Published online May 21, 2015. doi: 10.3748/wjg.v21.i19.5893
Peer-review started: November 25, 2014
First decision: December 11, 2014
Revised: January 12, 2015
Accepted: February 13, 2015
Article in press: February 13, 2015
Published online: May 21, 2015
Processing time: 177 Days and 5 Hours
AIM: To validate the value of aspartate aminotransferase to platelet ratio index (APRI) in assessment of liver fibrosis and prediction of postoperative prognosis of biliary atresia (BA) infants from Mainland China.
METHODS: Medical records of 153 BA infants who were hospitalized from January 2010 to June 2013 were reviewed. The efficacy of APRI for diagnosis of liver fibrosis was assessed using the receiver operator characteristic (ROC) curve compared to the pathological Metavir fibrosis score of the liver wedge specimens of 91 BA infants. The prognostic value of preoperative APRI for jaundice persistence, liver injury, and occurrence of cholangitis within 6 mo after KP was studied based on the follow-up data of 48 BA infants.
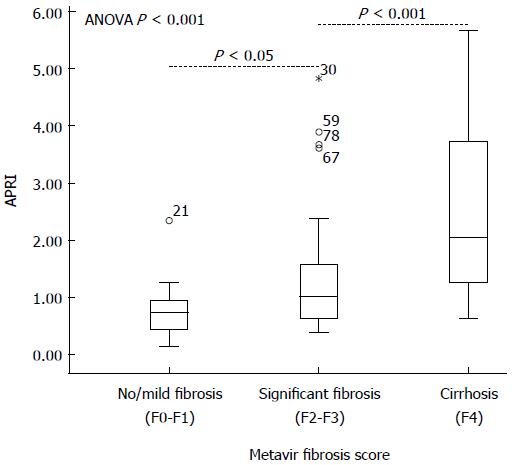
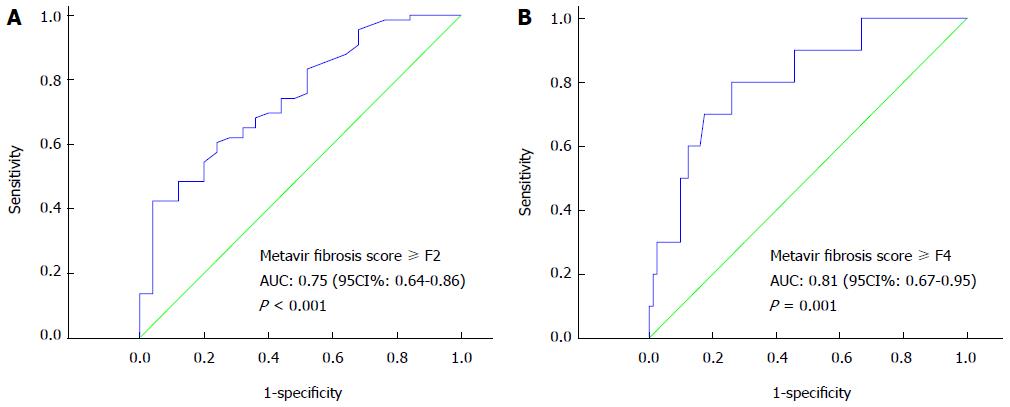
RESULTS: APRI was significantly correlated with Metavir scores (rs = 0.433; P < 0.05). The mean APRI value was 0.76 in no/mild fibrosis group (Metavir score F0-F1), 1.29 in significant fibrosis group (F2-F3), and 2.51 in cirrhosis group (F4) (P < 0.001). The area under the ROC curve (AUC) of APRI for diagnosing significant fibrosis and cirrhosis was 0.75 (P < 0.001) and 0.81 (P = 0.001), respectively. The APRI cut-off of 0.95 was 60.6% sensitive and 76.0% specific for significant fibrosis diagnosis, and a threshold of 1.66 was 70.6% sensitive and 82.7% specific for cirrhosis. The preoperative APRI in infants who maintained jaundice around 6 mo after KP was higher than that in those who did not (1.86 ± 2.13 vs 0.87 ± 0.48, P < 0.05). The AUC of APRI for prediction of postoperative jaundice occurrence was 0.67. A cut-off value of 0.60 showed a sensitivity of 66.7% and a specificity of 83.3% for the prediction of jaundice persistence. Preoperative APRI had no significant association with later liver injury or occurrence of cholangitis.
CONCLUSION: Our study demonstrated that APRI could diagnose significant liver fibrosis, especially cirrhosis in BA infants, and the elevated preoperative APRI predicts jaundice persistence after KP.
Core tip: There is still controversy over the role of aspartate aminotransferase to platelet ratio index (APRI) in the diagnosis of liver fibrosis and prognosis in biliary atresia. In this paper, we confirmed that APRI could help diagnose significant liver fibrosis, especially cirrhosis in a cohort of infants with biliary atresia from Mainland China, and preoperative APRI also could predict jaundice persistence after Kasai portoenterostomy. These results may have implications in the management of this disease.
- Citation: Yang LY, Fu J, Peng XF, Pang SY, Gao KK, Chen ZR, He LJ, Wen Z, Wang H, Li L, Wang FH, Yu JK, Xu Y, Gong ST, Xia HM, Liu HY. Validation of aspartate aminotransferase to platelet ratio for diagnosis of liver fibrosis and prediction of postoperative prognosis in infants with biliary atresia. World J Gastroenterol 2015; 21(19): 5893-5900
- URL: https://www.wjgnet.com/1007-9327/full/v21/i19/5893.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i19.5893
Biliary atresia (BA) is a severe, cholestatic disease of infancy of unknown cause characterized by fibrous obliteration of inter- and extra-hepatic biliary tree. In untreated cases, it would cause death by 2 years of age. It occurred with an incidence of 1 in 5000 to 1 in 19000 live births, and was more common in Asian countries[1-3]. Etiology and molecular networks underpinning such an expeditious fibrogenic process have not been well established. Immune and nonimmune factors were implicated in the pathogenesis of BA, and the resultant cholestasis and oxidative stress appeared to be the main triggers of hepatic fibrosis in this disease[4,5]. Early Kasai portoenterostomy (KP) is the only and palliative therapy with the hope of restoring bile flow to the gastrointestinal tract to alleviate injury of the liver caused by cholestasis and to prolong the survival with the native liver, but it cannot stop the progress of inflammation around bile ducts and hepatic fibrosis. Nearly one third of cases may develop persistent jaundice, recurrent cholangitis and other symptoms a short term post-operation and had worse outcome. Variable liver fibrosis, cirrhosis, and liver failure may still happen[6-8]. When KP had failed and severe complications occurred, liver transplantation is the only effective treatment. Hepatic fibrosis is not only a universal and prominent feature of BA, but also the most important predictor of outcome post KP. Therefore, assessment of hepatic fibrosis is critical for the management after KP[9-12].
In BA, assessment of liver fibrosis had traditionally been accomplished by subjective visual analysis of trichrome staining of liver tissue obtained at the time of KP or liver transplantation[13,14]. It cannot meet the needs of early and continual liver status assessment in BA patients, though pathological diagnosis is believed as the gold standard for determining the degree of liver fibrosis[15]. Less invasive techniques developed in adults had been applied in pediatric patients with chronic liver diseases[13,16,17]. Among the serological indicators, aspartate aminotransferase to platelet ratio index (APRI) is the simplest model based on aspartate transaminase (AST) and platelet count from routine hematological and biochemical tests and had obtained attention in BA patients during the last several years[18-21]. However, controversial results have been released when assessing the value of APRI in liver fibrosis diagnosis in BA children and post KP outcome prediction. Regarding that the biased results might be caused by small sample size in previous studies, we validated the value of APRI in assessment of liver fibrosis and prediction of postoperative prognosis of BA infants from Mainland China.
The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the ethics committee of Guangzhou Women and Children’s Medical Center in Guangzhou, China. Enrollment was proposed to all consecutive infants with diagnosed BA confirmed by cholangiogram at abdomen surgical or laparoscopic biliary duct exploration, who admitted to our center between January 2010 and June 2013. Basic information including sex, age at operation, and routine biochemical and hematological parameters were collected. Follow-up data which could evaluate persistence of jaundice (total bilirubin > 34 μmol/L), acute liver injury (ALT > 35 U/L), and occurrence of cholangitis within 6 mo after KP were collected for analysis of short-term outcomes.
Patients with incomplete data, or younger than 15 days, or diagnosed with co-occurring heart disease were excluded, because their platelet counts and AST levels might be influenced.
APRI was calculated using the formula of Wai et al[18]: [AST/upper limit of normal (ULN)]/platelet count (expressed as platelets × 109/L) × 100. AST and platelets tested within 1 wk before the operation were used in the analysis. Results closest to the date of operation were chosen if more than one set of laboratory data was available.
The ULN of AST was verified before calculation. AST levels of 150 age- and sex-matched healthy infants were tested according to document C28-A3 of the Clinical and Laboratory Standards Institute (CLSI)[22]. Their AST values showed normal distribution with 95% confidence interval (CI) of 28-60 U/L. Therefore, 60 U/L was used as the ULN of AST for APRI calculation in this study.
Liver sections of the enrolled patients were retrieved from sample bank in department of pathology and reviewed blindly by two experienced pathologists. Liver wedge tissue samples were obtained during surgical exploration or KP. Biopsy specimens were fixed in 10% neutral formalin and embedded in paraffin. Sections were stained with hematoxylin and eosin (HE), and Masson’s trichrome. The Metavir scoring system was applied to quantify liver fibrosis based on the architectural features of portal fibrosis, of which a 0-4 scale was staged as follows: F0 = no fibrosis, F1 = portal fibrosis without septa, F2 = portal fibrosis with rare septa, F3 = numerous septa and bridging fibrosis without cirrhosis, and F4 = cirrhosis[23]. Scoring F0-F1 was considered as no/mild fibrosis, F2-F3 as significant fibrosis, and F4 as cirrhosis.
Analyses were performed using IBM SSPS 19.0. Quantitative data are shown as mean ± SD unless otherwise stated. Continuous variables were compared using the Student’s t-test or ANOVA analysis. Correlation was evaluated by the Spearman correlation coefficient. The diagnostic efficacy of APRI was assessed using the receiver operator characteristic (ROC) curve. All of the possible cut-off values were associated with the probability of a true positive (sensitivity) and a true negative (specificity). The best cut-off point was defined as the highest value of sensitivity plus specificity. Values of the area under the ROC curves (AUC) near 1.0 indicated high diagnostic accuracy. P values less than 0.05 were regarded as statistically significant. The statistical methods of this study were reviewed by Jing Gu from the Department of Medical Statistics and Epidemiology, School of Public Health, Sun Yat-sen University.
One hundred and fifty-three BA infants (92 boys and 61 girls) were diagnosed at an average age of 85 ± 38 d (range: 1-241 d). Among them, 45.8% (70/153) underwent KP with a mean age of 73 ± 23 d at operation. After the exclusion criteria were considered, 91 and 48 BA infants who fulfilled the requirements of the clinical and laboratory data were entered into the study of APRI in liver fibrosis and postoperative prognosis, respectively.
Ninety-one infants, 55 boys and 36 girls, with a median age at surgery of 83 ± 36 d (range: 19-224 d), were enrolled for analysis of correlation between APRI and liver fibrosis severity. Metavir scores were F0 in 2, F1 in 23, F2 in 35, F3 in 21, and F4 in 10 patients. Clinical characteristics of the patients are summarized in Table 1. APRI in BA patients correlated with Metavir scores (rs = 0.433; P < 0.05), as well as original AST (rs = 0.266; P < 0.05) and PLT (-0.346; P < 0.05). Additionally, the age at the time of surgery and total bile acids (the cholestatic parameter) showed a significant positive correlation with the degree of fibrosis (rs = 0.289, 0.332, respectively; P < 0.01 for each). Comparison of clinical characteristics between these individuals showed a statistical significance for APRI by Metavir stage (P < 0.001); the median APRI in F4 was significantly different from any stage of F0-F3 with followed the least significant difference post-hoc test (P = 0.000-0.007) but no statistical difference in the stages of F0 to F3 (P = 0.050-0.703). When categorizing F0-F4 into groups of no/mild fibrosis (F0-F1), significant fibrosis (F2-F3) and cirrhosis (F4) by the degree of fibrosis, no/mild fibrosis was present in 27.5% of the biopsies, significant fibrosis in 61.5% and cirrhosis in 11.0%. The mean APRI values in groups of no/mild fibrosis, significant fibrosis, and cirrhosis were 0.76, 1.29, and 2.51, respectively (ANOVA P < 0.001; Figure 1). The APRI in the three groups significantly differed from each other and increased with the progression of liver fibrosis.
| Parameter, mean ± SD | All | Metavir stage | |||||
| (n = 91) | F0 | F1 | F2 | F3 | F4 | P value | |
| (n = 2) | (n = 23) | (n = 35) | (n = 21) | (n = 10) | |||
| Age (d) | 83 (36) | 45 (37) | 70 (37) | 84 (22) | 85 (34) | 110 (62) | 0.027 |
| AST (U/L) | 285 (182) | 233 (259) | 221 (114) | 310 (230) | 307 (169) | 306 (125) | NS |
| ALT (U/L) | 179 (105) | 127 (152) | 175 (128) | 197 (104) | 152 (78) | 198 (94) | NS |
| ALP (U/L) | 541 (221) | 366 (235) | 511 (207) | 569 (220) | 518 (242) | 597 (225) | NS |
| γ-GT (U/L) | 895 (685) | 892 (841) | 751 (632) | 1009 (810) | 972 (610) | 662 (404) | NS |
| TP (g/L) | 58.3 (4.8) | 57.6 (13.9) | 57.9 (5.7) | 58.8 (4.2) | 58.7 (4.0) | 56.5 (4.5) | NS |
| ALB (g/L) | 40.0 (4.7) | 41.4 (3.9) | 40.0 (3.8) | 40.6 (5.4) | 40.0 (4.2) | 38.2 (5.3) | NS |
| TBIL (μmol/L) | 202.8 (75.8) | 313.5 (44.6) | 192.5 (71.9) | 193.5 (60) | 222.5 (100.6) | 195.4 (65.6) | NS |
| DBIL (μmol/L) | 147.6 (55.2) | 133.6 (92.6) | 138.9 (52.9) | 144.2 (41.3) | 170.3 (75.6) | 134.9 (43.1) | NS |
| TBA (μmol/L) | 166.7 (68.5) | 71.9 (36.9) | 133.0 (53.8) | 175.2 (65.6) | 194.9 (64.0) | 177.2 (84.5) | 0.007 |
| PLT (× 109/L) | 447 (162) | 713 (127) | 498 (126) | 454 (151) | 435 (155) | 279 (165) | 0.000 |
| INR | 1.03 (0.26) | 1.69 (1.06) | 1.01 (0.13) | 1.00 (0.11) | 1.00 (0.12) | 1.18 (0.59) | 0.001 |
| AST/ALT | 1.84 (1.13) | 2.14 (0.52) | 1.84 (1.80) | 1.64 (0.65) | 2.21 (1.06) | 1.66 (0.52) | NS |
| APRI | 1.28 (1.05) | 0.50 (0.52) | 0.79 (0.45) | 1.26 (0.93) | 1.35 (0.93) | 2.51 (1.68) | 0.000 |
Based on two different thresholds of significant fibrosis (F2-F3) and cirrhosis (F4), ROC curves of APRI for the 91 subjects were constructed as F0-F1 vs F2-F4, and F0-F3 vs F4 (Figure 2). The area under the ROC curve (AUC) of APRI for diagnosing significant fibrosis (F2-F3) and cirrhosis (F4) were 0.75 (P < 0.001) and 0.81 (P = 0.001), respectively. For significant fibrosis, an APRI cut-off of 0.95 was 60.6% sensitive and 76.0% specific. A threshold of 1.66 was 70.6% sensitive and 82.7% specific for cirrhosis. The accuracies of APRI for diagnosing significant fibrosis and cirrhosis in the BA infants were 64.8% and 82.4%, respectively.
Forty-eight BA infants who had followed-up record were included in the prediction of postoperative outcomes. Twenty-four of them (50%) showed persistent jaundice 6 mo after KP and had higher preoperative APRI than the jaundice-free infants (1.86 ± 2.13 vs 0.87 ± 0.48, P < 0.05). The marginal cut-off value for preoperative APRI was 0.60 (AUC = 0.67; P < 0.05) in predicting persistence of jaundice, with a sensitivity of 66.7% and a specificity of 83.3%. The APRI value significantly predicted the persistence of jaundice after operation with an odds ratio of 7.0 (95%CI: 1.6-29.9) and accuracy of 68.9%. Clearance of jaundice was not achieved in any of the children with APRI > 2.0. 18/48 (37.5%) who developed cholangitis within 6 mo after KP, showed no difference in preoperative APRI from those did not (1.33 ± 1.20 vs 1.39 ± 1.84, P > 0.05). This suggested that there was no association between preoperative APRI and the development of postoperative cholangitis.
With a cut off value of APRI = 0.95, 24 (50%) patients were in the group of significant fibrosis and the others in non-significant fibrosis group. The significant fibrosis group had higher AST and ALT levels (parameters of liver injury) at the time of KP than the non-significant fibrosis group (P < 0.05 for each, Table 2). Six months after KP, these parameters decreased to the same levels as the non-significant fibrosis group. There was no obvious change of AST and ALT in non-significant fibrosis group before and after KP. With a cut off value of APRI = 1.66, 10 (26.3%) patients were in the cirrhosis group. At the time of KP, infants in the cirrhosis group also had much higher AST and ALT than those without (P < 0.01 for each). But six months after KP, these two parameters of those infants decreased to the same as in the non-cirrhosis group, and that before surgery. The change of AST and ALT before and after KP for all patients had no significant correlation with APRI (P > 0.05 for each).
| Parameter | APRI for significant fibrosis (cut-off = 0.95) | APRI for cirrhosis (cut-off = 1.66) | ||||||
| At KP | 6 mo after KP | At KP | 6 mo after KP | |||||
| ≤ 0.95(n = 24) | > 0.95(n = 24) | ≤ 0.95(n = 24) | > 0.95(n = 24) | ≤ 1.66(n = 38) | > 1.66(n = 10) | ≤ 1.66(n = 38) | > 1.66(n = 10) | |
| AST (U/L) | 194 (83) | 496 (721)1 | 210 (188) | 149 (93) | 234 (92) | 785 (1077)2 | 183 (159) | 165 (102) |
| ALT (U/L) | 136 (73) | 293 (327)1 | 163 (128) | 126 (96) | 164 (100) | 407 (474)2 | 139 (112) | 168 (121) |
APRI is an indirect biochemical marker of hepatic fibrosis, based on limited expense and widespread available laboratory parameters, reflecting alterations in hepatic function. It was developed by Wai et al[18] in 2003 when the correlation of serum AST and platelet counts with fibrosis was found in adult patients with chronic hepatitis C. It has been introduced to detect fibrosis and cirrhosis in adult patients with other liver diseases with promising results[24,25]. There are little data available on the use of APRI in pediatric patients mainly with chronic hepatitis (B or C), NAFLD, and intestinal failure[22,26,27]. Until 2007, association of APRI with BA was first described, and it was first reported to significantly correlate with fibrosis stages and have a significant AUC for the diagnosis of cirrhosis of 0.73 on biopsies from 33 children with a group of chronic liver diseases including nine presenting with BA[20]. Then, in a series of 35 BA children, Kim et al[19] observed a significant correlation between APRI and the degree of hepatic fibrosis (r = 0.77), and AUC of 0.92 and 0.91 for the determination of Metavir ≥ F3 and F4, respectively. However, it could not be proved when Lind et al[21] studied 31 BA patients. Nonetheless, the latest study showed that APRI at a cut-off of 1.22 (AUC = 0.83) could predict macroscopic cirrhosis with a sensitivity of 75% and a specificity of 84%[28], when macroscopic assessment of fibrosis were done in a larger population involving 260 BA infants.
Our study is the first to validate the performance of APRI in assessing liver fibrosis with a cohort of BA infants from Mainland China, a popular area with high morbidity for this disease[5,12]. Our findings validated that APRI could diagnose significant fibrosis and cirrhosis but with less accuracy than initially described for BA patients. In our study, APRI significantly correlated with Metavir stages in BA infants and increased with the progression of fibrosis, similar to that previously reported in pediatric liver diseases including BA[20]. We also observed that APRI was not sensitive enough to differentiate Metavir stages except cirrhosis and indicated the presence of mild fibrosis as Lind et al[21] reported. This was possibly related to the basis of APRI on the routine laboratory parameters which did not directly reflect fibrogenesis but rather the current state of hepatic damage due to fibrosis or cirrhosis. In spite of this, APRI could identify significant fibrosis and cirrhosis at AUC of 0.75 and 0.81, respectively, in the present study, which showed similar performance for staging hepatitis C-related fibrosis in a recent meta-analysis by Lin et al[29]. The cut-offs and diagnostic efficacy including sensitivity, specificity and accuracy were different from other studies about BA patients, which could be explained due to the difference in the number of patients enrolled and AST ULN used for calculation of APRI. Thereby, our study showed that APRI could be used as a non-invasive alternative index for assessing the severity of liver fibrosis in BA, especially for diagnosing significant fibrosis and cirrhosis.
BA is a progressive disease consistent with the effects of on-going cholangitis, cholestasis and fibrosis. APRI has provided a 5-year prognostic value in patients with chronic hepatitis in a prospective study[30]. The good correlation of APRI with the presence of varices in elder children post KP had been found by several studies[19,31,32]. And this correlation with poorer outcomes after surgery had been brought forward to preoperative APRI but no agreed conclusions have been drawn[19,21]. In the latest study, the infants that achieved clearance of jaundice had significantly lower median APRI at the time of KP, and jaundice persisted in any child with an initial APRI of > 3.0 and required a liver transplant to survive[28]. The survival rate of the native liver in BA infants with low APRI was significantly improved compared to those with a high APRI. With a similar rate of jaundice clearance to the above-mentioned study, our study showed that preoperative APRI (> 0.60) could predict jaundice persistence with an odds ratio of 7.0, but not worsened liver injury or occurrence of cholangitis after KP. Jaundice clearance was not achieved in any child with an APRI > 2.0. Therefore, determination of APRI before KP may help clinicians by providing beneficial information in decision of therapy and management of BA after surgery.
The present study has the following advantages compared to previous studies. First, the association of APRI originated parameters AST and PLT with Metavir fibrosis scores was verified, which strongly provided important preconditions for feasibility of the index in assessing liver fibrosis in BA. The results dispelled the suspicions whether APRI could apply in such fibrosis that dramatically progresses to cirrhosis within a few weeks after birth and may represent another type of fibrosis distinguishing what is involved in other chronic liver diseases. Second, a verified and age-matched ULN of AST was used in the calculation of APRI. The aminotransferase ULN varying with age and methods is thought to be a major disadvantage of APRI[33]. Different ULN used in the subjects with same age were noticed in previous studies, which may be partly responsible for the difference in results. It is worth noting that using a uniform ULN may benefit the comparability between different studies and clinical application of APRI. One limitation of this study is that this is a retrospective study including patients from a single center. The efficacy of cut-off values remains to be further validated in clinical prospective and multicenter studies. Moreover, other important postoperative outcomes resulting from liver fibrosis, including EV/GV and portal hypertension, were not focused in a short-term follow-up in the present study. This may limit the clarification of APRI for postoperative prognosis, since initial APRI had little predictive value for future variceal formation reported recently and several studies described that it could predict EV/GV in elder BA children because of short follow-up time[29]. This could be considered in a prospective study.
In conclusion, APRI is effective in diagnosing significant liver fibrosis, especially cirrhosis, in BA infants. The elevated preoperative APRI also could predict jaundice persistence after KP. Since the method is convenient, inexpensive, and non-invasive, it will benefit the management of BA infants particularly in regions with limited healthcare resources.
Biliary atresia (BA) is the most common cholestatic liver disease which results in progressive sclerosing fibrous obliteration of the extra-hepatic bile ducts in early infancy. Kasai portoenterostomy (KP) is the standard surgical management for BA patients. Full evaluation of liver fibrosis and determination of the prognosis after KP may be beneficial to clinical practice. There still lacks an appropriate non-invasive marker.
Aspartate aminotransferase to platelet ratio index (APRI) is the simplest serological model for evaluating liver fibrosis. It has been used to assess liver fibrosis and cirrhosis in adult patients and even children with liver-related diseases with promising results. It also has been found to be a predictor of poorer outcomes post KP. However, it is controversial that APRI could be valuable in diagnosing liver fibrosis and predicting postoperative prognosis after surgery for BA children.
This is the first study to investigate the diagnostic performance of APRI in assessing liver fibrosis and predicting postoperative prognosis in a larger cohort of infants with BA in Mainland China. The authors validated the association of APRI-originated parameters (AST and platelet count) with the degree of liver fibrosis in BA, which provided important preconditions for the use of APRI in this disease. Moreover, a verified and age-matched upper limit of normal of AST was used for calculation of APRI. This study suggests that APRI is effective in diagnosing significant liver fibrosis, especially cirrhosis, in infants with BA and also has predictive value in determining persistence of jaundice after KP.
APRI may decrease the need for staging liver biopsy specimens in highly suspected BA infants or in monitoring of fibrosis. It can help clinicians to select proper therapeutic strategy in the management of BA infants.
APRI is an indirect invasive parameter for assessing liver fibrosis. It does not reflect fibrogenesis directly but rather the current state of hepatic damage due to fibrosis or cirrhosis. It is calculated using the formula: (AST/upper limit of normal)/platelet count (expressed as platelets × 109/L) × 100.
The authors investigated the diagnostic performance of APRI in assessing liver fibrosis and predicting postoperative prognosis in a larger cohort of infants with BA in Mainland China. They found that it could identify significant fibrosis and cirrhosis in BA infants at the area under the receiver operator characteristic curve of 0.75 and 0.81, respectively, and preoperative APRI (> 0.60) could predict jaundice persistence but not worsened liver injury or occurrence of cholangitis after KP. The results are interesting and may help to make decisions in selection of therapeutic strategy and management of BA infants after KP.
P- Reviewer: Liang XS S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Liu XM
| 1. | Lien TH, Chang MH, Wu JF, Chen HL, Lee HC, Chen AC, Tiao MM, Wu TC, Yang YJ, Lin CC. Effects of the infant stool color card screening program on 5-year outcome of biliary atresia in Taiwan. Hepatology. 2011;53:202-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 131] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 2. | Yoon PW, Bresee JS, Olney RS, James LM, Khoury MJ. Epidemiology of biliary atresia: a population-based study. Pediatrics. 1997;99:376-382. [PubMed] |
| 3. | McKiernan PJ, Baker AJ, Kelly DA. The frequency and outcome of biliary atresia in the UK and Ireland. Lancet. 2000;355:25-29. [PubMed] |
| 4. | Mieli-Vergani G, Vergani D. Biliary atresia. Semin Immunopathol. 2009;31:371-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 5. | Xu Y, Yu J, Zhang R, Yin Y, Ye J, Tan L, Xia H. The perinatal infection of cytomegalovirus is an important etiology for biliary atresia in China. Clin Pediatr (Phila). 2012;51:109-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 6. | Otte JB, de Ville de Goyet J, Reding R, Hausleithner V, Sokal E, Chardot C, Debande B. Sequential treatment of biliary atresia with Kasai portoenterostomy and liver transplantation: a review. Hepatology. 1994;20:41S-48S. [PubMed] |
| 7. | Serinet MO, Broué P, Jacquemin E, Lachaux A, Sarles J, Gottrand F, Gauthier F, Chardot C. Management of patients with biliary atresia in France: results of a decentralized policy 1986-2002. Hepatology. 2006;44:75-84. [PubMed] |
| 8. | Shneider BL, Brown MB, Haber B, Whitington PF, Schwarz K, Squires R, Bezerra J, Shepherd R, Rosenthal P, Hoofnagle JH. A multicenter study of the outcome of biliary atresia in the United States, 1997 to 2000. J Pediatr. 2006;148:467-474. [PubMed] |
| 9. | Weerasooriya VS, White FV, Shepherd RW. Hepatic fibrosis and survival in biliary atresia. J Pediatr. 2004;144:123-125. [PubMed] |
| 10. | Lai MW, Chang MH, Hsu SC, Hsu HC, Su CT, Kao CL, Lee CY. Differential diagnosis of extrahepatic biliary atresia from neonatal hepatitis: a prospective study. J Pediatr Gastroenterol Nutr. 1994;18:121-127. [PubMed] |
| 11. | Pape L, Olsson K, Petersen C, von Wasilewski R, Melter M. Prognostic value of computerized quantification of liver fibrosis in children with biliary atresia. Liver Transpl. 2009;15:876-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Honsawek S, Chayanupatkul M, Chongsrisawat V, Vejchapipat P, Poovorawan Y. Increased osteopontin and liver stiffness measurement by transient elastography in biliary atresia. World J Gastroenterol. 2010;16:5467-5473. [PubMed] |
| 13. | Haafiz AB. Liver fibrosis in biliary atresia. Expert Rev Gastroenterol Hepatol. 2010;4:335-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Alisi A, de Vito R, Monti L, Nobili V. Liver fibrosis in paediatric liver diseases. Best Pract Res Clin Gastroenterol. 2011;25:259-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Manning DS, Afdhal NH. Diagnosis and quantitation of fibrosis. Gastroenterology. 2008;134:1670-1681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 309] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 16. | Honsawek S, Chayanupatkul M, Chongsrisawat V, Theamboonlers A, Praianantathavorn K, Udomsinprasert W, Vejchapipat P, Poovorawan Y. Serum adiponectin and transient elastography as non-invasive markers for postoperative biliary atresia. BMC Gastroenterol. 2011;11:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Adams LA, Bulsara M, Rossi E, DeBoer B, Speers D, George J, Kench J, Farrell G, McCaughan GW, Jeffrey GP. Hepascore: an accurate validated predictor of liver fibrosis in chronic hepatitis C infection. Clin Chem. 2005;51:1867-1873. [PubMed] |
| 18. | Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518-526. [PubMed] |
| 19. | Kim SY, Seok JY, Han SJ, Koh H. Assessment of liver fibrosis and cirrhosis by aspartate aminotransferase-to-platelet ratio index in children with biliary atresia. J Pediatr Gastroenterol Nutr. 2010;51:198-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 20. | de Lédinghen V, Le Bail B, Rebouissoux L, Fournier C, Foucher J, Miette V, Castéra L, Sandrin L, Merrouche W, Lavrand F. Liver stiffness measurement in children using FibroScan: feasibility study and comparison with Fibrotest, aspartate transaminase to platelets ratio index, and liver biopsy. J Pediatr Gastroenterol Nutr. 2007;45:443-450. [PubMed] |
| 21. | Lind RC, Verkade HJ, Porte RJ, Hulscher JB. Aspartate transaminase-to-platelet ratio index is not correlated with severity of fibrosis or survival in children with biliary atresia. J Pediatr Gastroenterol Nutr. 2012;54:698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | c-28a3 Calsic. Defining, establishing, and verifying reference intervals in the clinical laboratory, approved guideline. 3rd edn Wayne: Clsi 2008; . |
| 23. | Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289-293. [PubMed] |
| 24. | Shin WG, Park SH, Jang MK, Hahn TH, Kim JB, Lee MS, Kim DJ, Jun SY, Park CK. Aspartate aminotransferase to platelet ratio index (APRI) can predict liver fibrosis in chronic hepatitis B. Dig Liver Dis. 2008;40:267-274. [PubMed] |
| 25. | Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009;7:1104-1112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1205] [Cited by in RCA: 1168] [Article Influence: 73.0] [Reference Citation Analysis (1)] |
| 26. | Díaz JJ, Gura KM, Roda J, Perez-Atayde AR, Duggan C, Jaksic T, Lo CW. Aspartate aminotransferase to platelet ratio index correlates with hepatic cirrhosis but not with fibrosis in pediatric patients with intestinal failure. J Pediatr Gastroenterol Nutr. 2013;57:367-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 27. | Siberry GK, Cohen RA, Harris DR, Cruz ML, Oliveira R, Peixoto MF, Cervi MC, Hazra R, Pinto JA. Prevalence and predictors of elevated aspartate aminotransferase-to-platelet ratio index in Latin American perinatally HIV-infected children. Pediatr Infect Dis J. 2014;33:177-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 28. | Grieve A, Makin E, Davenport M. Aspartate Aminotransferase-to-Platelet ratio index (APRi) in infants with biliary atresia: prognostic value at presentation. J Pediatr Surg. 2013;48:789-795. [PubMed] |
| 29. | Lin ZH, Xin YN, Dong QJ, Wang Q, Jiang XJ, Zhan SH, Sun Y, Xuan SY. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepatology. 2011;53:726-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 821] [Cited by in RCA: 791] [Article Influence: 56.5] [Reference Citation Analysis (0)] |
| 30. | Ngo Y, Munteanu M, Messous D, Charlotte F, Imbert-Bismut F, Thabut D, Lebray P, Thibault V, Benhamou Y, Moussalli J. A prospective analysis of the prognostic value of biomarkers (FibroTest) in patients with chronic hepatitis C. Clin Chem. 2006;52:1887-1896. [PubMed] |
| 31. | Colecchia A, Di Biase AR, Scaioli E, Predieri B, Iughetti L, Reggiani ML, Montrone L, Ceccarelli PL, Vestito A, Viola L. Non-invasive methods can predict oesophageal varices in patients with biliary atresia after a Kasai procedure. Dig Liver Dis. 2011;43:659-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 32. | Chongsrisawat V, Vejapipat P, Siripon N, Poovorawan Y. Transient elastography for predicting esophageal/gastric varices in children with biliary atresia. BMC Gastroenterol. 2011;11:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 33. | Perazzo H, Pais R, Munteanu M, Ngo Y, Monneret D, Imbert-Bismut F, Moussalli J, Lebray P, Benhamou Y, Thabut D. Variability in definitions of transaminase upper limit of the normal impacts the APRI performance as a biomarker of fibrosis in patients with chronic hepatitis C: “APRI c’est fini ?”. Clin Res Hepatol Gastroenterol. 2014;38:432-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |