Published online May 21, 2015. doi: 10.3748/wjg.v21.i19.5901
Peer-review started: October 27, 2014
First decision: December 11, 2014
Revised: December 29, 2014
Accepted: January 21, 2015
Article in press: January 21, 2015
Published online: May 21, 2015
Processing time: 210 Days and 10.1 Hours
AIM: To investigate the correlations of pre-treatment positron emission tomography-computer tomography (PET-CT) metabolic quantifiers with clinical data of unstratified gastric cancer (GC) patients.
METHODS: Forty PET-CT scans utilising 18-fluorodeoxyglucose in patients who received no prior treatment were analysed. Analysis involved measurements of maximum and mean standardised uptake volumes (SUV), coefficient of variation (COV), metabolic tumour volumes and total lesion glycolysis of different thresholds above which the tumor volumes were identified. The threshold values were: SUV absolute value of 2.5, 30% of SUVmax, 40% of SUVmax, and liver uptake-based (marked 2.5, 30, 40 and liv, respectively). Clinical variables such as age, sex, clinical stage, performance index, weight loss, tumor histological type and grade, and CEA and CA19.9 levels were included in survival analysis. Patients received various treatment modalities appropriate to their disease stage and the outcome was defined by time to metastasis (TTM) and overall survival (OS). Clinical and metabolic parameters were evaluated by analysis of variance, receiver operating characteristics, univariate Kaplan-Meier, and multivariate Cox models. P < 0.05 was considered statistically significant.
RESULTS: Significant differences were observed between initially disseminated and non-disseminated patients in mean SUV (6.05 vs 4.13, P = 0.008), TLG2.5 (802 cm3vs 226 cm3; P = 0.031), and TLG30 (436 cm3vs 247 cm3, P = 0.018). Higher COV was associated with poor tumour differentiation (0.47 for G3 vs 0.28 for G1 and G2; P = 0.03). MTV2.5 was positively correlated to patient weight loss (< 5%, 5%-10% and > 10%: 40.4 cm3vs 123.6 cm3vs 181.8 cm3, respectively, P = 0.003). In multivariate Cox analysis, TLG30 was prognostic for OS (HR = 1.001, 95%CI: 1.0009-1.0017; P = 0.047) for the whole group of patients. In the same model yet only including patients without initial disease dissemination TLG30 (HR = 1.009, 95%CI: 1.003-1.014; P = 0.004) and MTV2.5 (HR = 1.02, 95%CI: 1.002-1.036; P = 0.025) were prognostic for OS; for TTM TLG30 was the only significant prognostic variable (HR = 1.006, 95%CI: 1.001-1.012; P = 0.02).
CONCLUSION: PET-CT in GC may represent a valuable diagnostic and prognostic tool that requires further evaluation in highly standardised environments such as randomised clinical trials.
Core tip: This study is one of the first to investigate the potential use of such a variety of radiotracer quantifiers, demonstrating their ability to differentiate locally advanced and disseminated tumours. This broad analysis can be utilized in clinical use to identify groups of patients with an unfavourable tumour prognosis who could possibly benefit from more aggressive treatment. Our database is being continuously updated and we plan to validate our findings in a larger and more homogeneous cohort.
- Citation: Grabinska K, Pelak M, Wydmanski J, Tukiendorf A, d’Amico A. Prognostic value and clinical correlations of 18-fluorodeoxyglucose metabolism quantifiers in gastric cancer. World J Gastroenterol 2015; 21(19): 5901-5909
- URL: https://www.wjgnet.com/1007-9327/full/v21/i19/5901.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i19.5901
Gastric adenocarcinoma is the third leading cause of cancer death in both sexes worldwide (723000 deaths, 8.8% of the total). The highest estimated mortality rates are in Eastern Asia (24 per 100000 in men, 9.8 per 100000 in women), and the lowest in Northern America (2.8 and 1.5, respectively). High mortality rates are also present in both sexes in Central and Eastern Europe and in Central and South America. Gastric cancer exhibits a high mortality to incidence ratio, which indirectly demonstrates the low curability of gastric cancer, suggesting that there is still significant room for improvement in its diagnosis and therapy[1].
Unspecific symptoms construed as indigestion or peptic ulcer disease are often ignored and contribute to late detection. The diagnosis of gastric cancer largely relies on endoscopy, which has been proven effective both medically and epidemiologically. However, due to changing approach to treatment of gastric cancer, which varies between local, locoregional, and metastatic disease, endoscopy should be accompanied by imaging modalities that help fully identify disease stage. This additional imaging has traditionally been achieved through contrast-enhanced computer tomography of the abdomen[2,3]. Recent studies have demonstrated high specificity and sensitivity of fusion positron emission tomography with computed tomography and its superiority over former methods in gastric cancer in detection of distant metastatic sites. However, reports demonstrate both advantages and limitations[4-7].
Currently, there are a number of reports indicating high usability of positron emission tomography- computer tomography (PET-CT) in determining disease stage[8-11], identification of recurrence following surgery[12], response to chemotherapy[8,9], prognostic value of 18Fluorodeoxyglucose (18FDG) metabolism quantifiers, risk of nodal and distant spread of tumour[11] and correlation between 18FDG uptake and tumour histopathology or grade[6].
Some of the limitations of PET-CT in gastric cancer as described by various studies include insufficient uptake in signet-ring histological subtype as well as in nodal and peritoneal metastasis. Insufficient uptake is also a problem for tumours less than 30 mm in diameter or those limited submucosa. The dependence of sensitivity on tumour infiltration level within the stomach wall has been reported for PET-CT. Reports have shown sensitivity of 44% in T1 and 92% in T2-T4 tumours.
However, false positive results with high tracer uptake are often observed in chronic mucosal inflammation[5]. Optimal study pre-conditioning (adequate filling of the gastric and dilating its wall) can increase imaging sensitivity[13,14].
Our study analysed the pattern of commonly measured and potentially clinically significant 18FDG metabolism quantifiers in PET-CT fusion studies in a cohort of previously untreated patients with gastric cancer and its correlations to clinical data. We also investigated the quality of the 18FDG variables in an attempt to select the variables most suitable for measurement in stomach cancer. The present study is one of a few studies that have evaluated such a large number of PET/CT parameters in gastric cancer. Earlier studies concentrated only on the SUV max value. Our analysis focused in particular on the differences between patients with local and metastatic disease and the possible impact of disease on their overall survival and time-to-metastasis.
All patients received no treatment prior to the examination and had undergone abdominal computed tomography with contrast-enhancement before PET/CT. The intention of the examination was to initially assess stage of disease for optimal selection of different treatment modalities. This study was approved by the local ethics committee (committee number-KB/493-59/09) in accordance with the Helsinki Declaration of 1975, as revised in 2000. The study protocol (established upon our institution experience) was uniform for all patients as follows: after a 6-h fast, patients were intravenously administered 7-15 mCi activity of 18FDG (0.1 mCi per kg body weight). CT data were acquired on exhaust breath phase not exceeding 9.6 s; PET acquisition times ranged from 17 to 20 min. All metabolic quantifiers were analysed on Siemens® Syngo.via™ PET-CT-dedicated workstations. Glucose metabolism-related factors that could possibly affect interpretation of results (blood glucose level at time of study and incidence of diabetes mellitus) were uniformly distributed in all groups. Clinical characteristics of the patients are summarised in Table 1.
| Variable | Overall (n = 40) number | Locally advanced (n = 23) number | Disseminated (n = 17) number | P value |
| Age: median/range (yr) | 63/37-79 | 69/37-79 | 62/42-79 | 0.38 |
| Sex | 0.66 | |||
| Male | 31 (77) | 17 (74) | 14 (82) | |
| Female | 9 (23) | 6 (26) | 3 (18) | |
| Performance status | 0.73 | |||
| 0 | 18 (45) | 11 (48) | 7 (41) | |
| 1-2 | 22 (55) | 12 (52) | 10 (59) | |
| Weight loss | 0.61 | |||
| < 5% | 11 (28) | 7 (30) | 4 (24) | |
| 5%-10% | 12 (30) | 7 (30) | 5 (29) | |
| > 10% | 17 (42) | 9 (40) | 8 (47) | |
| Tumour location | 0.85 | |||
| Upper third | 20 (50) | 12 (52) | 8 (47) | |
| Middle third | 15 (37.5) | 8 (35) | 7 (41) | |
| Lower third | 5 (12.5) | 3 (13) | 2 (12) | |
| Tumour clinical stage (AJCC 2010) | 0.39 | |||
| cT1-T3 | 32 (80) | 20 (87) | 12 (71) | |
| cT4 | 8 (20) | 3 (13) | 5 (29) | |
| Nodal involvement (AJCC 2010) | 0.014 | |||
| cN0 | 20 (50) | 16 (70) | 4 (23) | |
| cN1-N3 | 20 (50) | 7 (30) | 13 (77) | |
| Histology | 0.45 | |||
| Intestinal type | 32 (80) | 17 (74) | 15 (88) | |
| Diffuse type | 8 (20) | 6 (26) | 2 (12) | |
| Histological grade | 0.71 | |||
| G1-G2 | 12 (30) | 8 (35) | 4 (24) | |
| G3 | 18 (45) | 9 (39) | 9 (52) | |
| Not specified | 10 (25) | 6 (26) | 4 (24) | |
| CA19.9 median (range), IU/mL | 10.65 (2-159674) | 9.43 (2.0-12571) | 11.07 (2.0-159674) | 0.10 |
| CEA median (range), IU/mL | 2.86 (0.5-514) | 2.09 (0.5-117) | 9.04 (0.7-514) | 0.17 |
We retrospectively analyzed a set of 40 18FDG PET-CT scans performed in Maria Skłodowska-Curie Memorial Institute of Oncology between 2008 and 2014 by one of two hybrid PET-CT scanners (Philips® Gemini XL and Siemens® Biograph™ mCT) in patients who had histologically confirmed gastric cancer.
Both scanners were periodically calibrated against the same electronic phantom probe that guarantees identical baseline SUV readouts of the reference radiotracer activity. PET-CT studies were performed randomly 1 h after administration of 18FDG. Acquired DICOM images were analysed on Siemens Syngo.via PET-CT workstations. The following parameters were assessed for each primary gastric tumour: maximum standard uptake volume (SUVmax), mean SUV (SUVmean) and metabolic tumour volume (MTV). The latter was measured with four different thresholds, varying by the SUV above which voxels inside the three-dimensional region of interest (ROI) covering the visible tumour were considered the metabolic volume. The following metabolic volumes were listed: MTV2.5 (threshold: SUV = 2.5), MTV30 (≥ 30% of SUVmax), MTV40 (≥ 40% of SUVmax), MTVliv [≥ mean SUV of the patient’s liver + 2 standard deviations (SD)]. These values were measured directly on PET-CT workstations. Also analysed were the following composite parameters: Total lesion glycolysis (TLG = SUVmean* MTV) and coefficient of variation (COV = SD/SUVmean).
Statistical calculations were performed using Statistica 10 Software (StatSoft, Inc.). The group was compared with the independent sample t-test, analysis of variance or the Mann-Whitney U-test. Univariate ROC curve model[15] was used for assessment of the confidence of 18FDG metabolism quantifiers to identify metastatic and local disease. The impact of 18FDG metabolism quantifiers on overall survival (OS) and time-to-metastasis (TTM) was analysed by Cox and Kaplan-Meier models. Overall survival was defined as time from date of PET-CT study to patient death and (TTM) was defined as time from PET-CT study to first follow-up visit at which tumour dissemination was confirmed (by pathologic examination of specimen, most commonly probatory peritoneal biopsies or by clinically evident lesions in imaging studies); TTM was only assessed for patients who were not initially disseminated. Results within 95%CI (P < 0.05) were considered statistically significant.
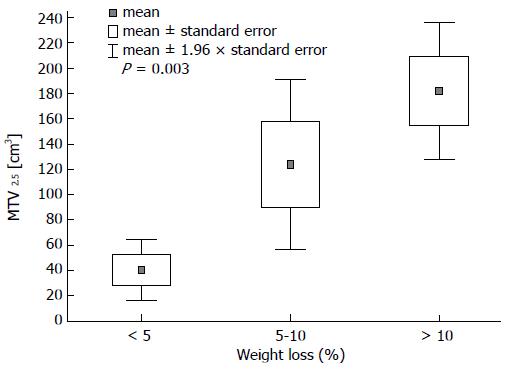
Analysis of 18FDG metabolism quantifiers revealed significant differences between three particular clinical groups of patients with stomach cancer. MTV2.5 was related to level of weight loss relative to starting weight: the average volume varied significantly among groups with: (1) less than 5% weight loss; and (2) 5% to 10% weight loss; (3) more than 10% weight loss: 40.4 cm3vs 123.6 cm3vs 181.8 cm3, respectively (P = 0.003, Figure 1).
Other significant metabolic variables included COV (0.21 vs 0.44 vs 0.44; P = 0.03) and TLGliv (4.63 cm3vs 17.45 cm3vs 19.52 cm3, P = 0.03). Another finding was the difference among different tumour grades with respect to COV, with higher COV observed in poorly differentiated G3 tumours than in better differentiated G1 and G2 tumours (0.46 vs 0.28, P = 0.03). Finally, almost all metabolic quantifiers differed between T1-T3 and T4 clinical stages. Tumour metabolic volumes (MTV2.5: 102.4 cm3vs 217.3 cm3, P = 0.009; MTV40: 46.7 cm3vs 107.5 cm3, P = 0.0007; MTVliv 57.8 cm3vs 166.3 cm3, P = 0.002) and the total lesion glycolysis volumes (TLG2.5 543.6 cm3vs 1827.1 cm3, P = 0.004; TLG30: 394.3 cm3vs 1086.1 cm3, P = 0.005; TLGliv: 12 cm3vs 26.1 cm3, P = 0.01) increased with clinical stage. A summary of this analysis is presented in Table 2.
| SUVmax | SUVmean | MTV2.5(cm3) | MTV30(cm3) | MTV40(cm3) | MTVliv(cm3) | COV | TLG2.5(cm3) | TLG30(cm3) | TLG40(cm3) | TLGliv(cm3) | |
| Sex | |||||||||||
| Male | 12.97 | 5.00 | 133.19 | 105.19 | 60.81 | 86.26 | 0.41 | 866.70 | 551.5 | 16.14 | 13.99 |
| Female | 10.88 | 4.76 | 98.91 | 85.45 | 52.06 | 56.03 | 0.28 | 571.58 | 384.9 | 10.21 | 9.22 |
| P value | 0.51 | 0.79 | 0.43 | 0.65 | 0.63 | 0.39 | 0.18 | 0.52 | 0.48 | 0.29 | 0.30 |
| Performance status | |||||||||||
| 0 | 11.16 | 4.39 | 109.79 | 124.00 | 68.8 | 65.79 | 0.37 | 582.67 | 570.26 | 15.14 | 13.35 |
| 1-2 | 13.60 | 5.41 | 138.31 | 81.73 | 50.69 | 90.63 | 0.39 | 978.35 | 478.41 | 11.09 | 15.99 |
| P value | 0.36 | 0.17 | 0.44 | 0.24 | 0.24 | 0.40 | 0.87 | 0.3 | 0.65 | 0.29 | 0.59 |
| Weight loss | |||||||||||
| < 5% | 7.71 | 4.31 | 40.41 | 61.38 | 38.98 | 23.98 | 0.211 | 209.67 | 243.88 | 7.52 | 4.631 |
| 5%-10% | 12.68 | 4.56 | 123.61 | 90.26 | 57.73 | 88.61 | 0.441 | 678.12 | 431.41 | 14.17 | 17.451 |
| > 10% | 15.47 | 5.63 | 181.81 | 133.62 | 72.48 | 108.90 | 0.441 | 1268.7 | 747.12 | 15.52 | 19.521 |
| P value | 0.051 | 0.28 | 0.0031 | 0.24 | 0.19 | 0.053 | 0.031 | 0.059 | 0.096 | 0.20 | 0.0271 |
| Tumour location | |||||||||||
| Upper third | 13.93 | 5.15 | 129.16 | 79.95 | 52.18 | 79.18 | 0.43 | 850.20 | 418.48 | 12.98 | 15.41 |
| Middle third | 11.11 | 4.83 | 142.17 | 74.72 | 54.09 | 88.87 | 0.29 | 878.94 | 727.09 | 13.95 | 15.01 |
| Lower third | 10.96 | 4.46 | 82.93 | 59.68 | 37.85 | 52.31 | 0.41 | 364.75 | 256.94 | 9.51 | 11.77 |
| P value | 0.56 | 0.82 | 0.67 | 0.19 | 0.22 | 0.75 | 0.24 | 0.68 | 0.2 | 0.77 | 0.89 |
| AJCC tumour clinical stage | |||||||||||
| cT1-T3 | 11.67 | 4.61 | 102.41 | 87.24 | 46.71 | 57.81 | 0.39 | 543.61 | 394.31 | 11.61 | 121 |
| cT4 | 15.82 | 6.28 | 217.31 | 154.79 | 107.51 | 166.31 | 0.34 | 1827.11 | 1086.11 | 18.24 | 26.101 |
| P value | 0.21 | 0.07 | 0.0091 | 0.13 | 0.00071 | 0.0021 | 0.63 | 0.0041 | 0.0051 | 0.16 | 0.011 |
| AJCC nodal involvement | |||||||||||
| cN0 | 11.32 | 4.49 | 97.33 | 70.88 | 46.16 | 66.48 | 0.40 | 600.96 | 376.09 | 11.11 | 12.04 |
| cN1-N3 | 13.68 | 5.41 | 153.62 | 130.61 | 71.52 | 91.43 | 0.36 | 999.63 | 668.3 | 14.73 | 17.58 |
| P value | 0.37 | 0.22 | 0.12 | 0.1 | 0.10 | 0.38 | 0.58 | 0.29 | 0.14 | 0.34 | 0.26 |
| Histology | |||||||||||
| Intestinal type | 13.34 | 5.17 | 138.79 | 106.51 | 60.93 | 91.90 | 0.40 | 902.61 | 560.06 | 13.85 | 17.01 |
| Diffuse type | 9.13 | 4.06 | 72.19 | 77.68 | 50.47 | 29.67 | 0.29 | 391.05 | 328.19 | 9.16 | 5.98 |
| P value | 0.21 | 0.24 | 0.14 | 0.53 | 0.59 | 0.09 | 0.31 | 0.28 | 0.38 | 0.32 | 0.07 |
| Histological grade | |||||||||||
| G1-G2 | 13.16 | 4.83 | 118.67 | 64.91 | 39.76 | 73.64 | 0.281 | 632.14 | 309.77 | 10.07 | 17.06 |
| G3 | 11.99 | 5.20 | 122.11 | 133.85 | 71.31 | 70.96 | 0.471 | 950.73 | 702.29 | 14.20 | 10.14 |
| P value | 0.61 | 0.73 | 0.91 | 0.14 | 0.36 | 0.89 | 0.031 | 0.59 | 0.11 | 0.42 | 0.16 |
A comparative analysis of the primary tumour metabolism of patients with local and disseminated disease revealed a statistically significant difference in SUVmean between the two groups: 4.13 vs 6.05, respectively, (P = 0.008). TLG2.5 and TLG30 also varied between local and disseminated disease: 225.87 cm3vs 802.17 cm3 (P = 0.03) for TLG2.5 and 247.33 cm3vs 435.61 cm3 (P = 0.01) for TLG30. Comparisons for all parameters are presented in Table 3.
| Metabolic quantifier | Overall (n = 40) | Locally advanced (n = 23) | Disseminated (n =17) | P value |
| SUVmax | 10.95 4.14-47.74 | 10.36 4.14-22.66 | 12.78 2.73-47.74 | 0.130 |
| SUVmean1 | 4.951 1.94-13.831 | 4.131 1.94-6.381 | 6.051 2.78-13.831 | 0.0081 |
| COV | 0.34 0-1.12 | 0.38 0.03-1.12 | 0.35 0-0.80 | 0.360 |
| MTV2.5(cm3) | 91.36 0.96-688.63 | 77.16 6.91-379.2 | 135.55 0.96-688.63 | 0.058 |
| MTV30(cm3) | 69.16 12.79-668.60 | 58.36 12.79-275.3 | 91.97 21.82-668.60 | 0.130 |
| MTV40(cm3) | 46.05 8.13-208.36 | 31.85 8.13-208.22 | 56.64 14.34-208.36 | 0.110 |
| MTVliv(cm3) | 47.31 0-352.25 | 31.56 1.18-337.38 | 87.12 0-352.25 | 0.140 |
| TLG2.5(cm3)1 | 445.071 2.62-59841 | 225.871 15.13-2419.91 | 802.171 2.62-59841 | 0.0311 |
| TLG30(cm3)1 | 352.651 46.7-29131 | 247.331 46.7-1756.41 | 435.611 113.9-29131 | 0.0181 |
| TLG40(cm3) | 8.35 0.56-49.26 | 7.61 1.41-45.77 | 8.82 0.56-49.26 | 0.760 |
| TLGliv(cm3) | 10.5 0-70.42 | 6.6 0.4-70.42 | 13.81 0-34.15 | 0.950 |
Analysis of all metabolic quantifiers using ROC curve model named TLG30, TLG2.5, MTV40 and SUVmean as fairly reliable quantifiers to identify tumour dissemination. Results, including identification of optimal cut-off values (best weighed between specifity and sensitivity) for each parameter are displayed in Table 4. Parameters are displayed in order according to overall grade, which is based on AUC (area under curve[15])-see table descriptions.
| Metabolic quantifier | AUC | SE | 95%CI | Cutoff value | Sensitivity of cutoff value | Specifity of cutoff value | Overall mark | P value |
| TLG30(cm3)1 | 0.781 | 0.071 | 0.63-0.921 | 390.531 | 65%1 | 78%1 | Fair1 | 0.00061 |
| SUVmean1 | 0.731 | 0.081 | 0.58-0.891 | 6.871 | 35%1 | 100%1 | Fair1 | 0.09001 |
| TLG2.5(cm3)1 | 0.721 | 0.081 | 0.55-0.881 | 802.171 | 53%1 | 83%1 | Fair1 | 0.03401 |
| MTV40(cm3)1 | 0.711 | 0.081 | 0.55-0.871 | 37.251 | 88%1 | 61%1 | Fair1 | 0.00101 |
| MTV30(cm3) | 0.69 | 0.08 | 0.53-0.86 | 83.54 | 65% | 74% | Poor | 0.0020 |
| MTV2.5(cm3) | 0.68 | 0.09 | 0.51-0.84 | 132.55 | 59% | 74% | Poor | 0.0600 |
| MTVliv(cm3) | 0.67 | 0.09 | 0.50-0.84 | 87.12 | 53% | 74% | Poor | 0.1000 |
| SUVmax | 0.61 | 0.09 | 0.43-079 | 14.44 | 47% | 78% | Poor | 0.4000 |
| TLGliv(cm3) | 0.61 | 0.09 | 0.43-0.79 | 7.42 | 71% | 56% | Poor | 0.3000 |
| TLG40(cm3) | 0.50 | 0.09 | 0.31-0.68 | 49.26 | 6% | 100% | Useless | - |
| COV | 0.50 | 0.09 | 0.27-0.63 | 1.13 | 0% | 96% | Useless | - |
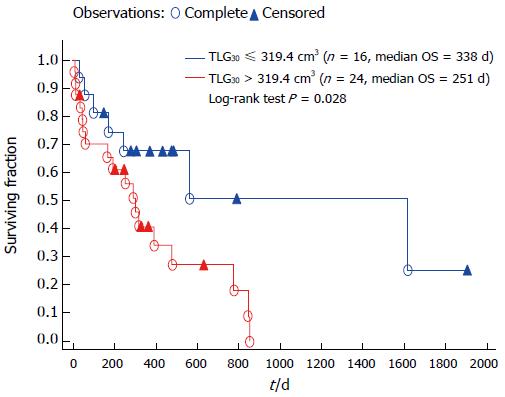
Clinical and metabolic variables were analysed using Cox multivariate models. Factors that significantly affected OS were as follows: male sex (HR = 0.13, 95%CI: 0.007-0.41; P = 0.005), initial tumour site in antrum (HR = 0.08, 95%CI: 0.007-0.39; P = 0.008), and TLG30 (HR = 1.001, 95%CI: 1.0009-1.0017; P = 0.047). The Kaplan-Meier univariate model comparing two groups of patients stratified by the ROC-calculated threshold for TLG30 confirmed TLG30 parameter to be a significant prognostic factor for OS (Figure 2). Predictably, there were more patients with disseminated disease who had TLG30 volumes above the threshold than non-disseminated ones (15/17 vs 9/23, P = 0.008). Notably though, the initial disease dissemination itself was not a significant survival predictor in univariate Kaplan-Meier model.
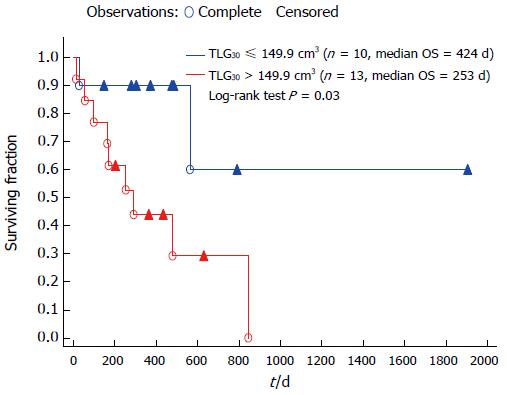
A separate analysis of OS and TTM was performed for patients in whom the PET-CT study identified local disease (n = 23). For OS, TLG30 was as well an independent prognostic factor (HR = 1.009, 95%CI: 1.003-1.014; P = 0.004), along with MTV2.5 (HR = 1.02, 95%CI: 1.002-1.036; P = 0.025). OS in the Kaplan-Meier model varied significantly for TLG30-stratified groups (Figure 3; note the different ROC threshold value resulting from lower TLG30 values in non-disseminated patients’ group). For TTM, the Cox proportional hazard model identified TLG30 as the only significant prognostic factor (HR = 1.006, 95%CI: 1.001-1.012; P = 0.02). This result was not significant when analysed in univariate Kaplan-Meier model. However, of all of parameters analysed in this model, TLG30 threshold was closest to reaching statistical significance (P = 0.06).
Other metabolic parameters were not observed to vary significantly across patient subgroups with respect clinical variables listed in Table 1, particularly between metastatic and non-metastatic patients. A summary of the average metabolic quantifier values for these variables is presented in Tables 2 and 3.
Two reports on usefulness of PET-CT imaging in gastric cancer (GC) have emerged recently, especially regarding its utilisation in disease staging[7,16]. These suggest that, despite its particular limitations in assessment of small, early tumours[17], PET-CT can reliably visualise advanced primary tumours and can detect tumour dissemination to (regional) lymph nodes and distant organs. The latter are often silent on contrast-enhanced CT[4,5,7]. One group reported 94% sensitivity in PET-CT diagnosis in advanced GC (stages III and IV)[18].
In addition to diagnostic value, many researchers have attempted to identify possible prognostic and predictive information derived from radiotracer metabolism quantification. In GC, the majority of available studies focused on SUVmax value. One group investigated 271 patients following gastrectomy and identified SUVmax > 8.2 as a negative prognostic factor favouring disease recurrence[19]. Another analysed 62 patients with disseminated GC. Their study described primary tumour SUVmax < 6.0 as a positive prognostic factor for OS and PFS. This value was calculated using an ROC model, and its significance out-powered median SUVmax value of the whole group (7.2)[8]. In a third study comprising 75 tumours limited to the stomach and 22 disseminated tumours, 25% of all tumours did not exhibit pathologic 18FDG uptake. The authors indirectly explained this by pointing at a significant share of tumours of low clinical stage and poor cellular differentiation (with both showing tendency for low 18FDG-uptake) in their group. In our study, no false negative results occurred. Tumours that did demonstrate pathologic 18FDG uptake, as well as T3/T4 tumours and those above 60 mm in diameter, exhibited a statistically worse OS, whereas a threshold of median SUVmax (6.7) was not prognostic for OS. This study is notable for demonstrating the superiority of PET-CT over contrast-enhanced CT in detection of metastatic lymph nodes; PET-CT-positive lymph nodes were a significant prognostic factor, whereas lymph nodes positive on CT were not prognostic[4]. Finally, it was reported that SUVmax > 8 was prognostic for worse OS in a group of 35 disseminated GC patients who underwent palliative chemotherapy[9].
In our study, despite a notable inequality in median SUVmax between limited and disseminated GC (10.36 vs 12.78), no significant difference in SUVmax was observed. The ROC model also failed to identify an SUVmax value that was significantly prognostic for tumour dissemination. However, we did identify other 18FDG metabolic quantifiers (TLG, MTV, SUVmean; these quantifiers are presented in Table 4) that significantly correlated with clinical variables, some of which have not been described previously in GC.
This study observed that MTV and TLG of almost all aforementioned thresholds were significantly elevated in T4 tumours. Other studies investigating the correlation between 18FDG metabolism and tumour stage have also demonstrated increased radiotracer uptake. One study reported that increased uptake was associated with worse OS but[4] another study did not[20]. Caution should be taken in the interpretation of our observations due to the significant differences in the sizes of the pT1-3 and pT4 groups (32 vs 8) and due to the lack of contrast enhancement of the low-dose CT layer in the PET-CT study. There have been numerous reports on high levels of 18FDG uptake by chronic gastric inflammation, which can significantly increase the number of false positive studies, as well as reports of predictions of inaccurately large tumour volumes in the context of chronic inflammation co-existing (common because gastritis chronica is an identified precancerous state)[21]. Dilation of the stomach with neutral fluid prior to the examination has been proposed by some who claim that it helps better visualise tumour borders and distinguishes physiological 18FDG uptake from tumor uptake and involved regional lymph nodes[13,22].
In our study, we observed that COV was significantly higher in poorly differentiated G3 tumours. This parameter has not been reported in GC, yet studies covering different tumour types describe elevated COV as an indicator of tumour heterogeneity and a predictive factor of a worse treatment outcome[23,24]. In our study, due to the variety of treatment modalities, we only investigated the correlation between COV and clinical and pathological variables. Our observation may indicate that tumours with higher COV exhibit a worse response to chemotherapy, consistent with the aforementioned studies. This hypothesis requires further study.
MTV2.5, TLGliv, and COV were significantly associated with the degree of weight loss, regardless of clinical stage. This trend may be explained by increased glucose metabolism in large and advanced tumours, leading to cachexia through deteriorating gastric function (through limiting its capacity, absorption and muscular activity) and secretion of pro-cachectic cytokines. No other study has investigated such correlations. One group included BMI in their analysis, but there was no correlation between BMI and tumours with high and low 18FDG uptake or according to lymph node involvement status[11].
Our study analysed 18FDG metabolism of primary tumours in an attempt to identify differences in metabolic quantifiers that differ significantly between metastatic and non-metastatic tumours. These metabolic quantifiers could help to identify patients in whom tumour dissemination is believed to have occurred. These quantifiers would be of particular interest in signet-ring carcinoma, which is characterised by exceptionally low 18FDG uptake (reportedly due to lower cellular density and lower GLUT-1 expression ) and peritoneal tumour spread with tumour foci below the limit of PET-CT resolution[25,26]. We have identified six metabolic quantifiers as potentially able to differentiate disseminated and limited disease. The investigated parameters were pre-selected to be suitable for tumours with relatively low radiotracer uptake (i.e., 50% SUVmax threshold was omitted from our analysis), and results confirmed the overall superiority of MTV and TLG with centre-weighed 30% SUVmax thresholds (78% AUC). Their potential to identify tumours likely to disseminate has not been well established for GC, but other studies in other cancers have described TLG as a useful prognostic biomarker[27-30].
Multi- and univariate analysis of OS and TTM in our study identified TLG30 as the most valuable survival predictor of all 18FDG metabolism quantifiers. The results of studies of other tumour types[27] n = 81 NSCLC patients[29] n = 86 oesophageal carcinoma patients; in both TLG2.5 prognostic for OS and recurrence-free survival[30]; n = 41 various solid tumours (n = 6 GC), TLG predictive for chemotherapy response are consistent with our observations regarding prognosis as well[27,29,30].
Our study was designed as a hypothesis generator to investigate general trends and correlations of 18FDG metabolism quantifiers with the clinical course of GC and should only be regarded as such. Patients were not standardised or stratified according to clinical stage, and they received a variety of treatment modalities (some received none except best supportive care). No minimal follow-up period was introduced (range: 6 d to 5.2 years, median-9.5 mo). Therefore, all results related to the survival analysis should be interpreted with caution. Lack of contrast enhancement in the CT layer in our PET/CT study protocol could also disturb interpretation of local lymph node involvement status (in PET image, lymph nodes often merge into a single high-radiotracer uptake region with the primary tumour) and, consecutively clinical stage.
In conclusion, PET-CT is a useful tool in the diagnosis of gastric cancer. When appropriate study protocol enhancements are applied, PET-CT can be used to more reliably stage patients and, as our study has demonstrated, identify patients with potentially worse prognoses and those at greater risk of peritoneal tumour spread. Radiotracer quantifiers with low 18FDG uptake are preferred for PET-CT; the results of our and other studies indicate that total lesion glycolysis with low radiotracer uptake with 2.5 absolute thresholds and 30% relative thresholds should always be included in the analysis. Initial and, optionally, intra-treatment PET-CT can be a valuable addition to randomised clinical trials to help properly stage patients and to assess radiotracer metabolism changes as a response to therapy. In a clinical trial with a standardised group of patients, the diagnostic, prognostic and possibly predictive values of PET-CT, as well as its limitations, can be more unambiguously confirmed, eventually permitting the widespread use of PET-CT in the therapeutic decision-making in GC patients.
Gastric cancer (GC) features a high mortality to incidence ratio, which indirectly demonstrates its low curability. Thus, there is still significant room for improvement in its diagnosis and therapy. Fusion 18-fluorodeoxyglucose positron emission tomography with computed tomography [18Fluorodeoxyglucose (18FDG)-positron emission tomography-computer tomography (PET-CT)] is not a routinely used diagnostic method in gastric cancer. However, many recent studies confirm its diagnostic potential, especially in tumours of higher clinical stage quantification.
In addition to visualising the primary tumour, locoregional and distant metastatic sites, studies also note its prognostic and predictive value.
In context s of predictive and prognostic value, most publications highlight the maximum standardized uptake value (SUV) value of the primary tumour. In our study, in addition to SUVmax, the authors analysed numerous radiotracer quantifiers, which have only been mentioned in few or none studies in gastric cancer. Despite a notable inequality in median SUVmax between limited and disseminated GC, no significant difference in SUVmax was observed. However, we did identify other 18FDG metabolic quantifiers measured in primary tumor (total lesion glycolysis (TLG), metabolic tumor volume (MTV), SUVmean) which allowed to differentiate between patients with local and metastatic disease and had a prognostic significance. The results confirmed the overall superiority of radiotracer uptake quantifiers that are not affected by relatively low radiotracer uptake of the tumour (which is generally the case for GC compared to other tumours), namely MTV and TLG with centre-weighed 30% SUVmax thresholds (78% AUC). Their potential to identify tumours likely to disseminate has not been well established for GC, but a number of studies in other cancers have described TLG as a useful prognostic biomarker.
This broad analysis can be clinically utilized to identify groups of patients with worse tumour prognosis and who could possibly benefit from more aggressive treatment. The study results suggest that PET-CT studies may have a firm place in the therapeutic decision-making in gastric cancer patients.
PET/CT is a tool of nuclear medicine. It is a combination of PET, a functional imaging technique that produces a three-dimensional image of metabolic processes in the body and CT, a 3-dimensional X-ray scan performed on the patient during the same session, in the same machine. 18FDG is an analogue of glucose labeled with a short-life radioactive isotope of fluorine, which is injected into a patient before PET scanning. The concentrations of radiotracer visualized indicate tissue metabolic activity by virtue of the regional glucose uptake. Radiotracer uptake above a certain level is considered as suspected for presence of a primary malignant tumor or metastasis, even despite its normal structural image in CT. The SUV is often used in PET imaging for a simple semi-quantitative analysis. The SUV represents the ratio of the radioactivity measured in a spatially defined part of the body at a certain time point to a hypothetically even distribution of the injected radioactivity across the whole body. MTV represents a measurable volume of a given cubic unit whose radioactivity exceeds a threshold assumed to differentiate between a normal tissue and a malignant tumor. TLG is defined as SUVmean x MTV; it represents a product of intensity and volume quantifiers describing the same spatially localized radioactivity.
Prognostic value and clinical correlations of 18-fluorodeoxyglucose metabolism quantifiers in gastric cancer is an excellent paper.
P- Reviewer: Garfield D, Kilickesmez O, Yen TC S- Editor: Qi Y L- Editor: A E- Editor: Zhang DN
| 1. | International Agency for Research on Cancer. GLOBOCAN project. Accessed 20 June. 2014; Available from: http://globocan.iarc.fr. |
| 2. | Minamimoto R, Senda M, Jinnouchi S, Terauchi T, Yoshida T, Murano T, Fukuda H, Iinuma T, Uno K, Nishizawa S. The current status of an FDG-PET cancer screening program in Japan, based on a 4-year (2006-2009) nationwide survey. Ann Nucl Med. 2013;27:46-57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 3. | Sehdev A, Catenacci DV. Gastroesophageal cancer: focus on epidemiology, classification, and staging. Discov Med. 2013;16:103-111. [PubMed] |
| 4. | Coupe NA, Karikios D, Chong S, Yap J, Ng W, Merrett N, Lin M. Metabolic information on staging FDG-PET-CT as a prognostic tool in the evaluation of 97 patients with gastric cancer. Ann Nucl Med. 2014;28:128-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Smyth EC, Shah MA. Role of ¹⁸F 2-fluoro-2-deoxyglucose positron emission tomography in upper gastrointestinal malignancies. World J Gastroenterol. 2011;17:5059-5074. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 42] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Makis W, Ciarallo A, Hickeson M, Rush C, Derbekyan V, Novales-Diaz JA, Laufer J, Stern J, Lisbona R. Spectrum of gastric malignancy on 18F-FDG PET/CT: a pictorial essay. Clin Imaging. 2012;36:432-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Namikawa T, Okabayshi T, Nogami M, Ogawa Y, Kobayashi M, Hanazaki K. Assessment of (18)F-fluorodeoxyglucose positron emission tomography combined with computed tomography in the preoperative management of patients with gastric cancer. Int J Clin Oncol. 2014;19:649-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Park JC, Lee JH, Cheoi K, Chung H, Yun MJ, Lee H, Shin SK, Lee SK, Lee YC. Predictive value of pretreatment metabolic activity measured by fluorodeoxyglucose positron emission tomography in patients with metastatic advanced gastric cancer: the maximal SUV of the stomach is a prognostic factor. Eur J Nucl Med Mol Imaging. 2012;39:1107-1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Chung HW, Lee EJ, Cho YH, Yoon SY, So Y, Kim SY, Lee MH, Kim JH, Lee SY, Sung IK. High FDG uptake in PET/CT predicts worse prognosis in patients with metastatic gastric adenocarcinoma. J Cancer Res Clin Oncol. 2010;136:1929-1935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 10. | Kim YH, Choi JY, Do IG, Kim S, Kim BT. Factors affecting 18F-FDG uptake by metastatic lymph nodes in gastric cancer. J Comput Assist Tomogr. 2013;37:815-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Oh HH, Lee SE, Choi IS, Choi WJ, Yoon DS, Min HS, Ra YM, Moon JI, Kang YH. The peak-standardized uptake value (P-SUV) by preoperative positron emission tomography-computed tomography (PET-CT) is a useful indicator of lymph node metastasis in gastric cancer. J Surg Oncol. 2011;104:530-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Zou H, Zhao Y. 18FDG PET-CT for detecting gastric cancer recurrence after surgical resection: a meta-analysis. Surg Oncol. 2013;22:162-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Lee SJ, Lee WW, Yoon HJ, Lee HY, Lee KH, Kim YH, Park do J, Kim HH, So Y, Kim SE. Regional PET/CT after water gastric inflation for evaluating loco-regional disease of gastric cancer. Eur J Radiol. 2013;82:935-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Kamimura K, Nagamachi S, Wakamatsu H, Fujita S, Nishii R, Umemura Y, Ogita M, Komada N, Sakurai T, Inoue T. Role of gastric distention with additional water in differentiating locally advanced gastric carcinomas from physiological uptake in the stomach on 18F-fluoro-2-deoxy-D-glucose PET. Nucl Med Commun. 2009;30:431-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Hopkins S, Yang GY. FDG PET imaging in the staging and management of gastric cancer. J Gastrointest Oncol. 2011;2:39-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 16. | Mukai K, Ishida Y, Okajima K, Isozaki H, Morimoto T, Nishiyama S. Usefulness of preoperative FDG-PET for detection of gastric cancer. Gastric Cancer. 2006;9:192-196. [PubMed] |
| 17. | Chen J, Cheong JH, Yun MJ, Kim J, Lim JS, Hyung WJ, Noh SH. Improvement in preoperative staging of gastric adenocarcinoma with positron emission tomography. Cancer. 2005;103:2383-2390. [PubMed] |
| 18. | Lee JW, Lee SM, Lee MS, Shin HC. Role of ¹⁸F-FDG PET/CT in the prediction of gastric cancer recurrence after curative surgical resection. Eur J Nucl Med Mol Imaging. 2012;39:1425-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Ha TK, Choi YY, Song SY, Kwon SJ. F18-fluorodeoxyglucose-positron emission tomography and computed tomography is not accurate in preoperative staging of gastric cancer. J Korean Surg Soc. 2011;81:104-110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Lin CY, Liu CS, Ding HJ, Sun SS, Yen KY, Hsieh TC, Lin CC, Kao CH. Positive correlation between standardized uptake values of FDG uptake in the stomach and the value of the C-13 urea breath test. Clin Nucl Med. 2006;31:792-794. [PubMed] |
| 21. | Ma Q, Xin J, Zhao Z, Guo Q, Yu S, Xu W, Liu C, Zhai W. Value of ¹⁸F-FDG PET/CT in the diagnosis of primary gastric cancer via stomach distension. Eur J Radiol. 2013;82:e302-e306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Willaime JM, Turkheimer FE, Kenny LM, Aboagye EO. Quantification of intra-tumour cell proliferation heterogeneity using imaging descriptors of 18F fluorothymidine-positron emission tomography. Phys Med Biol. 2013;58:187-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 23. | Bundschuh RA, Dinges J, Neumann L, Seyfried M, Zsótér N, Papp L, Rosenberg R, Becker K, Astner ST, Henninger M. Textural Parameters of Tumor Heterogeneity in 18F-FDG PET/CT for Therapy Response Assessment and Prognosis in Patients with Locally Advanced Rectal Cancer. J Nucl Med. 2014;55:891-897. [PubMed] |
| 24. | Yamada A, Oguchi K, Fukushima M, Imai Y, Kadoya M. Evaluation of 2-deoxy-2-[18F]fluoro-D-glucose positron emission tomography in gastric carcinoma: relation to histological subtypes, depth of tumor invasion, and glucose transporter-1 expression. Ann Nucl Med. 2006;20:597-604. [PubMed] |
| 25. | Stahl A, Ott K, Weber WA, Becker K, Link T, Siewert JR, Schwaiger M, Fink U. FDG PET imaging of locally advanced gastric carcinomas: correlation with endoscopic and histopathological findings. Eur J Nucl Med Mol Imaging. 2003;30:288-295. [PubMed] |
| 26. | Van de Wiele C, Kruse V, Smeets P, Sathekge M, Maes A. Predictive and prognostic value of metabolic tumour volume and total lesion glycolysis in solid tumours. Eur J Nucl Med Mol Imaging. 2013;40:290-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 132] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 27. | Zaizen Y, Azuma K, Kurata S, Sadashima E, Hattori S, Sasada T, Imamura Y, Kaida H, Kawahara A, Kinoshita T. Prognostic significance of total lesion glycolysis in patients with advanced non-small cell lung cancer receiving chemotherapy. Eur J Radiol. 2012;81:4179-4184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 28. | Lee JW, Kang CM, Choi HJ, Lee WJ, Song SY, Lee JH, Lee JD. Prognostic Value of Metabolic Tumor Volume and Total Lesion Glycolysis on Preoperative 18F-FDG PET/CT in Patients with Pancreatic Cancer. J Nucl Med. 2014;55:898-904. [PubMed] |
| 29. | Li YM, Lin Q, Zhao L, Wang LC, Sun L, Dai MM, Luo ZM, Zheng H, Wu H. Pre-treatment metabolic tumor volume and total lesion glycolysis are useful prognostic factors for esophageal squamous cell cancer patients. Asian Pac J Cancer Prev. 2014;15:1369-1373. [PubMed] |
| 30. | Larson SM, Erdi Y, Akhurst T, Mazumdar M, Macapinlac HA, Finn RD, Casilla C, Fazzari M, Srivastava N, Yeung HW. Tumor Treatment Response Based on Visual and Quantitative Changes in Global Tumor Glycolysis Using PET-FDG Imaging. The Visual Response Score and the Change in Total Lesion Glycolysis. Clin Positron Imaging. 1999;2:159-171. [PubMed] |