or intraoperative findings in patients who underwent reoperation. Predictors of early recurrence were compared in patients with pT1 and pT2-4a stage tumors. Pearson’s χ2 test and Fisher’s exact test were used to compare differences between categorical variables. Survival curves were constructed using the Kaplan-Meier method and compared via the log-rank test. Variables identified as potentially important for early recurrence using univariate analysis were determined by multivariate logistic regression analysis.
RESULTS: Of 417 gastric cancer patients, 80 (19.2%) were diagnosed with early gastric cancer and the remaining 337 (80.8%) were diagnosed with locally advanced gastric cancer. After a median follow-up period of 56 mo, 194 patients (46.5%) experienced recurrence. The mean time from curative surgery to recurrence in these 194 patients was 24 ± 18 mo (range, 1-84 mo). Additionally, of these 194 patients, 129 (66.5%) experienced recurrence within 2 years after surgery. There was no significant difference in recurrence patterns between early and late recurrence (P < 0.05 each). For pT1 stage gastric cancer, tumor size (P = 0.011) and pN stage (P = 0.048) were associated with early recurrence of gastric tumors. Patient age, pT stage, pN stage, Lauren histotype, lymphovascular invasion, intraoperative chemotherapy, and postoperative chemotherapy were independent predictors of early recurrence in patients with pT2-4a stage gastric cancer (P < 0.05 each).
CONCLUSION: Age, pT stage, pN stage, Lauren histotype, lymphovascular invasion, intraoperative chemotherapy, and postoperative chemotherapy are independent factors influencing early recurrence of pT2-4a stage gastric cancer.
Core tip: Few studies have assessed recurrence patterns or predictors of early recurrence after curative surgery in Chinese patients with gastric carcinoma. This study found that survival after gastric cancer recurrence was poor. Large tumor size and advanced pN stage were associated with early recurrence of tumor pT1 stage tumors. Age, pT stage, pN stage, Lauren histotype, lymphovascular invasion, intraoperative chemotherapy, and postoperative chemotherapy were independent predictors of early recurrence of pT2-4a stage tumors.
- Citation: Kang WM, Meng QB, Yu JC, Ma ZQ, Li ZT. Factors associated with early recurrence after curative surgery for gastric cancer. World J Gastroenterol 2015; 21(19): 5934-5940
- URL: https://www.wjgnet.com/1007-9327/full/v21/i19/5934.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i19.5934
Although it has declined somewhat, the overall age-standardized incidence of gastric cancer in China in 2009 was 17.85 per 100000 persons[1,2]. Despite improvements in diagnostic procedures and the introduction of multimodal treatment strategies, patient survival remains dismal owing to early recurrence originating from minimal residual disease[3-8]. More than 70% of recurrences and tumor-related deaths occur within 2 years after surgery[9-11], with tumor recurrence being the leading cause of death in patients who undergo curative surgery for gastric cancer.
Although several studies have sought to identify clinicopathological factors that predict early recurrence, their methodologies and definitions of early gastric cancer vary[11-13]. Few studies to date have focused on patterns and timing of recurrence, or on predictors of early recurrence, after curative surgery in Chinese patients.
Multimodal treatments, including intraoperative and/or postoperative chemotherapy, have been used for some patients undergoing D2 gastrectomy for locally advanced gastric cancer[14]. However, little is known about the effect of intraoperative and postoperative chemotherapy on early recurrence of gastric cancer. This study therefore retrospectively analyzed patterns and timing of recurrence in patients who underwent curative surgery for gastric cancer. Clinicopathological factors and therapeutic modalities significantly associated with early recurrence were identified to develop appropriate treatments and follow-up programs.
Between January 2002 and February 2008, 516 patients with gastric adenocarcinoma underwent radical gastrectomy and D2 lymphadenectomy in the Department of General Surgery, Peking Union Medical College Hospital (PUMCH), Chinese Academy of Medical Science and Peking Union Medical College. Patients with gastric stump cancer (n = 7), those who received neoadjuvant chemotherapy (n = 4) or postoperative radiotherapy (n = 2), patients with incomplete or inaccurate medical records (n = 10), patients lost to follow-up within 2 years after surgery (n = 68) and those who died of disease other than gastric cancer within 2 years after curative surgery (n = 8 cases) were excluded. The study therefore included a total of 417 patients. None of these patients had distant or peritoneal metastasis at the time of resection, as shown by chest X-ray or chest computed tomography (CT) scan and abdominal pelvic CT scan before surgery. Tumor (T) and node (N) status was reclassified according to the 7th edition of the American Joint Committee on Cancer (AJCC) tumor-node-metastasis (TNM) classification for carcinoma of the stomach[15,16].
Clinicopathological features and therapeutic modalities reviewed included sex, age at diagnosis, tumor size, Lauren histotype (intestinal or diffuse-mixed type)[17,18], lymphovascular invasion, AJCC pT stage of the primary tumor, AJCC pN stage, intraoperative chemotherapy and postoperative chemotherapy.
All patients in this study underwent curative (R0) resection and D2 lymphadenectomy as the primary treatment[19,20]. Of the 80 patients with early gastric cancer (pT1), 20 (25%) received intraoperative chemotherapy, and 3 (2 pT1N1M0 and 1 pT1N2M0) received six cycles of postoperative adjuvant chemotherapy. Of the 337 patients with locally advanced gastric cancer (pT2-4a), 190 (56.4%) received intraoperative chemotherapy, and 246 (73%) received postoperative adjuvant chemotherapy, with 200 (81.3%) of the latter completing at least six cycles.
Intraoperative chemotherapy consisted of intravenous administration of epirubicin 20 mg/m2, leucovorin 200 mg, 5-fluorouracil (5-FU) 600 mg/m2 (maximum ≤ 1000 mg), and mitomycin 5 mg/m2 (maximum ≤ 10 mg).
Three main postoperative adjuvant chemotherapy regimens were used: XELOX[21] (2-h intravenous infusion of oxaliplatin 130 mg/m2 on day 1 and oral capecitabine 1000 mg/m2 twice daily on days 1-14, with cycles every 21 d); FOLFOX4[22] (intravenous infusion of oxaliplatin 85 mg/m2 on day 1, leucovorin 200 mg/m2 as a 2-h infusion followed by bolus injection of 5-FU 400 mg/m2 on days 1, 2, and 22-h continuous intravenous infusion of 5-FU 600 mg/m2 on days 1, 2, every 2 wk for at least six cycles); and FOLFOX6[23] (intravenous infusion of oxaliplatin 100 mg/m2 on day 1, leucovorin 200 mg/m2 as a 2-h infusion followed by bolus injection of 5-FU 400 mg/m2 on day 1, and 46-h continuous intravenous infusion of 5-FU 3000 mg/m2 starting on day 1, every 2 wk for at least six cycles).
All patients were followed regularly from the date of surgery to death, emigration, or February 10, 2012, whichever came first. Survival data were obtained from both patients’ follow-up records and telephone follow-up. History and physical examinations were routinely performed every 3-6 mo for the first 3 years and every 6-12 mo thereafter. CT examinations were performed at least twice a year for the first 2 years, and annually thereafter. Gastroscopy with or without biopsy was performed every 1-2 years.
Recurrent gastric cancer was diagnosed based on clinical imaging, gastroscopy with biopsy, and/or cytological examination of ascites, or intraoperative findings in patients who underwent reoperation. Recurrences were classified as locoregional, hematogenous, peritoneal or distant lymphatic, according to the sites of relapse.
Categorical variables were compared using Pearson’s χ2 tests and Fisher’s exact tests. Variables differing significantly on univariate analysis were included in multivariate models of logistic regression analysis. Survival curves were constructed using the Kaplan-Meier method and compared by the log-rank test. Multicollinearity, defined as a tolerance < 0.1, was diagnosed by the linear regression model. All analyses were performed using SPSS 12.0 (SPSS, Chicago, Illinois, United States). The prognostic powers of covariates were expressed by calculating odds ratios (ORs) and 95% confidence internals (CIs). All P values were two-sided and P values < 0.05 were considered statistically significant.
The study was approved by the Ethics Committee of Chinese Academy of Medical Science and Peking Union Medical College, Beijing, China.
Of the 417 patients included in the current study, 80 (19.2%) were diagnosed with early gastric cancer and 337 (80.8%) with locally advanced gastric cancer. The median follow-up time of all 417 patients was 56 mo (range, 3-117 mo), during which gastric cancer recurrence was detected in 194 (46.5%) patients, with 184 patients dying of gastric cancer recurrence. Ten patients with recurrence remained alive after the end of follow-up. In contrast, 9 of 223 patients without recurrence died of other diseases within 2 years after surgery, with the other 214 patients remaining alive without recurrence during follow-up.
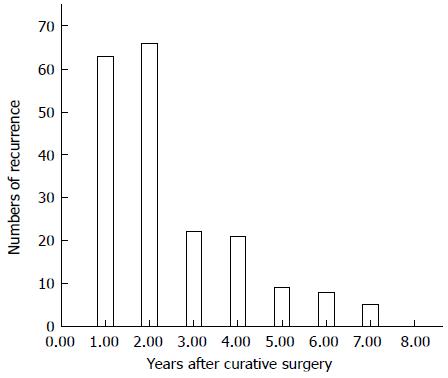
The mean time from curative surgery to recurrence in the 194 patients with recurrence was 24 ± 18 mo (range, 1-84 mo), with 129 (66.5%) of these patients experiencing recurrence within 2 years (Figure 1). Those 129 patients were classified as the early recurrence group, whereas the late/no recurrence group was defined as patients who lived without recurrence for more than 2 years after surgery.
Of the 194 patients with recurrence, 99 (51.0%) experienced locoregional recurrence, 86 had peritoneal dissemination (44.3%), 77 (39.7%) showed hematogenous metastases, and 11 (5.7%) had distant lymphatic recurrence (Table 1). Of the 129 patients with early recurrence, 56 (43.4%) had hematogenous recurrence, 59 (45.7%) had peritoneal recurrence, and 68 (52.7%) had locoregional recurrence. Recurrence patterns did not differ significantly in patients with early and late recurrence (Table 1).
| Pattern | Total recurrence | Early recurrence | Late recurrence | P value |
| n = 194 (100.0) | n = 129 (66.5) | n = 65 (33.5) | ||
| Hematogenous recurrence | 77 (39.7) | 56 (43.4) | 21 (32.3) | 0.136 |
| Liver | 55 (28.4) | 42 (32.6) | 13 (20.0) | 0.067 |
| Lung | 16 (8.2) | 10 (7.8) | 6 (9.2) | 0.724 |
| Bone | 14 (7.2) | 8 (6.2) | 6 (9.2) | 0.442 |
| Brain | 3 (1.5) | 2 (1.6) | 1 (1.5) | 0.995 |
| Locoregional recurrence | 99 (51.0) | 68 (52.7) | 31 (47.7) | 0.509 |
| Remnant stomach | 10 (5.2) | 6 (4.7) | 4 (6.2) | 0.734 |
| Anastomosis | 32 (16.5) | 25 (19.4) | 7 (10.8) | 0.127 |
| Perigastric area | 40 (20.6) | 27 (20.9) | 13 (20.0) | 0.880 |
| Peripancreatic area | 13 (6.7) | 10 (7.8) | 3 (4.6) | 0.410 |
| Abdominal wall | 2 (1.6) | 2 (1.9) | 0 (0.0) | 0.313 |
| Local lymph node | 34 (17.5) | 27 (20.9) | 7 (10.8) | 0.079 |
| Peritoneal recurrence | 86 (44.3) | 59 (45.7) | 27 (41.5) | 0.579 |
| Distant lymphatic recurrence | 11 (5.7) | 7 (5.4) | 4 (6.2) | 1.000 |
| Virchow’s node | 6 (3.1) | 4 (3.1) | 2 (3.1) | 1.000 |
| Inguinal lymph node | 1 (0.5) | 0 (0.0) | 1 (1.5) | 0.335 |
| Mediastinal lymph node | 3 (1.5) | 2 (1.6) | 1 (1.5) | 1.000 |
| Para-aortic lymph node | 2 (1.0) | 2 (1.6) | 0 (0.0) | 0.552 |
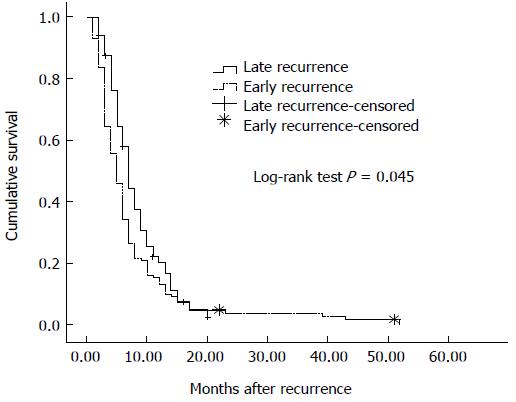
Median survival after recurrence in all patients with recurrence was 6 mo (95%CI: 5.32-6.68 mo), 5 mo (95%CI: 6.01-7.92 mo) in patients with early recurrence and 7 mo (95%CI: 4.21-5.80 mo) in patients with late recurrence (log rank test, P = 0.045) (Figure 2).
Of the 80 patients with early gastric cancer, 4 (5.0%) experienced early recurrence, as did 125 of the 337 patients (37.1%) with advanced gastric cancer. Analysis of predictors of early recurrence in patients with early gastric cancer (pT1) showed that larger tumor size (P = 0.011) and advanced AJCC pN stage (P = 0.048) were significantly associated with early recurrence (Table 2). Univariate analysis of clinicopathological factors predictive of recurrence in patients with locally advanced gastric cancer showed that age at diagnosis, tumor size, Lauren histotype, lymphovascular invasion, AJCC pT stage, AJCC pN stage, intraoperative systemic chemotherapy, and postoperative chemotherapy were significantly associated with early recurrences (Table 3). Multivariate analysis showed that age at diagnosis (P = 0.033), AJCC pT stage (P < 0.001), AJCC pN stage (P < 0.001), Lauren histotype (P < 0.001), lymphovascular invasion (P = 0.011), intraoperative chemotherapy (P < 0.001), and postoperative chemotherapy (P = 0.007) were independent predictors of early recurrence, whereas gender was not (Table 4). All tolerance values of significant factors were greater than 0.1.
| Variable | Early recurrence | Late/no recurrence | Univariate analysis |
| n = 4 | n = 76 | P value | |
| Gender | 0.623 | ||
| Women | 1 (25.0) | 35 (46.1) | |
| Men | 3 (75.0) | 41 (53.9) | |
| Age at diagnosis (yr) | 0.646 | ||
| ≤ 60 | 3 (75.0) | 45 (59.2) | |
| 60 | 1 (25.0) | 31 (40.8) | |
| Tumor size (cm) | 0.011 | ||
| ≤ 5.0 | 2 (50.0) | 74 (97.4) | |
| >5.0 | 2 (50.0) | 2 (2.6) | |
| Lauren histotype | 0.646 | ||
| Intestinal | 3 (75.0) | 45 (59.2) | |
| Diffuse-mixed | 1 (25.0) | 31 (40.8) | |
| Lymphovascular invasion | < 0.144 | ||
| Present | 1 (25.0) | 2 (2.6) | |
| Absent | 3 (75.0) | 74 (97.4) | |
| pT stage | < 0.639 | ||
| pT1a | 2 (50.0) | 47 (61.8) | |
| pT1b | 2 (50.0) | 29 (38.2) | |
| pN stage | 0.048 | ||
| N0 | 2 (50.0) | 70 (92.1) | |
| N1, N2 | 2 (50.0) | 6 (7.9) | |
| Intraoperative chemotherapy | 1.000 | ||
| Yes | 1 (25.0) | 19 (25.0) | |
| No | 3 (75.0) | 57 (75.0) |
| Variable | Early recurrence | Late/no recurrence | Univariate analysis |
| n = 125 | n = 212 | P value | |
| Gender | 0.683 | ||
| Women | 41 (32.8) | 65 (30.7) | |
| Men | 84 (67.2) | 147 (69.3) | |
| Age at diagnosis (yr) | 0.016 | ||
| ≤ 60 | 52 (41.6) | 117 (55.2) | |
| > 60 | 73 (58.4) | 95 (44.8) | |
| Tumor size (cm) | 0.019 | ||
| ≤ 5.0 | 76 (60.8) | 155 (73.1) | |
| > 5.0 | 49 (392) | 57 (26.9) | |
| Lauren histotype | 0.016 | ||
| Intestinal | 85 (68.0) | 169 (79.7) | |
| Diffuse-mixed | 40 (32.0) | 43 (20.3) | |
| Lymphovascular invasion | < 0.001 | ||
| Present | 21 (16.8) | 9 (4.2) | |
| Absent | 104 (83.2) | 203 (95.8) | |
| pT stage | < 0.001 | ||
| pT2, pT3 | 30 (24.0) | 105 (49.5) | |
| pT4a | 95 (76.0) | 107 (50.5) | |
| pN stage | < 0.001 | ||
| N0 | 13 (10.4) | 82 (38.7) | |
| N1 | 25 (20.0) | 52 (24.5) | |
| N2 | 30 (24.0) | 46 (21.7) | |
| N3 | 57 (45.6) | 32 (15.1) | |
| Intraoperative chemotherapy | < 0.001 | ||
| Yes | 54 (43.2) | 136 (64.2) | |
| No | 71 (56.8) | 76 (35.8) | |
| Postoperative chemotherapy | 0.036 | ||
| No | 42 (33.6) | 49 (23.1) | |
| 5-FU-based regimen | 83 (66.4) | 163 (76.9) |
| Factor | P value | OR | 95%CI |
| A Age at diagnosis | 0.033 | 1.813 | 1.050-3.131 |
| pT pT stage | < 0.001 | 2.865 | 1.603-5.123 |
| pN stage | < 0.001 | ||
| N0 | 1.00 (reference) | ||
| N1 | 0.001 | 4.029 | 1.708-9.500 |
| N2 | 0.001 | 4.425 | 1.889-10.365 |
| N3 | < 0.001 | 9.860 | 4.314-22.536 |
| Lauren histotype | < 0.001 | 3.492 | 1.810-6.736 |
| Lymphovascular invasion | 0.011 | 3.460 | 1.335-8.969 |
| Intraoperative chemotherapy | < 0.001 | 0.327 | 0.190-0.564 |
| Postoperative chemotherapy | 0.007 | 0.423 | 0.225-0.793 |
Many studies have reported that approximately 70% of patients with gastric cancer experience early tumor recurrence, defined as within 2 years after surgery[9,11,24]. Less is known, however, about patterns of recurrence and predictors of early recurrence, especially in Chinese patients. This study therefore investigated recurrence patterns and factors predicting early recurrence in patients undergoing curative surgery for gastric cancer.
In agreement with earlier findings[9,11], we found that most recurrences (129/194, 66.5%) were early, occurring within 2 years after curative surgery. The three main types of early recurrence were hematogenous (56/129, 43.4%), peritoneal (59/129, 45.7%), and locoregional (68/129, 52.7%). Patients with early recurrence had more liver metastases than patients with late recurrence (32.6% vs 20.0%), although the difference was not significant (P = 0.067). No other differences in recurrence patterns were observed between the early and late recurrence groups. As previously reported[12], survival after recurrence was poor, especially in patients with early recurrence.
Because 66.5% of recurrences occurred within 2 years after surgery and this study focused on factors associated with early recurrence, clinicopathologic characteristics were compared in patients with and without early recurrence within 2 years after surgery.
Age, lymphatic metastasis and submucosal invasion have been reported to be significantly associated with recurrence of gastric cancer[25]. In the current study, only 5% (4/80) of patients with early gastric cancer experienced early recurrence. Although larger tumor size and advanced AJCC pN stage were found to be significantly associated with early recurrence in this group, the number of patients was small, indicating that these results require further validation.
The early recurrence rate was significantly higher in patients with locally advanced than early gastric cancer. Age at diagnosis, AJCC pT stage, AJCC pN stage, Lauren histotype, lymphovascular invasion, intraoperative chemotherapy, and postoperative chemotherapy were found to be independent factors influencing timing of recurrence in patients who underwent curative surgery for locally advanced gastric cancer. No obvious multicollinearity was observed among these factors.
Interestingly, we found that more patients in the late- and non-recurrence groups received intraoperative chemotherapy than did patients in the early recurrence group, with intraoperative chemotherapy found to be an independent predictor of early recurrence [hazard ratio (HR) = 0.327, P < 0.001]. Most previous studies of intraoperative chemotherapy assessed only patients who received hyperthermic intraperitoneal chemotherapy, not intravenous chemotherapy, although intravenous 5-FU during surgery for advanced gastric cancer was first administered in 1987[26,27]. A randomized controlled clinical trial is required to confirm the benefits of intraoperative chemotherapy.
The current study also found that postoperative chemotherapy was an independent predictor of early recurrence (HR = 0.423, P < 0.007). Results of both the Capecitabine and Oxaliplatin Adjuvant Study in Stomach Cancer and the Adjuvant Chemotherapy Trial of TS-1 for Gastric Cancer trials suggested that FU-based adjuvant therapy improves survival of patients with gastric cancer following gastrectomy combined with D2 lymphadenectomy[28-31]. However, another study found that adjuvant chemotherapy was not a significant predictor of the timing of recurrence[12]. Further evaluation is necessary to assess the effect of adjuvant chemotherapy on early recurrence.
The main limitations of this study were its retrospective nature and relatively small sample size.
In summary, this retrospective study found that larger tumor size and advanced AJCC pN stage were significantly associated with early recurrence of early gastric cancer; whereas age at diagnosis, AJCC pT stage, AJCC pN stage, Lauren histotype, and lymphovascular invasion were all independent predictors of early recurrence in patients undergoing curative surgery for locally advanced gastric cancer. Chemotherapy, both intraoperative and postoperative, was associated with early recurrence of gastric carcinoma. Patients with gastric cancer should be closely monitored and actively followed for at least 2 years after surgery.
Tumor recurrence is the leading cause of death in patients who undergo curative surgery for gastric cancer. Few studies have assessed the patterns and timing of recurrence or predictors of early recurrence following surgery in Chinese patients. This retrospective study analyzed patterns and timing of recurrence in patients who underwent curative surgery for gastric cancer, and identified clinicopathological factors and therapeutic modalities, especially intraoperative chemotherapy, significantly associated with early recurrence, in order to develop appropriate treatments and follow-up programs.
This study was based on the experience of a large single center with intraoperative chemotherapy, curative surgery and postoperative chemotherapy for gastric cancer. Factors correlated with early recurrence were analyzed.
Intraoperative chemotherapy, as well as postoperative chemotherapy, age, pT stage, pN stage, Lauren histotype, and lymphovascular invasion were found to be independent predictors of early recurrence of pT2-4a stage gastric cancer.
Intraoperative chemotherapy combined with postoperative chemotherapy may reduce rates of early recurrence in patients with locally advanced gastric cancer.
This manuscript identified predictors of early recurrence after curative surgery and characterized patterns of recurrence and survival after gastric cancer recurrence. The study was very well designed and interesting.
P- Reviewer: Bell E, Ozdemir O S- Editor: Yu J L- Editor: Wang TQ E- Editor: Liu XM
| 1. | Chen W, Zheng R, Zhang S, Zhao P, Li G, Wu L, He J. Report of incidence and mortality in China cancer registries, 2009. Chin J Cancer Res. 2013;25:10-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 167] [Reference Citation Analysis (0)] |
| 2. | Chen W, Zheng R, Zhang S, Zhao P, Li G, Wu L, He J. The incidences and mortalities of major cancers in China, 2009. Chin J Cancer. 2013;32:106-112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 136] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 3. | Zou ZH, Zhao LY, Mou TY, Hu YF, Yu J, Liu H, Chen H, Wu JM, An SL, Li GX. Laparoscopic vs open D2 gastrectomy for locally advanced gastric cancer: a meta-analysis. World J Gastroenterol. 2014;20:16750-16764. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 72] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 4. | Seeliger H, Spatz H, Jauch KW. Minimal residual disease in gastric cancer. Recent Results Cancer Res. 2003;162:79-87. [PubMed] |
| 5. | Ubukata H, Motohashi G, Tabuchi T, Nagata H, Konishi S, Tabuchi T. Overt bone metastasis and bone marrow micrometastasis of early gastric cancer. Surg Today. 2011;41:169-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Zhang ZY, Dai ZL, Yin XW, Li SH, Li SP, Ge HY. Meta-analysis shows that circulating tumor cells including circulating microRNAs are useful to predict the survival of patients with gastric cancer. BMC Cancer. 2014;14:773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Ito H, Inoue H, Kimura S, Ohmori T, Ishikawa F, Gohda K, Sato J. Prognostic impact of the number of viable circulating cells with high telomerase activity in gastric cancer patients: a prospective study. Int J Oncol. 2014;45:227-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Uenosono Y, Arigami T, Kozono T, Yanagita S, Hagihara T, Haraguchi N, Matsushita D, Hirata M, Arima H, Funasako Y. Clinical significance of circulating tumor cells in peripheral blood from patients with gastric cancer. Cancer. 2013;119:3984-3991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 9. | Shiraishi N, Inomata M, Osawa N, Yasuda K, Adachi Y, Kitano S. Early and late recurrence after gastrectomy for gastric carcinoma. Univariate and multivariate analyses. Cancer. 2000;89:255-261. [PubMed] |
| 10. | Marrelli D, De Stefano A, de Manzoni G, Morgagni P, Di Leo A, Roviello F. Prediction of recurrence after radical surgery for gastric cancer: a scoring system obtained from a prospective multicenter study. Ann Surg. 2005;241:247-255. [PubMed] |
| 11. | Chiang CY, Huang KH, Fang WL, Wu CW, Chen JH, Lo SS, Hsieh MC, Shen KH, Li AF, Niu DM. Factors associated with recurrence within 2 years after curative surgery for gastric adenocarcinoma. World J Surg. 2011;35:2472-2478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Eom BW, Yoon H, Ryu KW, Lee JH, Cho SJ, Lee JY, Kim CG, Choi IJ, Lee JS, Kook MC. Predictors of timing and patterns of recurrence after curative resection for gastric cancer. Dig Surg. 2010;27:481-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Lee SE, Lee JH, Ryu KW, Nam B, Kim CG, Park SR, Kook MC, Kim YW. Changing pattern of postoperative body weight and its association with recurrence and survival after curative resection for gastric cancer. Hepatogastroenterology. 2012;59:430-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 14. | Meng QB, Yu JC, Ma ZQ, Kang WM, Zhou L, Ye X. Benefits of intra-operative systemic chemotherapy during curative surgery in patients with locally advanced gastric cancer. Chin Med J (Engl). 2013;126:3493-3498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol. 2010;17:3077-3079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 702] [Cited by in RCA: 814] [Article Influence: 58.1] [Reference Citation Analysis (0)] |
| 16. | Zhang J, Zhou Y, Jiang K, Shen Z, Ye Y, Wang S. Evaluation of the seventh AJCC TNM staging system for gastric cancer: a meta-analysis of cohort studies. Tumour Biol. 2014;35:8525-8532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31-49. [PubMed] |
| 18. | Patel SH, Kooby DA. Gastric adenocarcinoma surgery and adjuvant therapy. Surg Clin North Am. 2011;91:1039-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Waddell T, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D, European Society for Medical Oncology (ESMO), European Society of Surgical Oncology (ESSO); European Society of Radiotherapy and Oncology (ESTRO). Gastric cancer: ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Eur J Surg Oncol. 2014;40:584-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 147] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 20. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011;14:113-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1723] [Cited by in RCA: 1895] [Article Influence: 135.4] [Reference Citation Analysis (0)] |
| 21. | Orditura M, Galizia G, Sforza V, Gambardella V, Fabozzi A, Laterza MM, Andreozzi F, Ventriglia J, Savastano B, Mabilia A. Treatment of gastric cancer. World J Gastroenterol. 2014;20:1635-1649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 461] [Cited by in RCA: 483] [Article Influence: 43.9] [Reference Citation Analysis (5)] |
| 22. | Wang X, Wang Y, Qiu M, Li Q, Li ZP, He B, Xu F, Shen YL, Gou HF, Yang Y. Postoperative chemoradiotherapy in gastric cancer: a phase I study of radiotherapy with dose escalation of oxaliplatin, 5-fluorouracil, and leucovorin (FOLFOX regimen). Med Oncol. 2011;28 Suppl 1:S274-S279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Wu Y, Wei ZW, He YL, Schwarz RE, Smith DD, Xia GK, Zhang CH. Efficacy of adjuvant XELOX and FOLFOX6 chemotherapy after D2 dissection for gastric cancer. World J Gastroenterol. 2013;19:3309-3315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Sakar B, Karagol H, Gumus M, Basaran M, Kaytan E, Argon A, Ustuner Z, Bavbek SE, Bugra D, Aykan FN. Timing of death from tumor recurrence after curative gastrectomy for gastric cancer. Am J Clin Oncol. 2004;27:205-209. [PubMed] |
| 25. | Wu B, Wu D, Wang M, Wang G. Recurrence in patients following curative resection of early gastric carcinoma. J Surg Oncol. 2008;98:411-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 26. | Lelcuk S, Klausner JM, Inbar M, Kaplan O, Merhav A, Rozin RR. Gastrointestinal anastomotic healing during treatment with perioperative 5-fluorouracil. Am J Clin Oncol. 1987;10:317-319. [PubMed] |
| 27. | Yan TD, Black D, Sugarbaker PH, Zhu J, Yonemura Y, Petrou G, Morris DL. A systematic review and meta-analysis of the randomized controlled trials on adjuvant intraperitoneal chemotherapy for resectable gastric cancer. Ann Surg Oncol. 2007;14:2702-2713. [PubMed] |
| 28. | Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH, Lee KW, Kim YH, Noh SI, Cho JY. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012;379:315-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1290] [Article Influence: 99.2] [Reference Citation Analysis (0)] |
| 29. | Noh SH, Park SR, Yang HK, Chung HC, Chung IJ, Kim SW, Kim HH, Choi JH, Kim HK, Yu W. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:1389-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 783] [Cited by in RCA: 775] [Article Influence: 70.5] [Reference Citation Analysis (0)] |
| 30. | Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, Furukawa H, Nakajima T, Ohashi Y, Imamura H. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357:1810-1820. [PubMed] |
| 31. | Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T, Nashimoto A, Fujii M, Nakajima T, Ohashi Y. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29:4387-4393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 1089] [Article Influence: 77.8] [Reference Citation Analysis (0)] |